Hepatitis Delta Virus Infection: An Overview
Abstract
1. Introduction
2. Structure and Replication
3. Global Epidemiology of Hepatitis Delta Virus and Viral Genotypes
4. Patterns of Transmission
5. Diagnosis
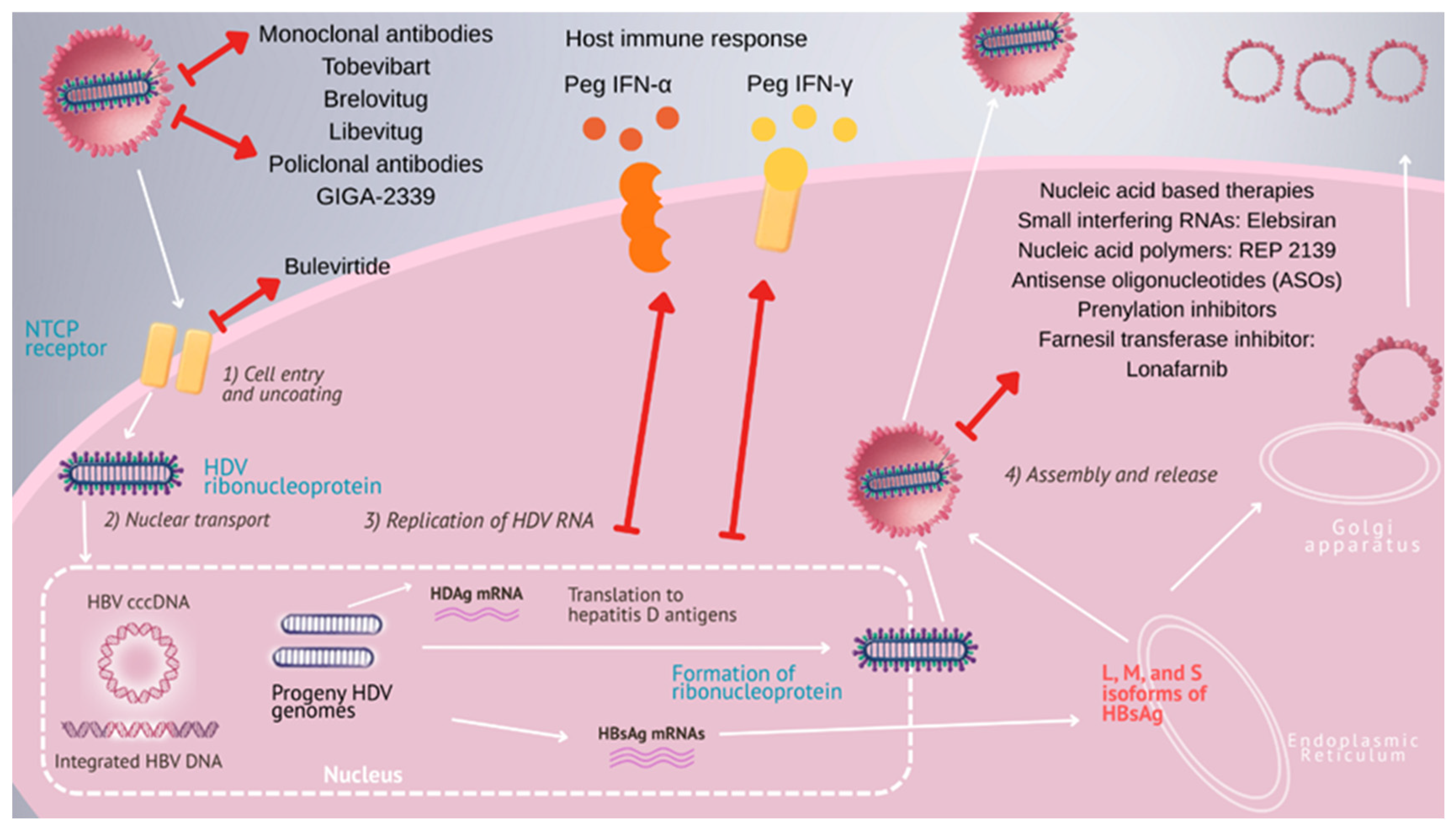
6. Overview of Hepatitis Delta Treatment
7. Small-Molecule Drug-Based Therapeutics
7.1. Bulevirtide
7.2. Lonafarnib
7.3. Nucleic Acid-Based Therapies
7.4. Antibody-Based Therapies
8. Liver Transplantation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizzetto, M.; Canese, M.G.; Aricò, S.; Crivelli, O.; Trepo, C.; Bonino, F.; Verme, G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977, 18, 997–1003. [Google Scholar] [CrossRef]
- Flores, R.; Owens, R.A.; Taylor, J. Pathogenesis by subviral agents: Viroids and hepatitis delta virus. Curr. Opin. Virol. 2016, 17, 87–94. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Babaian, A.; Bergner, L.M.; Dény, P.; Glebe, D.; Horie, M.; Koonin, E.V.; Krupovic, M.; Paraskevopoulou, S.; de la Peña, M.; et al. ICTV Virus Taxonomy Profile: Kolmioviridae 2024. J. Gen. Virol. 2024, 105, 001963. [Google Scholar] [CrossRef]
- Pearlman, B. Hepatitis Delta Infection: A Clinical Review. Semin. Liver Dis. 2023, 43, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Rizzetto, M. Hepatitis D Virus Infection. N. Engl. J. Med. 2023, 389, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Herrscher, C.; Roingeard, P.; Blanchard, E. Hepatitis B Virus Entry into Cells. Cells 2020, 9, 1486. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, Y.; Lempp, F.A.; Walter, L.; Mutz, P.; Bartenschlager, R.; Urban, S. Hepatitis D virus-induced interferon response and administered interferons control cell division-mediated virus spread. J. Hepatol. 2022, 77, 957–966. [Google Scholar] [CrossRef]
- Negro, F.; Lock, A.S. Hepatitis D: A Review. JAMA 2023, 330, 2376–2387. [Google Scholar] [CrossRef] [PubMed]
- Freitas, N.; Cunha, C.; Menne, S.; Gudima, S.O.; McFadden, G. Envelope proteins derived from naturally integrated hepatitis B virus DNA support assembly and release of infectious hepatitis delta virus particles. J. Virol. 2014, 88, 5742–5754. [Google Scholar] [CrossRef]
- Stockdale, A.J.; Kreuels, B.; Henrion, M.Y.R.; Giorgi, E.; Kyomuhangi, I.; Martel, C.; Hutin, Y.; Geretti, A.M. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J. Hepatol. 2020, 73, 523–532. [Google Scholar] [CrossRef]
- Montoya-Guzman, M.; Martinez, J.; Castro-Arroyave, D.; Rojas, C.; Navas, M.-C. Epidemiology and Genetic Diversity of Hepatitis B Virus and Hepatitis Delta Virus Infection in Indigenous Communities in Colombia. Microorganisms 2023, 11, 1739. [Google Scholar] [CrossRef]
- Rizzetto, M.; Hamid, S.; Negro, F. The changing context of hepatitis D. J. Hepatol. 2021, 74, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, T.; Ciancio, A.; Furlan, C.; Vinci, M.; Fontana, R.; Russello, M.; Colloredo, G.; Morisco, F.; Coppola, N.; Babudieri, S.; et al. Migratory flow and hepatitis delta infection in Italy: A new challenge at the beginning of the third millennium. J. Viral. Hepat. 2020, 27, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Béguelin, C.; Atkinson, A.; Boyd, A.; Falconer, K.; Kirkby, N.; Suter-Riniker, F.; Günthard, H.F.; Rockstroh, J.K.; Mocroft, A.; Rauch, A.; et al. Hepatitis delta infection among persons living with HIV in Europe. Liver Int. 2023, 43, 819–828. [Google Scholar] [CrossRef]
- Mello, F.; Barros, T.M.; Angelice, G.P.; Costa, V.P.; Mello, V.M.; Pardini, M.; Lampe, E.; Lago, B.V.; Villar, L.M.; Chao, D.-Y. Circulation of HDV Genotypes in Brazil: Identification of a Putative Novel HDV-8 Subgenotype. Microbiol. Spectr. 2023, 11, e0396522. [Google Scholar] [CrossRef]
- Aliasi-Sinai, L.; Worthington, T.; Lange, M.; Kushner, T. Maternal-to-Child Transmission of Hepatitis B Virus and Hepatitis Delta Virus. Clin. Liver Dis. 2023, 27, 917–935. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Chiu, K.W.; Chu, C.M.; Sheen, I.-S.; Huang, M.-J. Heterosexual transmission of hepatitis delta virus in the general population of an area endemic for hepatitis B virus infection: A prospective study. J. Infect. Dis. 1990, 162, 1170–1172. [Google Scholar] [CrossRef]
- Palom, A.; Sopena, S.; Riveiro-Barciela, M.; Carvalho-Gomes, A.; Madejón, A.; Rodriguez-Tajes, S.; Roade, L.; García-Eliz, M.; García-Samaniego, J.; Lens, S.; et al. One-quarter of chronic hepatitis D patients reach HDV-RNA decline or undetectability during the natural course of the disease. Aliment. Pharmacol. Ther. 2021, 54, 462–469. [Google Scholar] [CrossRef]
- WHO 2024. Guidelines for the Prevention, Diagnosis, Care and Treatment for People with Chronic Hepatitis B Infection. Available online: https://www.who.int/publications/i/item/9789240090903 (accessed on 18 July 2025).
- Yurdaydin, C.; Abbas, Z.; Buti, M.; Cornberg, M.; Esteban, R.; Etzion, O.; Gane, E.J.; Gish, R.G.; Glenn, J.S.; Hamid, S.; et al. Treating chronic hepatitis delta: The need for surrogate markers of treatment efficacy. J. Hepatol. 2019, 70, 1008–1015. [Google Scholar] [CrossRef]
- European Medicines Agency. Hepcludex (Bulevirtide). Product Information. 2023. Available online: https://www.ema.europa.eu/en/documents/product-information/hepcludex-epar-product-information_en.pdf (accessed on 18 July 2025).
- Wedemeyer, H.; Aleman, S.; Brunetto, M.R.; Blank, A.; Andreone, P.; Bogomolov, P.; Chulanov, V.; Mamonova, N.; Geyvandova, N.; Morozov, V.; et al. A phase 3, randomized trial of bulevirtide in chronic hepatitis D. N. Engl. J. Med. 2023, 389, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Aleman, S.; Brunetto, M.; Blank, A.; Andreone, P.; Bogomolov, P.; Chulanov, V.; Mamonova, N.; Geyvandova, N.; Morozov, V.; et al. Bulevirtide monotherapy in patients with chronic HDV: Efficacy and safety results through week 96 from a phase III randomized trial. J. Hepatol. 2024, 81, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Schöneweis, K.; Bogomolov, P.; Blank, A.; Voronkova, N.; Stepanova, T.; Sagalova, O.; Chulanov, V.; Osipenko, M.; Morozov, V.; et al. Safety and efficacy of bulevirtide in combination with tenofovir disoproxil fumarate in patients with hepatitis B virus and hepatitis D virus coinfection (MYR202): A multicentre, randomised, parallel-group, open-label, phase 2 trial. Lancet Infect. Dis. 2023, 23, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Chulanov, V.; Lampertico, P.; Wedemeyer, H.; Streinu-Cercel, A.; Pântea, V.; Lazar, S.; Placinta, G.; Gherlan, G.S.; Bogomolov, P.; et al. Bulevirtide Combined with Pegylated Interferon for Chronic Hepatitis D. N. Engl. J. Med. 2024, 391, 133–143. [Google Scholar] [CrossRef]
- Messaoudi, S.E.; Brichler, S.; Fougerou-Leurent, C.; Gordien, E.; Gerber, A.; Kortebi, A.; Lagadic, G.; Subic-Levrero, M.; Metivier, S.; Pol, S.; et al. Effect of Peg-IFN on the viral kinetics of patients with HDV infection treated with bulevirtide. JHEP Rep. 2024, 6, 101070. [Google Scholar] [CrossRef]
- Allweiss, L.; Volmari, A.; Suri, V.; Wallin, J.J.; Flaherty, J.F.; Mannuilov, D.; Downie, B.; Lütgehetmann, M.; Bockmann, J.-H.; Urban, S.; et al. Blocking viral entry with bulevirtide reduces the number of HDV-infected hepatocytes in human liver biopsies. J. Hepatol. 2024, 80, 882–891. [Google Scholar] [CrossRef]
- Koh, C.; Canini, L.; Dahari, H.; Zhao, X.; Uprichard, S.L.; Haynes-Williams, V.; A Winters, M.; Subramanya, G.; Cooper, S.L.; Pinto, P.; et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: A proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect. Dis. 2015, 15, 1167–1174. [Google Scholar] [CrossRef]
- Yurdaydin, C.; Keskin, O.; Kalkan, C.; Karakaya, F.; Caliskan, A.; Karatayli, E.; Karatayli, S.; Bozdayi, A.M.; Koh, C.; Heller, T.; et al. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatology 2018, 67, 1224–1236. [Google Scholar] [CrossRef]
- Yurdaydin, C.; Keskin, O.; Yurdcu, E.; Çalişkan, A.; Önem, S.; Karakaya, F.; Kalkan, Ç.; Karatayli, E.; Karatayli, S.; Choong, I.; et al. A phase 2 dose-finding study of lonafarnib and ritonavir with or without interferon alpha for chronic delta hepatitis. Hepatology 2022, 75, 1551–1565. [Google Scholar] [CrossRef]
- Etzion, O.; Hamid, S.S.; Asselah, T.; Gherlan, G.S.; Turcanu, A.; Petrivna, T.; Weissfeld, L.; Choong, I.; Hislop, C.; Apelian, D.; et al. Week 48 results of the phase 3 D-LIVR study, a randomized double-blind, placebo-controlled trial evaluating the safety and efficacy of Lonafarnib-boosted with Ritonavir with or without Peginterferon Alfa in patients with chronic hepatitis delta. J. Hepatol. 2023, 78, S10. [Google Scholar] [CrossRef]
- Vaillant, A. Oligonucleotide-Based Therapies for Chronic HBV Infection: A Primer on Biochemistry, Mechanisms and Antiviral Effects. Viruses 2022, 14, 2052. [Google Scholar] [CrossRef]
- Collotta, D.; Bertocchi, I.; Chiapello, E.; Collino, M. Antisense Oligonucleotides: A Novel Frontier in Pharmacological Strategy. Front. Pharmacol. 2023, 14, 1304342. [Google Scholar] [CrossRef]
- Boulon, R.; Blanchet, M.; Lemasson, M.; Vaillant, A.; Labonté, P. Characterization of the Antiviral Effects of REP 2139 on the HBV Lifecycle In Vitro. Antivir. Res. 2020, 183, 104853. [Google Scholar] [CrossRef]
- Bazinet, M.; Pântea, V.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Albrecht, J.; Schmid, P.; Le Gal, F.; Gordien, E.; Krawczyk, A.; et al. Safety and Efficacy of REP 2139 and Pegylated Interferon Alfa-2a for Treatment-Naive Patients with Chronic Hepatitis B Virus and Hepatitis D Virus Co-Infection (REP 301 and REP 301-LTF): A Non-Randomised, Open-Label, Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2017, 2, 877–889. [Google Scholar] [CrossRef]
- Bazinet, M.; Pântea, V.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Anderson, M.; Gersch, J.; Holzmayer, V.; Elsner, C.; Krawczyk, A.; et al. Persistent Control of Hepatitis B Virus and Hepatitis Delta Virus Infection Following REP 2139-Ca and Pegylated Interferon Therapy in Chronic Hepatitis B Virus/Hepatitis Delta Virus Coinfection. Hepatol. Commun. 2021, 5, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Lim, Y.S.; Yoon, K.T.; Lim, T.-H.; Heo, J.; Tangkijvanich, P.; Tak, W.Y.; Thanawala, V.; Cloutier, D.; Mao, S.; et al. VIR-2218 (Elebsiran) plus Pegylated Interferon-Alfa-2a in Participants with Chronic Hepatitis B Virus Infection: A Phase 2 Study. Lancet Gastroenterol. Hepatol. 2024, 9, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Streinu-Cercel, A.; Jucov, A.; Gane, E.J.; Wedemeyer, H.; Lampertico, P.; Chattergoon, M.A.; Wu, P.; Maciejewski, S.; Pilowa, C.; et al. OS-127 Efficacy and Safety of Tobevibart (VIR-3434) Alone or in Combination with Elebsiran (VIR-2218) in Participants with Chronic Hepatitis Delta Virus Infection: Preliminary Results from the Phase 2 SOLSTICE Trial in Non-Cirrhotic and Compensated Cirrhotic Participants. J. Hepatol. 2024, 80, S75–S76. [Google Scholar] [CrossRef]
- Agarwal, K.; Buti, M.; Van Bommel, F.; Lampertico, P.; Janczewska, E.; Bourliere, M.; Vanwolleghem, T.; Lenz, O.; Verbinnen, T.; Kakuda, T.N.; et al. JNJ-73763989 and Bersacapavir Treatment in Nucleos(t)ide Analogue-Suppressed Patients with Chronic Hepatitis B: REEF-2. J. Hepatol. 2024, 81, 404–414. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Gane, E.J.; Agarwal, K.; Tabak, F.; Forns, X.; Akarca, U.; Viacheslav, M.; Aleman, S.; Buti, M.; Yilmaz, G.; et al. Treatment with siRNA JNJ-73763989 plus Nucleos(t)ide Analogue (NA) Decreases HBsAg and HDV RNA Levels in Patients with Chronic Hepatitis D (CHD): Part 1 of the REEF-D Study. J. Hepatol. 2023, 78, S34. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Lampertico, P.; Gane, E.J.; Agarwal, K.; Tabak, F.; Akarca, U.; Aleman, S.; Buti, M.; Sprinzl, K.; Donohue, K.; et al. LBP-044 Robust Reduction of HBsAg and HDV RNA Levels with Low Risk for ALT Elevations in JNJ-73763989 Treated Patients with Chronic Hepatitis D (CHD) and Baseline HBsAg Levels below 10,000 IU/mL: Part 2 of the REEF-D Study. J. Hepatol. 2024, 80, S100. [Google Scholar] [CrossRef]
- Yuen, M.F.; Lim, S.G.; Plesniak, R.; Tsuji, K.; Janssen, H.L.A.; Pojoga, C.; Gadano, A.; Popescu, C.P.; Stepanova, T.; Asselah, T.; et al. Efficacy and Safety of Bepirovirsen in Chronic Hepatitis B Infection. N. Engl. J. Med. 2022, 387, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Lempp, F.A.; Volz, T.; Cameroni, E.; Benigni, F.; Zhou, J.; Rosen, L.E.; Noack, J.; Zatta, F.; Kaiser, H.; Bianchi, S.; et al. Potent Broadly Neutralizing Antibody VIR-3434 Controls Hepatitis B and D Virus Infection and Reduces HBsAg in Humanized Mice. J. Hepatol. 2023, 79, 1129–1138. [Google Scholar] [CrossRef]
- Zhou, J.; Kaiser, H.; Rocha, E.; Terrell, A.N.; Corti, D.; Purcell, L.A.; Lempp, F.A.; Puschnik, A.S. Therapy with Murinized Tobevibart and Elebsiran Is Efficacious in a Liver-Chimeric Mouse Model of HDV Infection. JHEP Rep. 2025, 7, 101400. [Google Scholar] [CrossRef]
- Jucov, A.; Asselah, T.; Streinu-Cercel, A.; Gane, E.J.; Wedemeyer, H.; Lampertico, P.; Chattergoon, M.A.; Bullard, B.; Huang, C.; Acosta, R.; et al. THU-243 SOLSTICE Week 24 Subgroup Analysis: Impact of Baseline Viral Parameters and Cirrhosis Status on Virological and Biochemical Responses in Participants with Chronic Hepatitis Delta Virus Infection Treated with Tobevibart and Elebsiran. J. Hepatol. 2025, 82, S829–S830. [Google Scholar] [CrossRef]
- A Global, Randomized, Open-Label, Multicenter, Phase 2b/3 Trial Evaluating BJT-778 vs. Delayed Treatment for the Treatment of Chronic Hepatitis Delta Infection (AZURE-1). Available online: https://clinicaltrials.gov/study/NCT06907290 (accessed on 18 August 2025).
- Wang, X.; Chi, X.; Zhang, Y.; Gu, Y.; Xiao, L.; Qi, Y.; Zou, L.; Wen, J.; Zhang, Y.; Chen, P.; et al. Safety and Efficacy of Anti-Pre-S1 Domain Monoclonal Antibody (HH-003) Treatment in Patients with Co-Infection of Chronic Hepatitis B Virus (HBV) and Hepatitis D Virus (HDV): A Single Center, Open-Label, Phase 2 Trial. J. Hepatol. 2023, 78, S117. [Google Scholar] [CrossRef]
- Keating, S.; Higgins, B.; Niedecken, A.; Chiang, Y.; Witte, P.; Sharda, R.; Lopez, Y.; Lucifora, J.; Durantel, D.; Vainorius, E.; et al. OS-074 Recombinant Polyclonal Antibody GIGA-2339 Potently Neutralizes Hepatitis B and Hepatitis D Virus. J. Hepatol. 2025, 82, S53. [Google Scholar] [CrossRef]
- Gish, R.G.; Wong, R.J.; Di Tanna, G.L.; Kaushik, A.; Kim, C.; Smith, N.J.; Kennedy, P.T. Association of hepatitis delta virus with liver morbidity and mortality: A systematic literature review and meta-analysis. Hepatology 2024, 79, 1129–1140. [Google Scholar] [CrossRef]
- Martini, S.; Tandoi, F.; Romagnoli, R.; Rizzetto, M. Liver Transplantation in Hepatitis B/Hepatitis D (Delta) Virus Coinfected Recipients. Transplantation 2022, 106, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Duvoux, C.; Belli, L.S.; Fung, J.; Angelico, M.; Buti, M.; Coilly, A.; Cortesi, P.; Durand, F.; Féray, C.; Fondevila, C.; et al. 2020 position statement and recommendations of the European Liver and Intestine Transplantation Association (ELITA): Management of hepatitis B virus-related infection before and after liver transplantation. Aliment. Pharmacol. Ther. 2021, 54, 583–605. [Google Scholar] [CrossRef] [PubMed]
- Lenci, I.; Tariciotti, T.; Angelico, R.; Milana, M.; Signorello, A.; Manzia, T.M.; Toti, L.; Tisone, G.; Angelico, M.; Baiocchi, L. Successful clinical and virological outcomes of liver transplantation for HDV/HBV-related disease after long-term discontinuation of hepatitis B immunoglobulins. Clin. Transplant. 2023, 37, e14971. [Google Scholar] [CrossRef]
| Class | Drugs/Regimens | Route | Drug Sponsor | Mechanism of Action | Development | * Doses/Regimens |
|---|---|---|---|---|---|---|
| Immunomodulators | PegIFN-α-2a/−2b | SC | PharmaEssentia | Immune activation | Approved off-label HDV | 180 mcg qwk/1.5 mcg/kg qwk − 48 wk |
| Immunomodulators | PegIFN-λ | SC | Eiger InnoTherapeutics | Immune activation, Type III IFN | Phase 2/3 (stopped) | 120 to 180 mcg qwk − 48 wk |
| Entry inhibitors | Bulevirtide | SC | Gilead | NTCP receptor inhibition | ** Approved 2 mg qd | 2 to 10 mg qd |
| Entry inhibitors | Bulevirtide + PegIFN-α | SC | – | NTCP receptor inhibition + immune activation | Phase 2 | 2 to 10 mg qd + PegIFN-α − 96 wk |
| Assembly/secretion inhibitors | Lonafarnib + Ritonavir | PO | Eiger InnoTherapeutics | Inhibition of L-HDAg prenylation | Phase 3 | 50 mg BID/Ritonavir − 48 wk |
| Assembly/secretion inhibitors | Lonafarnib + Ritonavir + PegIFN-α | PO + SC | – | Inhibition of L-HDAg prenylation + immune activation | Phase 3 | 50 mg BID/Ritonavir + PegIFN-α − 48 wk |
| Nucleic acid-directed therapies | Elesbiran + Tobevibart | SC | Vir Biotechnology | siRNA (HBV mRNA silencing) + HBsAg targeting mAb (HBsAg neutralization) | Phase 2–3 | 100/200 mg q1m + Tobevibart − 48 wk |
| Nucleic acid-directed therapies | JNJ-3989 | SC | GlaxoSmithKline Pharmaceuticals | siRNA (all HBV RNA transcription silencing) | Phase 2 | 100 mg q4 wk + NA − 144 wk |
| Nucleic acid-directed therapies | REP 2139-Ca/-Mg | IV | Replicor | NAP (binds host chaperones + blocks assembly and HBsAg secretion) | Phase 2 | REP 2139-Ca − 500 mg IV qwk 15 wk, then 250 mg IV qwk + PegIFN-α 15 wk (until 33 wk) REP 2139-Mg − 250 mg IV qwk ± PegIFN-α, for 48 wk |
| Antibody-based therapies—Monoclonal | Tobevibart | SC | Vir Biotechnology | anti-HBsAg (HBsAg neutralization) | Phase 2/3 | 300 mg q2 wk |
| Antibody-based therapies—Monoclonal | Brelovitug | SC | Bluejay | anti-HBsAg (HBsAg neutralization) | Phase 2b/3 | 300 mg qwk or 900 mg q1m |
| Antibody-based therapies—Monoclonal | Libevitug | IV | HuaHei | Pre-S1 domain (HBsAg neutralization) | Phase 2 | 20 mg/kg q2wks − 24 wk, and 24 wk follow-up |
| Antibody-based therapies—Monoclonal | RG-6349; 2H5-A14, E6F6; H3B-6520; CM1239(20) | SC | – | Anti-HBsAg; preS1-NTCP block; HBsAg clearance; direct NTCP block | Phase 1 | — |
| Antibody-based therapies—Polyclonal | GIGA-2339 | IV | IgaGen | Anti-HBsAg (broad neutralization) | Phase 1 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duque, V.; Duque, D. Hepatitis Delta Virus Infection: An Overview. Pathogens 2025, 14, 899. https://doi.org/10.3390/pathogens14090899
Duque V, Duque D. Hepatitis Delta Virus Infection: An Overview. Pathogens. 2025; 14(9):899. https://doi.org/10.3390/pathogens14090899
Chicago/Turabian StyleDuque, Vitor, and Diana Duque. 2025. "Hepatitis Delta Virus Infection: An Overview" Pathogens 14, no. 9: 899. https://doi.org/10.3390/pathogens14090899
APA StyleDuque, V., & Duque, D. (2025). Hepatitis Delta Virus Infection: An Overview. Pathogens, 14(9), 899. https://doi.org/10.3390/pathogens14090899






