From Hyperendemic to Low Endemicity: The Effect of Hepatitis B Vaccination on HBV and HDV Prevalence in the Brazilian Amazon
Abstract
1. Introduction
2. Materials and Methods
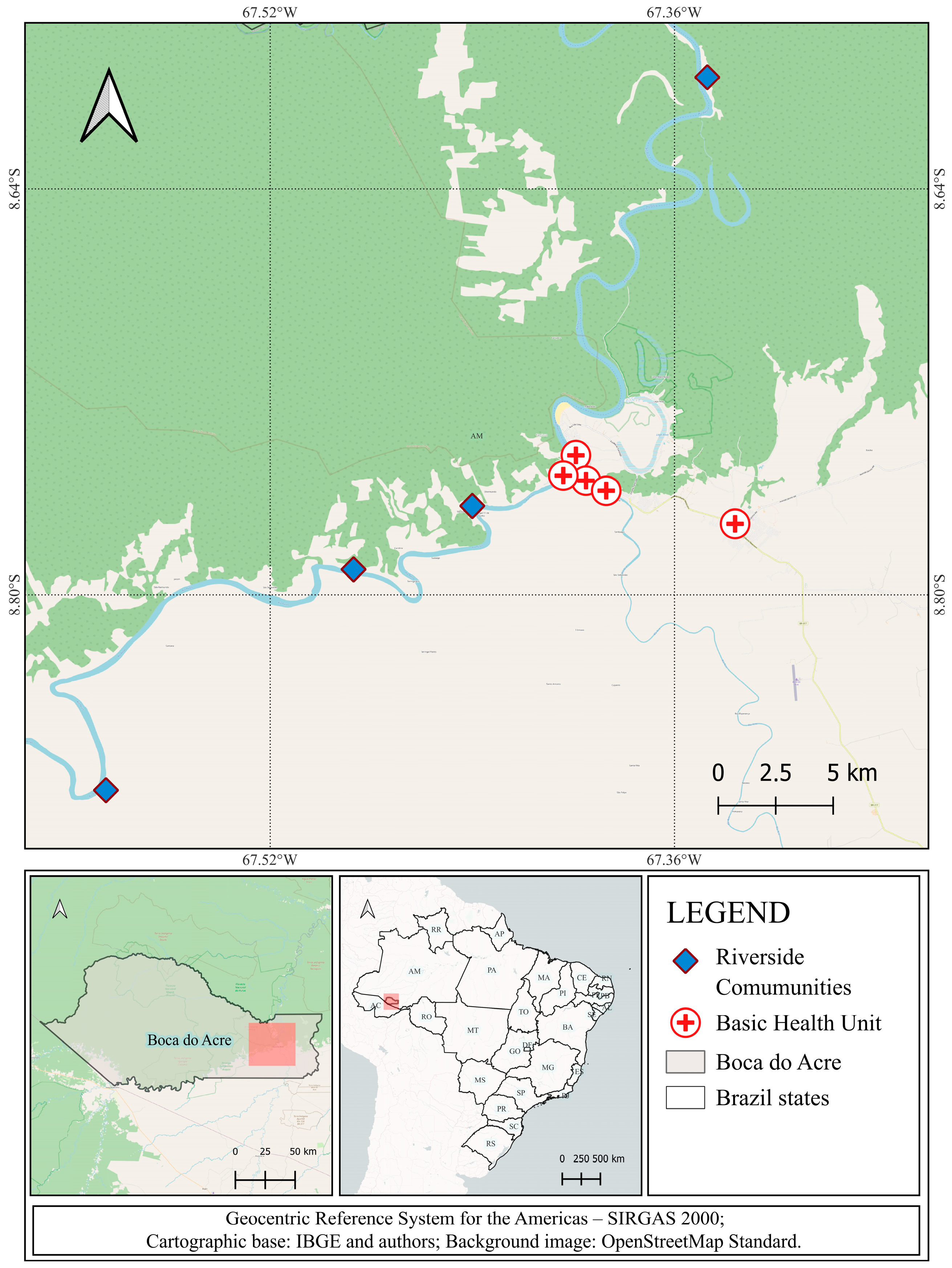
2.1. Study Design
2.2. Serology
- HBsAg (Biolisa HBsAg, BIOLISA, Guayaquil, Ecuador; HBsAg ELISA, Wiener Lab, Rosario, Argentina);
- Total anti-HBc (Monolisa Anti-HBc PLUS, BIO-RAD, California, CA, USA; AFG Bioscience, Illinois, IL, USA; anti-HBc ELISA, Wiener Lab, Rosario, Argentina);
- Quantitative anti-HBs (Biolisa anti-HBs, Bioclin, Minas Gerais, Brazil).
2.3. Molecular Biology
2.4. Statistics
2.5. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBV | hepatitis B virus |
| HDV | hepatitis D virus |
| HBsAg | hepatitis B surface antigen |
| anti-HBc | antibody to hepatitis B core antigen |
| anti-HBs | antibody to hepatitis B surface antigen |
| anti-HDV | antibody to hepatitis D virus |
| HBV-DNA | Hepatitis B Virus DNA |
| HDV-RNA | Hepatitis D Virus RNA |
| IEC | Evandro Chagas Institute |
| CAAE | Certificate of Ethical Appreciation Presentation |
| IBGE | Brazilian Institute of Geography and Statistics |
| HBeAg | hepatitis B e antigen |
| anti-HBe | antibody to hepatitis B e antigen |
| OBI | occult hepatitis B infection |
| COVID-19 | CoronaVirus Disease 2019 |
| WHO | World Health Organization |
| MTCT | Mother-to-Child Transmission |
References
- Hadler, S.C.; De Monzon, M.; Ponzetto, A.; Anzola, E.; Rivero, D.; Mondolfi, A.; Bracho, A.; Francis, D.P.; Gerber, M.A.; Thung, S.; et al. Delta virus infection and severe hepatitis. An epidemic in the Yucpa Indians of Venezuela. Ann. Intern. Med. 1984, 100, 339–344. [Google Scholar] [CrossRef]
- Buitrago, B.; Hadler, S.C.; Popper, H.; Thung, S.N.; Gerber, M.A.; Purcell, R.H.; Maynard, J.E. Epidemiologic aspects of Santa Marta hepatitis over 40 years. Hepatology 1986, 6, 1292–1296. [Google Scholar] [CrossRef]
- Fay, O. Hepatitis B in Latin America: Epidemiological patterns and eradication strategy. Vaccine 1990, 8, S100–S106. [Google Scholar] [CrossRef]
- Cabezas S., C.; Suárez J., M.; Romero C., G.; Carrillo P., C.; García, M.P.; Reátegui S., J.; Vallenas G., F.; Torres T., L. Hiperendemicidad de hepatitis viral B y delta en pueblos indígenas de la amazonía peruana. Rev. Peru. Med. Exp. Salud Publica 2006, 23, 114–122. [Google Scholar]
- Braga, W.S.M.; Castilho, M.C.; Borges, F.G.; Leão, J.R.D.T.; Martinho, A.C.d.S.; Rodrigues, I.S.; de Azevedo, E.P.; Júnior, G.M.d.B.; Paraná, R. Hepatitis D virus infection in the Western Brazilian Amazon—Far from a vanishing disease. Rev. Soc. Bras. Med. Trop. 2012, 45, 691–695. [Google Scholar] [CrossRef]
- Alvarado-Mora, M.V.; Pinho, J.R. Epidemiological update of hepatitis B, C, and delta in Latin America. Antivir. Ther. 2013, 18, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.B.; Moraes, M.A.P. Hepatite de Lábrea. Rev. Inst. Med. Trop. São Paulo 1973, 15, 86–93. [Google Scholar]
- Bensabath, G.; Dias, L.B. Labrea hepatitis (Labrea black fever) and other fulminant forms of hepatitis in Sena Madureira, Acre, and Boca do Acre, Amazonas, Brazil. Rev. Inst. Med. Trop. Sao Paulo 1983, 25, 182–194. [Google Scholar] [PubMed]
- Dias Junior, L.B.; Alves, V.A.; Kanamura, C.; Oikawa, R.T.; Wakamatsu, A. Fulminant hepatic failure in northern Brazil: Morphological, immunohistochemical and pathogenic aspects of Labrea hepatitis and yellow fever. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 831–836. [Google Scholar] [CrossRef]
- Bensabath, G.; Hadler, S.C.; Soares, M.C.; Fields, H.; Dias, L.B.; Popper, H.; Maynard, J.E. Hepatitis delta virus infection and Labrea hepatitis. Prevalence and role in fulminant hepatitis in the Amazon Basin. JAMA 1987, 258, 479–483. [Google Scholar] [CrossRef]
- Bensabath, G.; Boshel, J. Presença do antígeno “Austrália” (Ag) em populações do interior do Estado do Amazonas-Brasil. Rev. Inst. Med. Trop. Sao Paulo 1973, 15, 284–288. [Google Scholar] [PubMed]
- Bensabath, G.; Hadler, S.C.; Soares, M.C.; Fields, H.; Maynard, J.E. Epidemiologic and serologic studies of acute viral hepatitis in Brazil’s Amazon Basin. Bull. Pan Am. Health Organ. 1987, 21, 16–27. [Google Scholar]
- Fonseca, J.C.; Simonetti, S.R.; Schatzmayr, H.G.; Castejón, M.J.; Cesário, A.L.; Simonetti, J.P. Prevalence of infection with hepatitis delta virus (HDV) among carriers of hepatitis B surface antigen in Amazonas State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 469–471. [Google Scholar] [CrossRef]
- Bensabath, G.; Leão, R.N.Q. Epidemiologia na Amazônia brasileira. In Roberto Focaccia, Tratado de Hepatites Virais; Atheneu: São Paulo, Brazil, 2003; pp. 11–26. [Google Scholar]
- Bensabath, G.; Soares, M.C.P. A evolução do conhecimento sobre as hepatites virais na região amazônica: Da epidemiologia e etiologia à prevenção. Rev. Soc. Bras. Med. Trop. 2004, 37, 14–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brasil, Ministério da Saúde. Secretaria de Vigilância em Saúde. Programa Nacional de Imunizações—30 anos. 2003. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/livro_30_anos_pni.pdf (accessed on 10 July 2025).
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde e Ambiente. Departamento do Programa Nacional de Imunizações. Manual de Normas e Procedimentos para Vacinação. ISBN 978-65-5993-616-8. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/manual_normas_procedimentos_2edrev.pdf (accessed on 10 July 2025).
- Ximenes, R.A.A.; Figueiredo, G.M.; Cardoso, M.R.A.; Stein, A.T.; Moreira, R.C.; Coral, G.; Crespo, D.; dos Santos, A.A.; Montarroyos, U.R.; Braga, M.C.; et al. Population-Based Multicentric Survey of Hepatitis B Infection and Risk Factors in the North, South, and Southeast Regions of Brazil, 10–20 Years After the Beginning of Vaccination. Am. J. Trop. Med. Hyg. 2015, 93, 1341–1348. [Google Scholar] [CrossRef]
- IBGE. Available online: https://www.ibge.gov.br/cidades-e-estados/am/boca-do-acre.html (accessed on 7 June 2025).
- Gomes-Gouvea, M.S.; Soares, M.C.P.; Mello, I.M.; Brito, E.M.F.; Moia, L.d.J.M.P.; Bensabath, G.; Nunes, H.M.; Carrilho, F.J.; Pinho, J.R.R. Hepatitis D and B virus genotypes in chronically infected patients from the Eastern Amazon Basin. Acta Trop. 2008, 106, 149–155. [Google Scholar] [CrossRef] [PubMed]
- The jamovi Project. jamovi (Version 2.6) [Computer Software]. 2025. Available online: https://www.jamovi.org (accessed on 1 August 2025).
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B Vaccines. J. Infect. Dis. 2021, 224 (Suppl. S4), S343–S351. [Google Scholar] [CrossRef]
- Ni, Y.H.; Chang, M.H.; Jan, C.F.; Hsu, H.Y.; Chen, H.L.; Wu, J.F.; Chen, D.S. Continuing Decrease in Hepatitis B Virus Infection 30 Years After Initiation of Infant Vaccination Program in Taiwan. Clin. Gastroenterol. Hepatol. 2016, 14, 1324–1330. [Google Scholar] [CrossRef]
- Chang, K.C.; Chang, M.H.; Chen, H.L.; Wu, J.F.; Chang, C.H.; Hsu, H.Y.; Ni, Y.H. Universal Infant Hepatitis B Virus (HBV) Vaccination for 35 Years: Moving Toward the Eradication of HBV. J. Infect. Dis. 2022, 225, 431–435. [Google Scholar] [CrossRef]
- He, W.Q.; Guo, G.N.; Li, C. The impact of hepatitis B vaccination in the United States, 1999–2018. Hepatology 2022, 75, 1566–1578. [Google Scholar] [CrossRef]
- Ye, X.; Li, T.; Xu, X.; Du, P.; Zeng, J.; Zhu, W.; Yang, B.; Li, C.; Allain, J.-P. Characterisation and follow-up study of occult hepatitis B virus infection in anti-HBc-positive qualified blood donors in southern China. Blood Transfus. 2016, 15, 6–12. [Google Scholar] [CrossRef]
- Ye, X.; Li, T.; Li, Y.; Zeng, J.; Li, R.; Xu, X.; Guan, X.; Li, L. Comparative analysis of hepatitis B virus infections in blood donors born before and after the implementation of universal HBV vaccination in southern China. Transfus. Med. 2023, 33, 81–89. [Google Scholar] [CrossRef]
- Razavi-Shearer, D.; Gamkrelidze, I.; Pan, C.; Jia, J.; Berg, T.; Gray, R.; Lim, Y.-S.; Chen, C.-J.; Ocama, P.; Desalegn, H.; et al. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: A modelling study. Lancet Gastroenterol. Hepatol. 2023, 8, 879–907. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, C.; Trujillo, O.; Balbuena, J.; Peceros, F.d.M.; Terrazas, M.; Suárez, M.; Marin, L.; Apac, J.; Ramírez-Soto, M.C. Decrease in the prevalence of hepatitis B and D virus infections in an endemic area in Peru 23 years after the introduction of the first pilot vaccination program against hepatitis B. PLoS ONE 2020, 15, e0236993. [Google Scholar] [CrossRef]
- Garcia, D.; Porras, A.; Mendoza, A.R.; Alvis, N.; Navas, M.C.; De La Hoz, F.; De Neira, M.; Osorio, E.; Valderrama, J.F. Hepatitis B infection control in Colombian Amazon after 15 years of hepatitis B vaccination. Effectiveness of birth dose and current prevalence. Vaccine 2018, 36, 2721–2726. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Su, W.J.; Chen, H.L.; Chen, S.F.; Liu, Y.L.; Wang, T.A.; Ho, Y.C.; Chang, M.H. Optimization of Mother-to-Child Hepatitis B Virus Prevention Program: Integration of Maternal Screening and Infant Post-Vaccination Serologic Testing. Clin. Infect. Dis. 2024, 79, 690–700. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wu, C.C.; Chen, X.W.; Li, X.; Li, J.; Lu, M.J. Genetic variation of hepatitis B virus and its significance for pathogenesis. World J. Gastroenterol. 2016, 22, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Saitta, C.; Pollicino, T.; Raimondo, G. Occult Hepatitis B Virus Infection: An Update. Viruses 2022, 14, 1504. [Google Scholar] [CrossRef]
- Nguwoh, P.S.; Ngounouh, C.T.; Essomba, R.G.; Olinga, P.Z.; Likeng, J.L.N.; Nguepidjo, G.; Douyong, S.C.T.; Tchoffo, D.; Nlend, A.E.N.; Assoumou, M.C.O.; et al. Effect of hepatitis B vaccination on HBV-infection among school children in Yaounde; ten years after the introduction of HBV vaccine into routine Immunization Program in Cameroon. Pan Afr. Med. J. 2024, 47, 169. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Dionne, M.; Leroux-Roels, G.; Van Der Meeren, O.; Di Paolo, E.; Salaun, B.; Kiran, P.S.; Folschweiller, N. Persistence of HBsAg-specific antibodies and immune memory two to three decades after hepatitis B vaccination in adults. J. Viral Hepat. 2019, 26, 1066–1075. [Google Scholar] [CrossRef]
- Broeckhoven, E.; Dallmeier, K. Mission 2030: Toward universal hepatitis B immunization. Hum. Vaccin. Immunother. 2025, 21, 2473222. [Google Scholar] [CrossRef] [PubMed]
| N | % | |
|---|---|---|
| Area | ||
| Urban | 849 | 77.2 |
| Rural/Riverside | 251 | 22.8 |
| Age group | ||
| 0–4 | 42 | 3.8 |
| 5–9 | 80 | 7.3 |
| 10–14 | 100 | 9.1 |
| 15–19 | 95 | 8.6 |
| 20–29 | 162 | 14.7 |
| 30–39 | 174 | 15.8 |
| 40–49 | 165 | 15.0 |
| 50–59 | 129 | 11.7 |
| 60–69 | 81 | 7.4 |
| 70–79 | 43 | 3.9 |
| >80 | 29 | 2.6 |
| Sex | ||
| Female | 662 | 60.2 |
| Male | 438 | 39.8 |
| Total | 1100 | 100 |
| N | HBsAg + | Total a-HBc + and a-HBs > 10 mUI/mL | Total a-HBc Positive Alone (HBsAg and a-HBs Negative) | a-HBs > 10 mUI/mL (with HBsAg and a-HBc Negative) | All Negative | p Value | |
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Area | |||||||
| Urban | 849 | 12 (1.4) | 247 (29.1) | 86 (7.8) | 208 (24.5) | 296 (34.9) | 0.150 |
| Rural/Riverside | 251 | 4 (1.6) | 93 (37.1) | 27 (2.5) | 53 (21.1) | 74 (29.5) | |
| Age group | |||||||
| 0–4 | 42 | 0 | 0 | 0 | 29 (69.0) | 13 (31.0) | <0.001 |
| 5–9 | 80 | 0 | 1 (1.3) | 0 | 30 (37.5) | 49 (61.3) | |
| 10–14 | 100 | 0 | 1 (1.0) | 0 | 25 (25.0) | 74 (74) | |
| 15–19 | 95 | 0 | 0 | 0 | 21 (22.1) | 74 (77.9) | |
| 20–29 | 162 | 1 (0.6) | 18 (11.1) | 11 (6.8) | 39 (24.1) | 93 (57.4) | |
| 30–39 | 174 | 5 (2.9) | 63 (36.2) | 15 (8.6) | 60 (34.5) | 31 (17.8) | |
| 40–49 | 165 | 5 (3.0) | 94 (57.0) | 23 (13.9) | 28 (17.0) | 15 (9.1) | |
| 50–59 | 129 | 2 (1.6) | 79 (61.2) | 23 (17.8) | 13 (10.1) | 12 (9.3) | |
| 60–69 | 81 | 1 (1.2) | 48 (59.3) | 21 (25.9) | 8 (9.9) | 3 (3.7) | |
| 70–79 | 43 | 1 (2.3) | 23 (53.5) | 11 (25.6) | 4 (9.3) | 4 (9.3) | |
| 80–89 | 21 | 1 (4.8) | 12 (57.1) | 6 (28.6) | 4 (4.8) | 1 (4.8) | |
| 90–99 | 8 | 0 | 1 (12.5) | 3 (37.5) | 3 (37.5) | 1 (12.5) | |
| Sex | |||||||
| Female | 662 | 10 (1.5) | 201 (30.4) | 61 (9.2) | 160 (24.2) | 230 (34.7) | 0.600 |
| Male | 438 | 6 (1.4) | 139 (31.7) | 52 (11.9) | 101 (23.1) | 140 (32.0) | |
| Total | 1100 | 16 (1.5) | 340 (30.9) | 113 (10.3) | 261 (23.7) | 370 (33.6) |
| Age Group | N | HBsAg + (%) | p | a-HBc + (%) | p | a-HBs + Isolate (%) | p |
|---|---|---|---|---|---|---|---|
| <25 years † | 417 | 1 (0.2) | 0.015 | 17 (4.1) | <0.001 | 130 (31.2) | <0.001 |
| 26–44 years ‡ | 329 | 8 (2.4) | 159 (48.3) | 94 (28.6) | |||
| >45 years § | 354 | 7 (2.0) | 292 (82.5) | 37 (10.5) | |||
| Total | 1100 | 16 | 468 | 261 |
| Year | Localities | Age | Doses |
|---|---|---|---|
| 1989 | For high HBV prevalence cities in Amazonas state | Under 10 years old | BD + 2 HB or 3 HB |
| 1991 | For all cities in Amazonas state | Under 1 year old | BD + 2 HB or 3 HB |
| 1992 | Vaccination expanded to the entire Legal Amazon, Paraná, Espírito Santo, Santa Catarina, and the Federal District. | Under 5 years old | BD + 2 HB or 3 HB |
| 1998 | For all states of Brazil | Under 1 year old | BD + 2 HB or 3 HB |
| Legal Amazon, Paraná, Espírito Santo, Santa Catarina, and the Federal District | Under 15 years old | BD + 2 HB or 3 HB | |
| 2012 | For all states of Brazil | Under 7 years old | BD + 3 pentavalent or 3 HB |
| Above 7 years to 29 years | 3 HB | ||
| 2015-until now | For all states of Brazil | All age groups | BD + 3 doses pentavalente or 3 HB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malheiros, A.P.; Gomes-Gouvêa, M.S.; Ribeiro, L.B.; Souza, A.J.S.d.; Azevedo, R.S.; Brito, D.C.N.d.; Oliveira, C.M.A.d.; Nunes, H.M.; Pinho, J.R.R. From Hyperendemic to Low Endemicity: The Effect of Hepatitis B Vaccination on HBV and HDV Prevalence in the Brazilian Amazon. Pathogens 2025, 14, 1089. https://doi.org/10.3390/pathogens14111089
Malheiros AP, Gomes-Gouvêa MS, Ribeiro LB, Souza AJSd, Azevedo RS, Brito DCNd, Oliveira CMAd, Nunes HM, Pinho JRR. From Hyperendemic to Low Endemicity: The Effect of Hepatitis B Vaccination on HBV and HDV Prevalence in the Brazilian Amazon. Pathogens. 2025; 14(11):1089. https://doi.org/10.3390/pathogens14111089
Chicago/Turabian StyleMalheiros, Andreza Pinheiro, Michele Soares Gomes-Gouvêa, Leidiane Barbosa Ribeiro, Alex Junior Souza de Souza, Raymundo Soares Azevedo, Dickson Ciro Nascimento de Brito, Candida Maria Abrahão de Oliveira, Heloisa Marceliano Nunes, and João Renato Rebello Pinho. 2025. "From Hyperendemic to Low Endemicity: The Effect of Hepatitis B Vaccination on HBV and HDV Prevalence in the Brazilian Amazon" Pathogens 14, no. 11: 1089. https://doi.org/10.3390/pathogens14111089
APA StyleMalheiros, A. P., Gomes-Gouvêa, M. S., Ribeiro, L. B., Souza, A. J. S. d., Azevedo, R. S., Brito, D. C. N. d., Oliveira, C. M. A. d., Nunes, H. M., & Pinho, J. R. R. (2025). From Hyperendemic to Low Endemicity: The Effect of Hepatitis B Vaccination on HBV and HDV Prevalence in the Brazilian Amazon. Pathogens, 14(11), 1089. https://doi.org/10.3390/pathogens14111089






