Abstract
Resistance to anthelmintics in gastrointestinal nematodes (GINs) is highly prevalent, as these parasites have been treated with anthelmintics for decades in ruminants and horses. Anthelmintics belong to different classes, each with a different mode of action. The most used are benzimidazoles and macrocyclic lactones and, to a lesser extent, levamisole and pyrantel in herbivores, as estimated from the literature. Combining these classes should be effective in controlling GIN. However, several farmers’ practices tend to promote GIN resistance. Therefore, it is unclear whether the use of anthelmintic associations is a sustainable solution for controlling resistance in natural conditions. It is not easy to establish the association of anthelmintic resistances on farms since rarely several anthelmintics and their combinations are used on a single farm. Composed probability calculations were employed when literature data indicated the presence of resistance (to benzimidazoles, levamisole, or macrocyclic lactones) in several ruminant GIN cases. The efficacy of different anthelmintics (benzimidazoles, pyrantel, or macrocyclic lactones) was evaluated in terms of the correlation between faecal nematode egg counts in horses in the available literature. No associations of anthelmintic resistance were found between the different classes of anthelmintics in either ruminants or horses. However, the association between anthelmintic resistance in GIN may appear in the long term. It is presumed that combining drugs may reduce the development of resistance and allow better control of infection on farms where resistance is already established to a low level.
1. Introduction
Resistance to anthelmintics of gastrointestinal nematodes has been a pervasive issue for an extended period [1,2]. Such resistance has been recorded in sheep [3,4,5,6] and goats [7,8] across numerous countries. Furthermore, evidence of resistance to anthelmintics has been documented in cattle, albeit to a lesser extent [9,10]. Extensive documentation of such cases has been recorded also in horses [11,12,13,14,15]. Most resistance studies are grounded in the faecal egg reduction test (FECRT). According to [16], a reduction of less than 90% of faecal egg counts following treatment was indicative of resistance in horses, whereas a 95% cut-off has been proposed for ruminants [17]. Resistance is typically evaluated in a control group of sheep and cattle, whereas a simple before-and-after treatment approach is recommended for horses [18]. However, it should be noted that the results of FECRT are susceptible to variation according to factors such as sampling, drug, calculation of efficacy, and level of infection [19]. In light of these variations, recent recommendations have been made to reduce this variability [17]. Another problem may obscure the evaluation of efficacy: broad-spectrum anthelmintics have a dose-limiting parasite (DLP). It means that among target parasite species listed on a product’s label, there is always one—designated as the DLP—that requires a higher dose of the active compound than the others in order to meet the efficacy standards specified on the label. Lack of efficacy may thus be due to the presence of one particular species that requires a higher dosage in a farm; it is not then a case of resistance. Bias due to DLP is found in non-strongylid nematodes of horses (Parascaris equorum and Oxyuris equi) [15].
Several facts suggest an absence of association between resistance to different anthelmintics. The targets for anthelmintic resistance mechanisms of nematodes appear to differ for benzimidazoles, imidazothiazoles, and macrocyclic lactones [20,21]. Consequently, it is anticipated that resistance to one drug should not affect resistance to another. Nevertheless, it should be noted that the precise mechanisms of resistance are not always fully elucidated, as evidenced by studies of nicotine-sensitive acetylcholine receptors [22].
Conversely, some other facts (general mechanisms of resistance and wrong use of anthelmintics on farms) suggest the presence of association between resistance to different anthelmintics. In cases where multidrug resistance mechanisms are involved [23], it is anticipated that a shared selection may occur in part and that a certain degree of association of resistance may emerge in field conditions. The apparent co-selection of benzimidazole and macrocyclic lactone in the gastrointestinal Haemonchus contortus [24] or Onchocerca volvulus in man [25] may militate furthermore for association between resistances. The management of gastrointestinal strongyles is also important for co-selecting resistant nematodes. In ovine models, Silvestre et al. [26] identified the number and timing of treatments as pivotal factors in the development of resistance. The most efficacious treatment was identified as that administered at the conclusion of winter, prior to the animals being moved again to pastures. In addition, Sallé et al. [27] identified additional risks in horses, with pastures characterised by high stocking rates and infrequent rotations being associated with resistance. Consequently, it can be hypothesised that analogous management practices may promote resistance against all anthelmintics.
A large-scale development of resistance has favoured the marketing of drug combinations. Proposals have been made for the registration of anthelmintic combinations [28]. The primary rationales for employing these combinations are twofold: firstly, to ensure effective management of nematodes in the context of single or multiple drug resistance, and secondly, to curtail the emergence of resistance to the constituent anthelmintic classes. These combinations have been commercially available for an extended period in Australia, New Zealand, South Africa, and Latin America and include dual combinations of levamisole and macrocyclic lactones, levamisole and benzimidazole, or derquantel and macrocyclic lactones. Additionally, triple combinations of benzimidazole, levamisole, and macrocyclic lactones have been utilised [29]. It has been hypothesised that these combinations may be harmful if resistances are co-selected, or, conversely, protective if there is enhanced synergy between drug metabolism [30]. Synergistic effects were identified in conjunction with derquantel (a nicotinic antagonist) and abamectin [31]. It has also been shown that the pharmacology of each drug is altered when used in combination [32], and this may modify the interest in combining drugs. The restoration of 100% efficacy of macrocyclic lactones with other classes of anthelmintics was recorded in cattle [33]. Wrigley et al. [34] observed that the combination of abamectin, levamisole, and oxfendazole exhibited high efficacy against all three species of sheep abomasal nematodes, while the combination of ivermectin, levamisole, and albendazole was not. However, it has been noticed that in cases of high resistance, the combination of anthelmintics was not effective [35]. Consequently, the outcomes of such combinations do not always align with the anticipated results. Then, there is a need to evaluate in the field how combinations are effective and relate their efficacy to the level of resistance for each drug.
It is finally difficult to assess if resistances are associated or not from similar resistance mechanisms studied in the laboratory, if these associations are due to gastrointestinal management practices in the field, or both. In fact, there are many records of multidrug resistance cases, but it is not known if it is only due to chance or if it is a true association of resistances among different anthelmintics in the field. The aim of this paper is to assess if there is a real association of anthelmintic resistances by means of statistical or probability calculations from the few selected published studies in field evaluations. Due to the limited sample size used for assessing the relation between anthelmintic associations, we compared our survey with the main traits of anthelmintic resistance to evaluate the generality of our findings.
2. Material and Methods
First, I will evaluate the main traits of anthelmintic resistance and their associations in a large body of literature. Then, I will present the dataset concerning ruminants, followed by the dataset concerning horses.
2.1. Evaluation of the Main Traits of Anthelmintic Resistance in the Literature
We concentrated on benzimidazoles, levamisole, and ivermectin that were used for decades and thus prone to high levels of anthelmintic resistance. The literature on anthelmintic resistance was screened with the Open Alex database. The analysis of the references was performed with VosViewer-1.6.20 [36]. We checked which words were associated with anthelmintic resistance, how the evolution of resistance occurred over the years, and where the most common anthelmintics were used in different countries. The percentage of papers on each anthelmintic per species of host and on resistance was obtained from screening Google Scholar.
2.2. The Set of Data Relating to Ruminants
The primary data are presented in Table 1. They were chosen when at least one anthelmintic had a reduced efficacy and when there was an association of anthelmintics tested in each farm. The datasets on resistance are large for anthelmintic resistance in livestock (32,700 on Google Scholar) but our criteria of selection drastically reduced the number of available papers (16). The presence of resistance was indicated when the faecal egg reduction test result was below 90%. The 95% cut-off was utilised in two surveys conducted in New Zealand [37], and a comparison was made between these two cut-offs. The absence of associations between resistances was equally found whatever the cut-off, 90 or 95% efficacy (rs = 0.99, p = 0.001, n = 8 [37]. The observed associations were compared to calculated ones. The latter followed the theorem of composed probabilities, which stipulates that an event A is independent of an event B if the probability of A is independent of B [38]). Therefore, the calculated associations were based on the independence of probabilities of single resistance and are outlined below. Similarly, the independence of three events (A, B, C) is determined by the condition that their joint probability is equivalent to the product of their individual probabilities. The calculated probability of association between Bz (Benzimidazoles) and Lev (Levamisole) resistance in Table 1 was determined by multiplying the frequency of Bz resistance in farms by the frequency of Lev resistance in farms. For instance, in survey one (Table 1), this is 0.56 × 0.53, i.e., 0.30 or 30%. This was then compared with an observed value of 35%. The differences between expected and observed frequencies were subjected to a statistical evaluation using a chi-square test. Their relation was also evaluated with a Spearman rho (rs).

Table 1.
Anthelmintic use and resistance based on references in Google Scholar.
2.3. The Set of Data Relating to Horses (Table 2)
The literature did provide FECRT for each main anthelmintic used in individual farms, but the observed associated resistances were not available in most cases. To detect the association of resistances, the FECRT of each drug in a farm or in a group of farms from the same area was correlated (Spearman rs). They were chosen when at least one anthelmintic had a reduced efficacy and when there was an association of anthelmintics tested in each farm. The datasets on resistance are large for anthelmintic resistance in horses (13,600 in Google Scholars) but our criteria of selection drastically reduced the numbers of available papers (11 for cyathostomins and 11 for Parascaris). A correlation could exist when both anthelmintics are fully efficient or when they are both poorly efficient, and that is why we selected data in which at least one anthelmintic had insufficient efficacy. The association was also compared in terms of binary data (presence or absence of resistance); benzimidazoles and pyrantel phi coefficient of correlation were calculated.

Table 2.
Gastrointestinal resistance to the three main groups of anthelmintics in 1265 ruminant farms (Bz: benzimidazoles, Lev: levamisole/tetramisole imidazothiazoles, Ml: ivermectin, a macrocyclic lactone).
Table 2.
Gastrointestinal resistance to the three main groups of anthelmintics in 1265 ruminant farms (Bz: benzimidazoles, Lev: levamisole/tetramisole imidazothiazoles, Ml: ivermectin, a macrocyclic lactone).
| Survey | Resistance Based on FECRT < 90% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| % of Farms with Resistance to | % of Farms with Association Between Resistances | ||||||||
| Host | No Farms | Country (Reference) | Bz | Lev | Ml | BzLev | BzMl | LevMl | BzLevMl |
| Goats | 34 | Brazil [39] | 56 | 53 | nd * | 35 | nd | nd | nd |
| 18 | France [40] | 67 | 0 | nd | 6 | nd | nd | nd | |
| Sheep and goats | 25 | Brazil [41] | 92 | 68 | 52 | 65 | 48 | 44 | 40 |
| Sheep | 10 | Algeria [42] | 50 | nd | 8 | nd | 8 | nd | nd |
| 10 | Morocco [43] | 50 | 10 | 1 | 3 | 1 | 0 | 0 | |
| 336 | Australia [4] | 86 | 65 | 0 | 56 | 0 | 0 | 0 | |
| 118 | Australia [4] | 93 | 72 | 0 | 69 | 0 | 0 | 0 | |
| 56 | Australia [4] | 89 | 29 | 0 | 28 | 0 | 0 | 0 | |
| 315 | Australia [4] | 81 | 67 | 0 | 57 | 0 | 0 | 0 | |
| 56 | Australia [4] | 88 | 80 | 0 | 73 | 0 | 0 | 0 | |
| 80 | New-Zealand [37] | 41 | 24 | 25 | 18 | 13 | 10 | 8 | |
| 85 | Spain [44] | 13 | 9 | 16 | 1 | 0 | 2 | 0 | |
| 23 | France [45] | 61 | 17 | 0 | 13 | 0 | 0 | 0 | |
| Cattle | 13 | Argentina [46] | 8 | 0 | 54 | 0 | 8 | 0 | 0 |
| 25 | Brazil [47] | 20 | 8 | 70 | 0 | 12 | 0 | 8 | |
| 61 | New-Zealand [37] | 76 | 8 | 92 | 8 | 74 | 8 | 5 | |
* nd: not done.
3. Results
3.1. Main Characteristics of Anthelmintic Use and Resistance
For Figure 1, the options in VosViewer were to search the queries ‘anthelmintic’ and ‘resistance’ in OpenAlex, to perform a full-text analysis, and to create a co-occurrence network visualisation. The size of the spheres and labels was related to the number of times the word occurred. The lines joining words indicate the number of associations between them. Only the main anthelmintics (benzimidazoles and ivermectin) are present in the figure. This figure shows the words associated with anthelmintic resistance: nematode (such as Haemonchus), benzimidazole, and mode of action. The term was also included in more general disciplines, such as zoology and ecology. Anthelmintics are associated with ivermectin, flocks, pasture, and pharmacology but not so much with anthelmintic resistance.
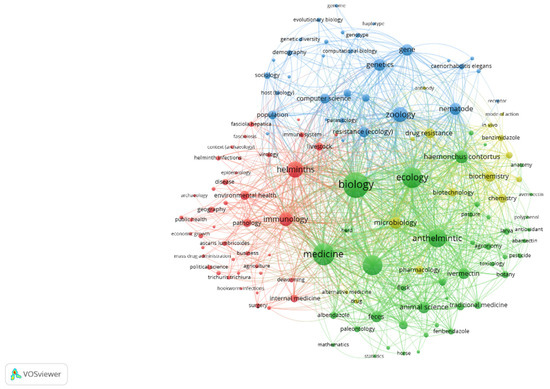
Figure 1.
Words associated with anthelmintic and resistance with OpenAlex.
The prevalence of different anthelmintics in publications on anthelmintics in OpenAlex was as follows: benzimidazoles (55%), ivermectin (34%), moxidectin (7%), pyrantel (3%) and levamisole (1%). The main anthelmintics used for all species were benzimidazoles and ivermectin, both of which were considered in our selected surveys. The countries involved (2001–2025) were primarily the USA, China, India, the United Kingdom, and Germany for benzimidazoles; the USA, Brazil, and Germany for levamisole; the USA, the United Kingdom, Brazil, and India for ivermectin; and the USA, Brazil and Germany for pyrantel. Figure 2A,B (similar VosViewer conditions to Figure 1 but with the indication of countries) show how the number of publications from different countries had evolved over time: they were concentrated in a few countries in the first period (the USA, the United Kingdom, and Australia) and then became more widely distributed in recent years. It was probably indicative of the spread of anthelmintic resistance, given that it concerned publications by researchers from many more countries. The majority of the surveys we selected for further analysis were from the first few years (1960–2000) and the beginning of the 2000s for ruminants. This bias is related to the necessity of obtaining field joint evaluations of single and associated resistances on farms only present at that period.
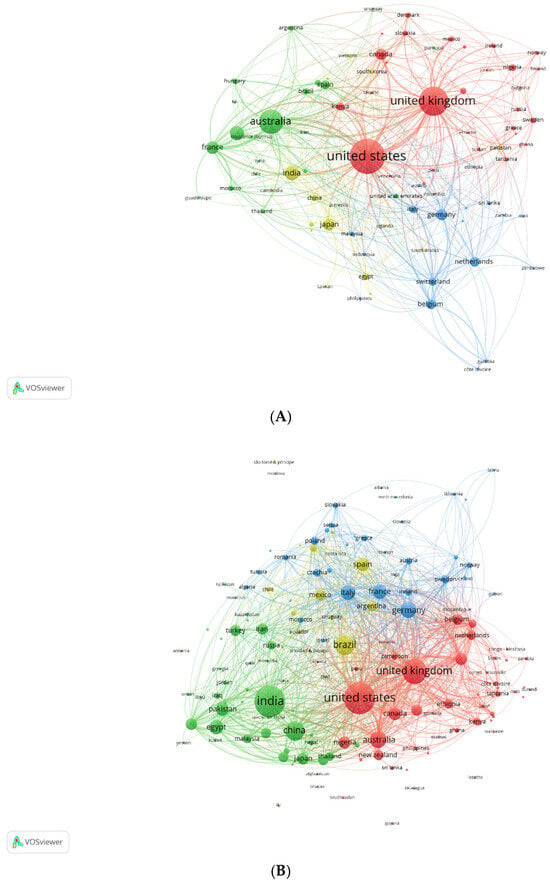
Figure 2.
Countries publications on anthelmintics with OpenAlex: (A) 1960–2000 and (B) 2001–2025.
The importance of resistance among the different hosts was evaluated based on Google Scholar references (Table 1). The total percentage of uses does not reach 100% since some drugs (e.g., moxidectin, closantel, etc.) were not investigated.
The lowest records in the literature were for levamisole in goats and cattle and for pyrantel in horses. Resistance was frequently recorded for all four drugs: 56–100%. Combination therapy in herbivores was cited in 3980 Google Scholar results, including 799 reviews. However, in research papers, the combination was often only cited as a possibility and not investigated. This means that I had to select manually the references of interest.
3.2. Anthelmintic Associations in Ruminants (Table 2)
The observed and calculated frequencies of all associated resistance were highly related (see Figure 3 for all drug associations in all surveys). The chi-square for each type of association was not significant (p = 0.89 to 0.99). Thus, we calculated for all the associations a general Spearman rs; it was 0.90 (p < 0.0001; n = 55): the observed and calculated associations were highly similar.
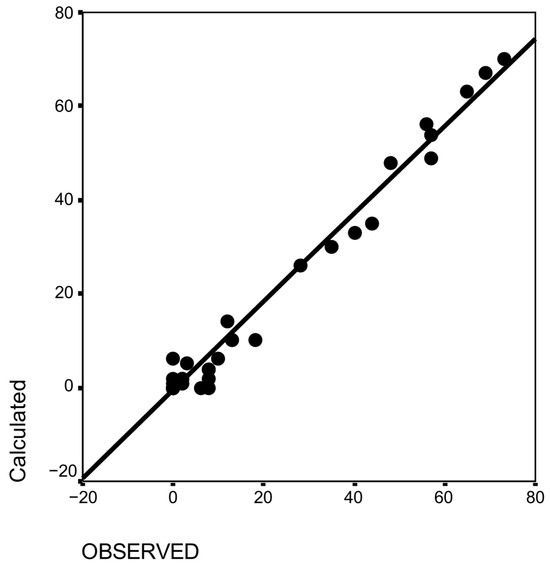
Figure 3.
Relationship between observed and calculated associations of anthelmintic resistances in sheep.
3.3. Anthelmintic Associations in Horses
3.3.1. Strongyles
Strongyles were mostly cyathostomins. A calculation similar to that of ruminant on observed and calculated association of resistance was performed on the data of Table 3. There was no significant difference even for the Denmark survey [48].

Table 3.
Strongyle resistance to two anthelmintics (Bz: benzimidazole, Pyr: pyrantel) in 66 horse farms.
The results of the FECRT for each farm are presented in Table 4. The FECRT results for ivermectin were high on all farms and could therefore not be correlated with the other resistances. There was no significant relationship between pyrantel and benzimidazole FECRT (rs = −0.06; p > 0.05; n = 55): see Figure 4. Analysing the data in Table 4 in terms of resistance or not, based on FECRT < 90%, showed no relationship between resistance to Bz and Pyr (Phi coefficient 0.27, p > 0.05).

Table 4.
Efficacy on gastrointestinal nematodes of the three main groups of anthelmintics in 56 individual horse farms (Bz: benzimidazoles, Pyr: pyrantel, Ml: ivermectin, a macrocyclic lactone).
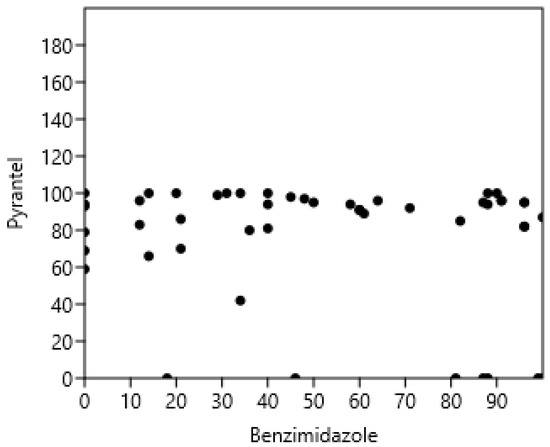
Figure 4.
Absence of correlation between pyrantel and benzimidazoles faecal strongyle egg reduction test in horses.
3.3.2. Parascaris
The data on Parascaris are presented in Table 5.

Table 5.
Efficacy on Parascaris of three main groups of anthelmintics in 60 horse farms.
The Spearman correlations between FECRT of the three drugs (rs = −0.03, n = 22, p = 0.92) were not significant (Figure 5). The correlations between benzimidazoles and pyrantel (rs = −0.12), benzimidazoles and ivermectin (rs = −0.22), and pyrantel and ivermectin (rs = 0.54) were not significant.
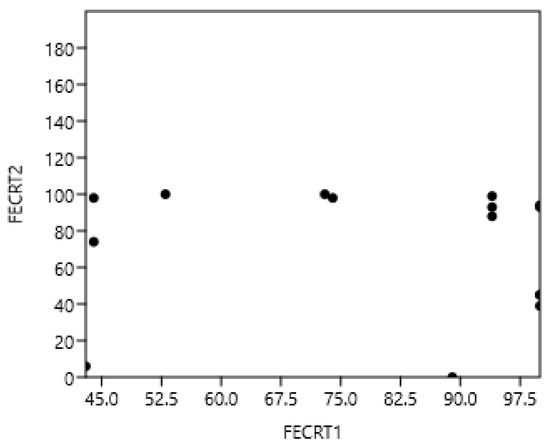
Figure 5.
Absence of correlation between benzimidazoles, pyrantel (1), and ivermectin (2) in the faecal Parascaris egg reduction test (FECRT) in horses.
4. Discussion
First, I will discuss the necessity of combining anthelmintics due to the increase in resistance. As few new anthelmintics appear on the market, molecules released many years ago, such as levamisole and pyrantel, may be included in these combinations. Secondly, I will present ways to evaluate the efficacy of these combinations in field conditions. Two methods will be employed and evaluated: a combined probability method and a regression method. Thirdly, I will present the absence of a relationship between resistance to the different tested anthelmintics, based on our data. Fourthly, the pros and cons of combining anthelmintics will be described.
4.1. The Necessity of Combining Anthelmintics
Since anthelmintic resistance is a growing concern worldwide (Figure 2A,B, ref. [1,68]) and new anthelmintics available are scarce; a combination of existing drugs has been proposed. The examination of the literature gives a false idea of the importance of the works on combinations of anthelmintics. Many are presented in review articles and based on the difference in mode of action of each anthelmintic class. The most studied are benzimidazoles and macrocyclic lactones (such as ivermectin and moxidectin), which are used in all species of hosts (Table 1). Levamisole (mostly in sheep) and pyrantel (only in horses) did represent a very small part (a few percent of the literature) (see Table 1) and were more cited in older works. They regained interest, probably due to nowadays anthelmintic associations used to cope against GIN resistance. I selected benzimidazoles, ivermectin, and levamisole in ruminants and benzimidazole, ivermectin, and pyrantel in horses because I could obtain data with measured individual and associated drug resistances. I chose to evaluate drugs that have been used for decades because they give an indication of the potential for joint resistance to build up.
4.2. How to Evaluate Efficacy of Combinations?
Assessing the efficacy of combinations of different classes of anthelmintics is not a straightforward task. When two drugs are combined, they may behave like a third, introducing a range of uncertainties. The efficacy of oxfendazole and pyrantel was relatively low, whereas abamectin and morantel showed high efficacy in horses [67], depending on the history of resistance to these drugs. In theory, the resulting Combination Index (CI), based on the Chou-Talalay theorem, provides a quantitative definition of the additive effect (CI = 1), synergism (CI < 1), or antagonism (CI > 1) of drug combinations [69,70]. The proposed method for assessing the CI is experimental and requires efficacy measurements at various doses of each drug and their combinations, expressed as percentages [69]. This approach was applied in an animal model to evaluate the most effective drug combinations for treating Trichuris in humans [71], but it is not feasible in routine practice.
In many current trials, each drug’s efficacy is assessed separately at the recommended dose, which means no information is obtained on the efficacy of combinations or resistance patterns. However, when drugs are used concurrently against gastrointestinal nematodes (GIN) and prevalence of resistance is established in a large number of farms, I demonstrated that the efficacy of the combination can be estimated using probability calculations and compared to the actual results. This was true for many of the ruminant studies I examined. In contrast, combination efficacy studies in horses were limited; only three were comparable to those in ruminants (Table 3). Therefore, alternative strategies were employed for horses, such as correlating the faecal egg count reduction test (FECRT) results for different drugs in a farm. The correlation of FECRT should be used with caution since if all drugs are efficient or none, the correlation is high; I avoided correlation due to the absence of resistance by selecting farms with at least one resistant anthelmintic. One issue with my evaluation of associations is that the FECRTs were not evaluated using the same methods, which could have introduced bias into the efficacy results. This was probably more significant for the correlation method than the probability method: the former considered the exact value of the FECRT, whereas the latter assessed resistance as efficacy below 90%. The probability method can also be understood in the context of repeated experiences, where variability is divided by the square root of the number of experiences (here, four for ruminants). The ruminant results are more reliable than the cyathostomin horse results (larger number of farms and better evaluation of associations). The data concerning Parascaris might be the least reliable since this species is a dose-limiting parasite [15]. This selection of surveys was performed on an automatic selection (Google Scholar and AlexOpen) and then screened manually to find the information I needed.
4.3. The Independence of Resistance to Major Anthelmintics
In all cases (Figure 3, Figure 4 and Figure 5), resistance to different classes of anthelmintics and for different nematodes (GIN of ruminants, Cyathostomins, and Parascaris in horses) was not associated in the field as it might be expected based on their molecular targets in nematodes [21]. Although it is based on a limited number of references, it encompasses the results of more than 1200 ruminant farms and 120 horse farms, making it representative of the actual situation regarding resistance. However, cases in New Zealand cattle suggest that the resistance to oxfendazole, levamisole, and macrocyclic lactones could emerge simultaneously under the strong selective pressure of these drugs [72]. There is also a record of associated resistance between eprinomectin and doramectin in the USA [73]. In the long term, it is possible that associations of resistance to multiple classes of anthelmintics will emerge: (i) if no new anthelmintic is available, and (ii) an intensive use of the available anthelmintics is promoted as the only manner of controlling GIN, then (iii) resistance will then appear progressively in all anthelmintics after being sequentially used.
The appearance of resistance is due to human (choice of anthelmintics, frequency of treatments, etc.), genetic (selection of mutations in worms), and environmental factors (choice of pasture to preserve refugia). The farmers’ practices concerning anthelmintic use and animal management frequently do not follow the requirements based on research [74]. In theory, this should have led to the appearance of drug resistance, but this was not evident in our data. Most factors influencing the emergence of resistance—such as treatment frequency [73] and timing in relation to worms in refugia [75]—are based on benzimidazole resistance and may differ for other drug classes. It is also difficult to pinpoint the most probable reason for the appearance of resistance. In dairy ewes in south-east France, for example, transhumance was identified as the main risk factor for the emergence of resistance to the macrocyclic lactone eprinomectin, likely due to the spread of resistant GIN between flocks [76]. Hence, the various husbandry management scenarios may explain why anthelmintic resistances are not necessarily related.
4.4. The Pros and Cons of Combining Anthelmintics
The principle that combinations of chemotherapeutic agents can benefit infected hosts by maintaining drug efficacy in the presence of resistance has been repeatedly demonstrated for various pathogens, drawing on insights from insecticide and pesticide use [28]. In ruminants—particularly sheep—combinations of two or more anthelmintics are primarily used to manage resistance [77]. They may delay the emergence and spread of resistance [28] and help maintain control of GIN populations despite existing resistance [29]. Modelling studies have also indicated that combinations provide significant advantages for both drugs when used together, especially if they initially have high efficacy [78]. Using anthelmintics in combination has been shown to be superior to using them individually, whether in sequence or rotation, even when some cross-resistance exists between the two classes [79]. However, concerns remain regarding the use of combinations, particularly the potential risk of selecting for multi-class resistance if (i) refugia (GIN not submitted to anthelmintics) are insufficient and (ii) the initial frequency of resistance alleles is too high on certain farms to justify introducing combination treatments [79,80]. When resistance is high for several anthelmintics, farmers should not rely on the sole anthelmintic treatment and turn to other control methods (among others: use of predatory hyphomycetes or plants extracts [81].
5. Conclusions
It is difficult to establish associations—whether positive or negative—between anthelmintic resistances across different drug classes in field conditions. Such associations can be explored by calculating the probability of cross-resistance (when tests on individual anthelmintics and their combinations are available in different farms) or by cautiously evaluating the correlation between drug efficacy (when no combination is tested), as determined by faecal egg count reduction tests. No clear association of resistance has been demonstrated under farming conditions, either in ruminants or in horses. Therefore, the use of anthelmintic combinations may be of interest as a strategy to possibly slow the development of resistance and to maintain effective control of GIN infections when resistance has not yet reached a high level. Research should focus on designing the most effective anthelmintic combinations in relation to pre-existing resistance status and on understanding their potential pharmacological implications on efficacy.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available within this paper.
Acknowledgments
I thank R.J. Martin, C. Charvet, and C. Neveu for interesting discussions on the subject.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Vineer, H.R.; Morgan, E.R.; Hertzberg, H.; Bartley, D.J.; Bosco, A.; Charlier, J.; Chartier, C.; Claerebout, C.; de Waal, T.; Hendrickx, G.; et al. Increasing importance of anthelmintic resistance in European livestock: Creation and meta-analysis of an open database. Parasite 2020, 27, 69. [Google Scholar] [CrossRef]
- Wolstenholme, A.J.; Fairweather, I.; Prichard, R.; von Samson-Himmelstjerna, G.; Sangster, N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004, 20, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Geurden, T.; Hoste, H.; Jacquiet, P.; Traversa, D.; Sotiraki, S.; di Regalbono, A.F.; Tzanidakis, N.; Kostopoulou, D.; Gaillac, C.; Privat, S.; et al. Anthelmintic resistance and multidrug resistance in sheep gastro-intestinal nematodes in France, Greece and Italy. Vet. Parasitol. 2014, 201, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Overend, D.J.; Phillips, M.L.; Poulton, A.L.; Foster, C. Anthelmintic resistance in Australian sheep nematode populations. Aust. Vet. J. 1994, 71, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, T.S.; Leathwick, D.M.; Rhodes, A.P.; Lawrence, K.E.; Jackson, R.; Pomroy, W.E.; West, D.M.; Moffat, J.R. Prevalence of anthelmintic resistance on sheep farms in New Zealand. N. Z. Vet. J. 2006, 54, 271–277. [Google Scholar] [CrossRef]
- Echevarria, F.; Borba, M.F.; Pinheiro, A.C.; Waller, P.J.; Hansen, J.W. The prevalence of anthelmintic resistance in nematode parasites of sheep in Southern Latin America: Brazil. Vet. Parasitol. 1996, 62, 199–206. [Google Scholar] [CrossRef]
- Baudinette, E.; O’Handley, R.; Trengove, C. Anthelmintic resistance of gastrointestinal nematodes in goats: A systematic review and meta-analysis. Vet. Parasitol. 2022, 15, 109809. [Google Scholar] [CrossRef]
- Cabaret, J. Anthelmintic resistance in goats: From fiction to facts. In 7th Conférence Internationale sur les Caprins; Institut de l’Élevage: Paris, France, 2000. [Google Scholar]
- Mejía, M.; Igartúa, B.M.; Schmidt, E.; Cabaret, J. Multispecies and multiple anthelmintic resistance on cattle nematodes in a farm in Argentina: The beginning of high resistance? Vet. Res. 2003, 34, 461–467. [Google Scholar] [CrossRef]
- Baiak, B.H.; Lehnen, C.R.; da Rocha, R.A. Anthelmintic resistance in cattle: A systematic review and meta-analysis. Livestock Sci. 2018, 217, 127–135. [Google Scholar] [CrossRef]
- Borgsteede, F.H.; Roos, M.H.; Smith, G.; Prichard, R.K. Anthelminthics resistance. Vet. Parasitol. 1996, 64, 129–132. [Google Scholar] [CrossRef]
- Drudge, J.H.; Elam, G. Preliminary observations on the resistance of the horse strongyles to phenothiazine. J. Parasitol. 1961, 47, 38–39. [Google Scholar]
- Pereira, M.C.; Kohek Junior, I.; Campos, R.; Lima, S.B.; Foz, R.P. A field evaluation of anthelmintics for control of cyathostomes of horses in Brazil. Vet. Parasitol. 1991, 38, 121–129. [Google Scholar] [CrossRef]
- Traversa, D.; von Samson-Himmelstjerna, G.; Demeler, J.; Milillo, P.; Schürmann, S.; Barnes, H.; Otranto, D.; Perrucci, S.; di Regalbono, A.F.; Beraldo, P.; et al. Anthelmintic resistance in cyathostomin populations from horse yards in Italy, United Kingdom and Germany. Parasites Vectors 2009, 2, S2. [Google Scholar] [CrossRef] [PubMed]
- Reinemeyer, C.R.; Craig, R. Diagnosis and control of anthelmintic-resistant Parascaris equorum. Parasites Vectors 2009, 2, S8. [Google Scholar] [CrossRef]
- Bauer, C.; Merkt, J.C.; Janke-Grimm, G.; Bürger, H.J. Prevalence and control of benzimidazole-resistant small strongyles on German thoroughbred studs. Vet. Parasitol. 1986, 21, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Coles, G.C.; Jackson, F.; Pomroy, W.E.; Prichard, R.K.; von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.A.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M.; Klei, T.R.; Lyons, E.T.; Lester, G.; Courtney, C.H.; French, D.D.; Tolliver, S.C.; Vidyashankar, A.N.; Zhao, Y. Prevalence of anthelmintic resistant cyathostomes on horse farms. J. Am. Vet. Med. Assoc. 2004, 225, 903–910. [Google Scholar] [CrossRef]
- Cabaret, J. Reliable phenotypic evaluations of anthelmintic resistance in herbivores: How and when should they be done. In Anthelmintics: Clinical Pharmacology, Uses in Veterinary Medicine and Efficacy; Nova Science Publisher: New York, NY, USA, 2014; pp. 1–26. [Google Scholar]
- Jabbar, A.; Iqbal, Z.; Kerboeuf, D.; Muhammad, G.; Khan, M.N.; Afaq, M. Anthelmintic resistance: The state of play revisited. Life Sci. 2006, 79, 2413–2431. [Google Scholar] [CrossRef]
- Robertson, A.P.; Buxton, S.K.; Puttachary, S.; Williamson, S.M.; Wolstenholme, A.J.; Neveu, C.; Cabaret, J.; Charvet, C.L.; Martin, R.J. Antinematodal drugs—Modes of action and resistance: And worms will not come to thee (Shakespeare: Cymbeline: IV, ii). In Parasitic Helminths: Targets, Screens, Drugs and Vaccines; Caffrey, C.R., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 233–249. [Google Scholar]
- Charvet, C.L.; Guégnard, F.; Courtot, E.; Cortet, J.; Neveu, C. Nicotine-sensitive acetylcholine receptors are relevant pharmacological targets for the control of multidrug resistant parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 540–549. [Google Scholar] [CrossRef]
- Kerboeuf, D.; Blackhall, W.; Kaminsky, R.; von Samson-Himmelstjerna, G. P-glycoprotein in helminth: Function and perspectives for anthelmintic treatment and reversal of resistance. Int. J. Antimicrob. Agents. 2003, 22, 332–346. [Google Scholar] [CrossRef]
- Mottier, M.D.; Prichard, R.K. Genetic analysis of a relationship between macrocyclic lactone and benzimidazole anthelmintic selection on Haemonchus contortus. Pharmacogenet. Genom. 2008, 18, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.K.L.; Blackhall, W.J.; Osei-Atweneboana, M.Y. Ivermectin selection on beta-tubulin: Evidence in Onchocerca volvulus and Haemonchus contortus. Mol. Biochem. Parasitol. 2006, 150, 229–235. [Google Scholar] [CrossRef]
- Silvestre, A.; Leignel, V.; Berrag, B.; Gasnier, N.; Humbert, J.F.; Chartier, C.; Cabaret, J. Sheep and goat nematode resistance to anthelmintics: Pro and cons among breeding management factors. Vet. Res. 2002, 33, 465–480. [Google Scholar] [CrossRef]
- Sallé, G.; Cortet, J.; Bois, I.; Guyot-Sionest, Q.; Larrieu, C.; Landrin, V.; Majorel, G.; Wittreck, S.; Woringer, E.; Couroucé, A.; et al. Risk factor analysis of equine strongyle resistance to anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 407–415. [Google Scholar] [CrossRef]
- Geary, T.G.; Hosking, B.C.; Skuce, P.J.; von Samson-Himmelstjerna, G.; Maeder, S.; Holdsworth, P.; Pomroy, W.; Vercruysse, J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) Guideline: Anthelmintic combination products targeting nematode infections of ruminants and horses. Vet. Parasitol. 2012, 190, 306–316. [Google Scholar] [CrossRef]
- Bartram, D.J.; Leathwick, D.M.; Taylor, M.A.; Geurden, T.; Maeder, S.J. The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet. Parasitol. 2012, 186, 151–158. [Google Scholar] [CrossRef]
- Alvarez, L.; Lifschitz, A.; Entrocasso, C.; Manazza, J.; Mottier, L.; Borda, B.; Virkel, G.; Lanusse, L. Evaluation of the interaction between ivermectin and albendazole following their combined use in lambs. J. Vet. Pharmacol. Therap. 2008, 31, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Abongwa, M.; Buxton, S.K.; Robertson, A.P.; Martin, R.J. Curiouser and Curiouser: The Macrocyclic Lactone, Abamectin, Is also a Potent Inhibitor of Pyrantel/Tribendimidine Nicotinic Acetylcholine Receptors of Gastro-Intestinal Worms. PLoS ONE 2016, 11, e0146854. [Google Scholar] [CrossRef] [PubMed]
- Suarez, G.; Alvarez, L.; Castells, D.; Moreno, L.; Fagiolino, P.; Lanusse, C. Evaluation of pharmacological interactions after administration of a levamisole, albendazole and ivermectin triple combination in lambs. Vet. Parasitol. 2014, 201, 110–119. [Google Scholar] [CrossRef]
- Allworth, M.B.; Goonan, B.; Nelson, J.E.; Kelly, G.; McGrath, S.R.; Woodgate, R.G. Comparison of the efficacy of macrocyclic lactone anthelmintics, either singly or in combination with other anthelmintic(s), in nine beef herds in southern NSW. Aust. Vet. J. 2023, 101, 293–295. [Google Scholar] [CrossRef]
- Wrigley, J.; McArthur, M.; McKenna, P.B.; Mariadass, B. Resistance to a triple combination of broad-spectrum anthelmintics in naturally acquired Ostertagia circumcincta infections in sheep. N. Z. Vet. J. 2006, 54, 47–49. [Google Scholar] [CrossRef]
- Anziani, O.S.; Muchiut, S. Resistencia antihelmíntica múltiple (closantel, fenbendazol, ivermectina y levamisol) en Haemonchus spp parasitando ovinos en la provincia de Santa Fe. Ineficacia de una triple combinación de estas drogas para su control. Rev. Med. Vet. 2014, 95, 23–27. [Google Scholar]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Waghorn, T.S.; Leathwick, D.M.; Rhodes, A.P.; Jackson, R.; Pomroy, W.E.; West, D.M.; Moffat, J.R. Prevalence of anthelmintic resistance on 62 beef cattle farms in the North Island of New Zealand. N. Z. Vet. J. 2006, 54, 278–282. [Google Scholar] [CrossRef]
- Ventsel, H. Théorie des Probabilités; Editions Mir: Moscow, Russia, 1973; 563p. [Google Scholar]
- Silva Vieira, L.; Rocha Cavalcante, A.C. Resistencia anti-helmíntica em rebanhos caprinos no Estado do Ceara. Pesq. Vet. Bras. 1999, 19, 99–103. [Google Scholar] [CrossRef]
- Chartier, C.; Soubirac, F.; Pors, I.; Silvestre, A.; Hubert, J.; Couquet, C.; Cabaret, J. Prevalence of anthelmintic resistance in gastrointestinal nematodes of dairy goats under extensive management conditions in southwestern France. J. Helm. 2001, 75, 325–330. [Google Scholar] [CrossRef]
- Melo, A.C. Resistance to Benzimidazoles in Nematode Haemonchus contortus in Ceara State. Ph.D. Thesis, Universida de Ceara, Fortaleza, Brazil, 2005; 170p. [Google Scholar]
- Bentounsi, B.; Attir, B.; Meradi, S.; Cabaret, J. Repeated treatment faecal egg counts to identify gastrointestinal nematode resistance in a context of low-level infection of sheep on farms in eastern Algeria. Vet. Parasitol. 2007, 144, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Zuiten, H. Résistance des Nématodes aux Antiparasitaires Etude Comparée chez les Ovins et les Chevaux. Ph.D. Thesis, Faculté des Sciences, Université Mohamed V-Agdal, Rabat, Morrocco, 2006; 137p. [Google Scholar]
- Álvarez-Sánchez, M.A.; Pérez-García, J.; Cruz-Rojo, M.A.; Rojo-Vázquez, F.A. Anthelmintic resistance in trichostrongylid nematodes of sheep farms in Northwest Spain. Parasitol. Res. 2006, 99, 78–83. [Google Scholar] [CrossRef]
- Chartier, C.; Pors, I.; Hubert, J.; Rocheteau, D.; Benoit, C.; Bernard, N. Prevalence of anthelmintic resistant nematodes in sheep and goats in Western France. Small Rum. Res. 1998, 29, 33–41. [Google Scholar] [CrossRef]
- Cristel, S.L.; Suárez, V.H. Resistencia antihelmíntica: Evaluación de la prueba de reducción del conteo de huevos. Rev. Investig. Agropecu. 2006, 35, 29–43. [Google Scholar]
- Soutello, R.G.V.; Seno, M.C.Z.; Amarante, A.F.T. Anthelmintic resistance in cattle nematodes in northwestern Sao Paulo state, Brazil. Vet. Parasitol. 2007, 148, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Craven, J.; Bjørn, H.; Barnes, E.H.; Henriksen, S.A.; Nansen, P. A comparison of in vitro tests and a faecal egg count reduction test in detecting anthelmintic resistance in horse strongyles. Vet. Parasitol. 1999, 85, 49–59. [Google Scholar] [CrossRef]
- Ihler, C.F. A Field Survey on Anthelmintic Resistance in Equine Small Strongyles in Norway. Acta Vet. Scand. 1995, 36, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Lind, E.O.; Rautalinko, E.; Uggla, A.; Waller, P.J.; Morrison, D.A.; Höglund, J. Parasite control practices on Swedish horse farms. Acta Vet. Scand. 2007, 49, 25. [Google Scholar] [CrossRef]
- Canever, R.J.; Braga, P.R.C.; Boeckh, A.; Grycajuck, M.; Bier, D.; Molento, M.B. Lack of Cyathostomin sp. reduction after anthelmintic treatment in horses in Brazil. Vet. Parasitol. 2013, 194, 35–39. [Google Scholar] [CrossRef]
- Pagnon, R. Résistance aux Anthelminthiques des Strongles chez les Équidés: Enquête dans un Centre Équestre du Sud de la France. Ph.D. Thesis, Ecole Nationale Vétérinaire, Toulouse, France, 2005; 121p. [Google Scholar]
- Traversa, D.; Castagna, P.; von Samson-Himmelstjerna, G.; Meloni, S.; Bartolini, R.; Geurden, T.; Pearce, M.C.; Woringer, E.; Besognet, B.; Milillo, P.; et al. Efficacy of major anthelmintics against horse cyathostomins in France. Vet. Parasitol. 2012, 188, 294–300. [Google Scholar] [CrossRef]
- Traversa, D.; Klei, R.T.; Iorio, R.; Paoletti, B.; Lia, R.P.; Otranto, D.; Sparagano, O.A.E.; Giangaspero, A. Occurrence of anthelmintic resistant equine cyathostome populations in central and southern Italy. Prev.Vet. Med. 2007, 82, 314–320. [Google Scholar] [CrossRef]
- Guerrero, C. (Departamento de Parasitologia, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autonoma, Mexico City, Mexico). Personal communication, 2010.
- Zouiten, H.; Berrag, B.; Oukessou, M.; Sadak, A.; Cabaret, J. Poor efficacy of the most commonly used anthelmintics in sport horse nematodes in Morocco in relation to resistance. Parasite 2005, 12, 347–351. [Google Scholar] [CrossRef]
- Kuzmina, T.A.; Kharchenko, V.O. Anthelmintic resistance in cyathostomins of brood horses in Ukraine and influence of anthelmintic treatments on strongylid community structure. Vet. Parasitol. 2008, 154, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.; West, E.; Norat-Collazo, L.; Vargas, J. A combination treatment strategy using pyrantel pamoate and oxibendazole demonstrates additive effects for controlling equine cyathostomins. Equine Vet. Edu. 2014, 26, 485–491. [Google Scholar] [CrossRef]
- Cooper, L.G.; Caffe, G.; Cerutti, J.; Nielsen, M.K.; Anziani, O.S. Reduced efficacy of ivermectin and moxidectin against Parascaris spp. in foals from Argentina. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100388. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.K.; Woodgate, R.G.; Gough, S.; Heller, J.; Sangster, N.C.; Hughes, K.J. The efficacy of ivermectin, pyrantel and fenbendazole against Parascaris equorum infection in foals on farms in Australia. Vet. Parasitol. 2014, 205, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Molento, M.B.; Antunes, J.; Bentes, R.N.; Coles, G.C. Anthelmintic resistant nematodes in Brazilian horses. Vet. Rec. 2008, 162, 384. [Google Scholar] [CrossRef]
- Lignon, J.S.; Pappen, F.G.; Böhm, B.C.; Pinto, D.M. Antiparasitic action of ivermectin, moxidectin and piperazine against Parascaris spp. in foals at southern Rio Grande do Sul, Brazil. Ciência Anim. 2023, 33, 41–47. [Google Scholar]
- Lassen, B.; Peltola, S.-M. Anthelmintic resistance of intestinal nematodes to ivermectin and pyrantel in Estonian horses. J. Helm. 2015, 89, 760–763. [Google Scholar] [CrossRef]
- Aromaa, M.; Hautala, K.; Oksanen, A.; Sukura, A.; Näreaho. Parasite infections and their risk factors in foals and young horses in Finland. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 35–38. [Google Scholar] [CrossRef]
- Lyons, E.T.; Tolliver, S.C.; Ionita, M.; Collins, S.S. Evaluation of parasiticidal activity of fenbendazole, ivermectin, oxibendazole, and pyrantel pamoate in horse foals with emphasis on ascarids (Parascaris equorum) in field studies on five farms in Central Kentucky in 2007. Parasitol. Res. 2008, 103, 287–291. [Google Scholar] [CrossRef]
- Lyons, E.T.; Tolliver, S.C.; Kuzmina, T.A.; Collins, S.S. Further evaluation in field tests of the activity of three anthelmintics (fenbendazole, oxibendazole, and pyrantel pamoate) against the ascarid Parascaris equorum in horse foals on eight farms in Central Kentucky (2009–2010). Parasitol. Res. 2011, 109, 1193–1197. [Google Scholar] [CrossRef]
- Reinemeyer, C.R. Anthelmintic resistance in non-strongylid parasites of horses. Vet. Parasitol. 2012, 185, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Voigt, K.; Geiger, M.; Jäger, M.C.; Knubben-Schweizer, G.; Strube, C.; Zablotski, Y. Effectiveness of anthelmintic treatments in small ruminants in Germany. Animals 2022, 12, 1501. [Google Scholar] [CrossRef]
- Chou, T.C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Martin, N. CompuSyn Software for Drug Combinations and for General Dose Effect Analysis, and User’s Guide; ComboSyn, Inc.: Paramus, NJ, USA, 2007; Available online: https://www.combosyn.com/ (accessed on 26 August 2025).
- Keiser, J.; Tritten, L.; Adelfio, T.; Vaegas, M. Effect of combinations of marketed human anthelmintic drugs against Trichuris muris in vitro and in vivo. Parasites Vectors 2012, 5, 292. [Google Scholar] [CrossRef] [PubMed]
- Sauermann, C.; Waghorn, T.; Miller, C.; Leathwick, D. Simultaneous resistance to multiple anthelmintic classes in nematode parasites of cattle in New Zealand. Vet. Parasitol. 2024, 325, 110079. [Google Scholar] [CrossRef]
- Gianchini, L.S.; Paras, K.L.; George, M.M.; Howell, S.B.; Storey, B.; Denwood, M.J.; Ray, M. Kaplan RM. Multiple-species resistance to avermectin anthelmintics on beef cattle farms in Georgia, USA. Vet. Parasitol. 2025, 336, 110435. [Google Scholar] [CrossRef]
- Cabaret, J.; Nicourt, C. Farmers’ and Experts’ Knowledge Coping with Sheep Health, Control and Anthelmintic Resistance of Their Gastrointestinal Nematodes. Pathogens 2024, 13, 297. [Google Scholar] [CrossRef]
- Van Wyk, J.A. Refugia-overlooked as perhaps the most potent factor concerning the development of anthelmintic. Onderstepoort J. Vet. Res. 2001, 68, 55–67. [Google Scholar]
- Jouffroy, S. Resistance to Eprinomectin in Haemonchus Contortus: Diagnostic, Risk factors and Solutions for Dairy Sheep Farms in France. Ph.D. Thesis, Ecole Vétérinaire de Toulouse, Toulouse, France, 2024; 250p. [Google Scholar]
- Le Jambre, L.F.; Martin, P.J.; Johnston, A. Efficacy of combination anthelmintics against multiple resistant strains of sheep nematodes. Anim. Prod. Sci. 2010, 50, 946–952. [Google Scholar] [CrossRef]
- Leathwick, D.M. Modelling the benefits of a new class of anthelmintic in combination. Vet. Parasitol. 2012, 186, 96–100. [Google Scholar]
- Abbas, G.; Ghafar, A.; Hurley, J.; Bauquier, J.; Beasley, A.; Wilkes, E.J.; Jacobson, C.; El-Hage, C.; Cudmore, L.; Carrigan, P.; et al. Cyathostomin resistance to moxidectin and combinations of anthelmintics in Australian horses. Parasites Vectors 2021, 14, 597. [Google Scholar] [CrossRef]
- Lanusse, C.; Canton, C.; ∙Virkel, G.; Alvarez, L.; Costa-Junior, L. Adrian Lifschitz. A. Strategies to Optimize the Efficacy of Anthelmintic Drugs in Ruminants. Trends Parasitol. 2018, 34, 664–682. [Google Scholar] [CrossRef]
- de la Crúz-Crúz, H.A.; Higuera-Piedrahita, R.I.; Zamilpa, A.; Alcalá-Canto, Y.; Ocampo-Gutiérrez, A.Y.; Arango-de la Pava, L.D.; López-Arellano, M.E.; Hernandez-Patlan, D.; Cuéllar-Ordaz, J.A.; Mendoza-de Gives, P. Using an Aqueous Suspension of Duddingtonia flagrans Chlamydospores and a Hexane Extract of Artemisia cina as Sustainable Methods to Reduce the Fecal Egg Count and Larvae of Haemonchus contortus in the Feces of Periparturient Ewes. Pathogens 2025, 14, 105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).