Impact of pH, Temperature and Exogenous Proteins on Aspartic Peptidase Secretion in Candida auris and the Candida haemulonii Species Complex
Abstract
1. Introduction
2. Material and Methods
2.1. Microorganisms
2.2. Growth Conditions
2.3. Plate Testing Assay
2.4. Modulation of Sap Production in Response to Different Protein Substrates
2.5. Quantification of Sap Activity Using Fluorogenic Peptide Substrate
2.6. Statistical Analyzes
3. Results
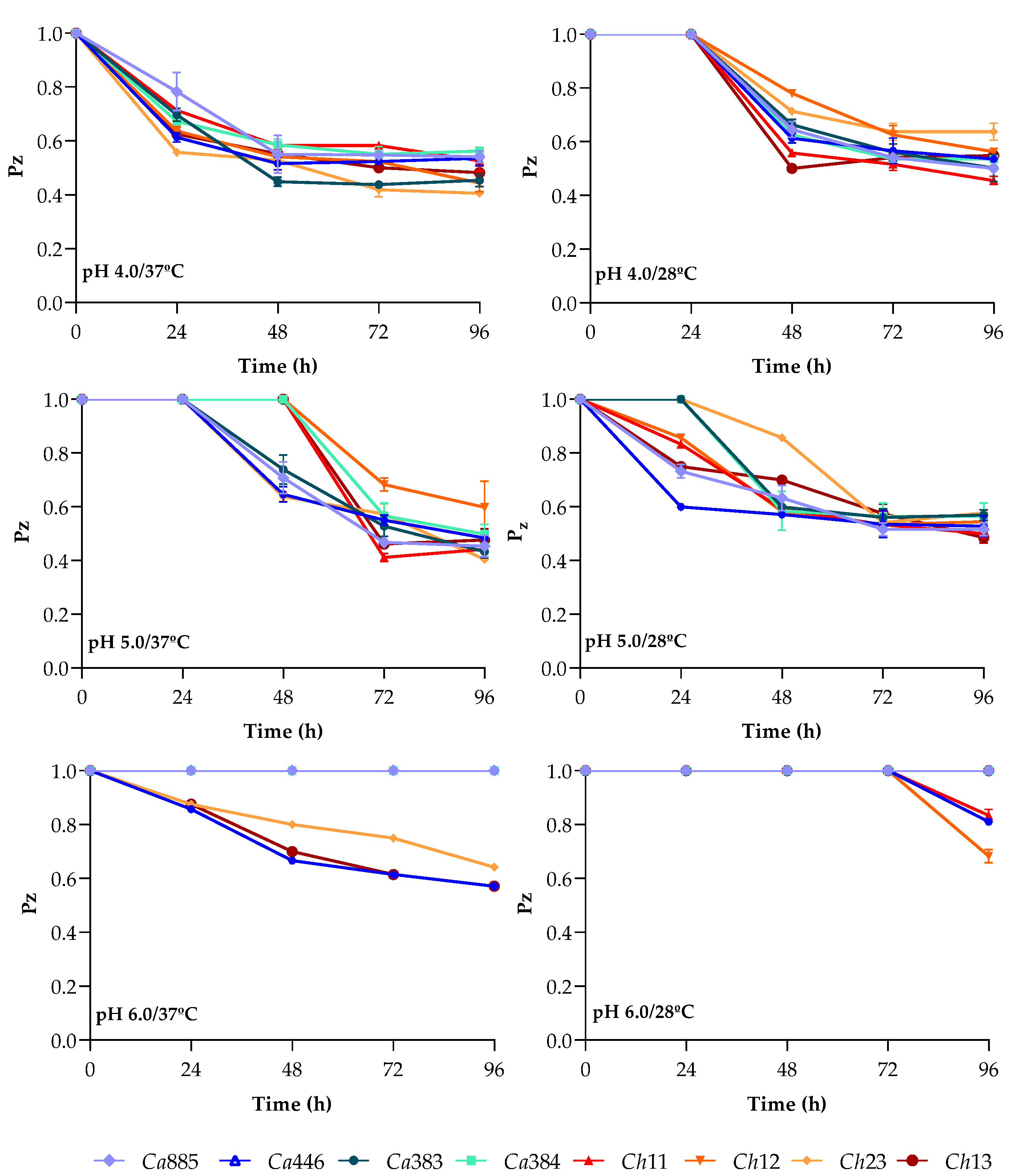
3.1. Modulation of Sap Production Across Different pHs and Temperatures over Time
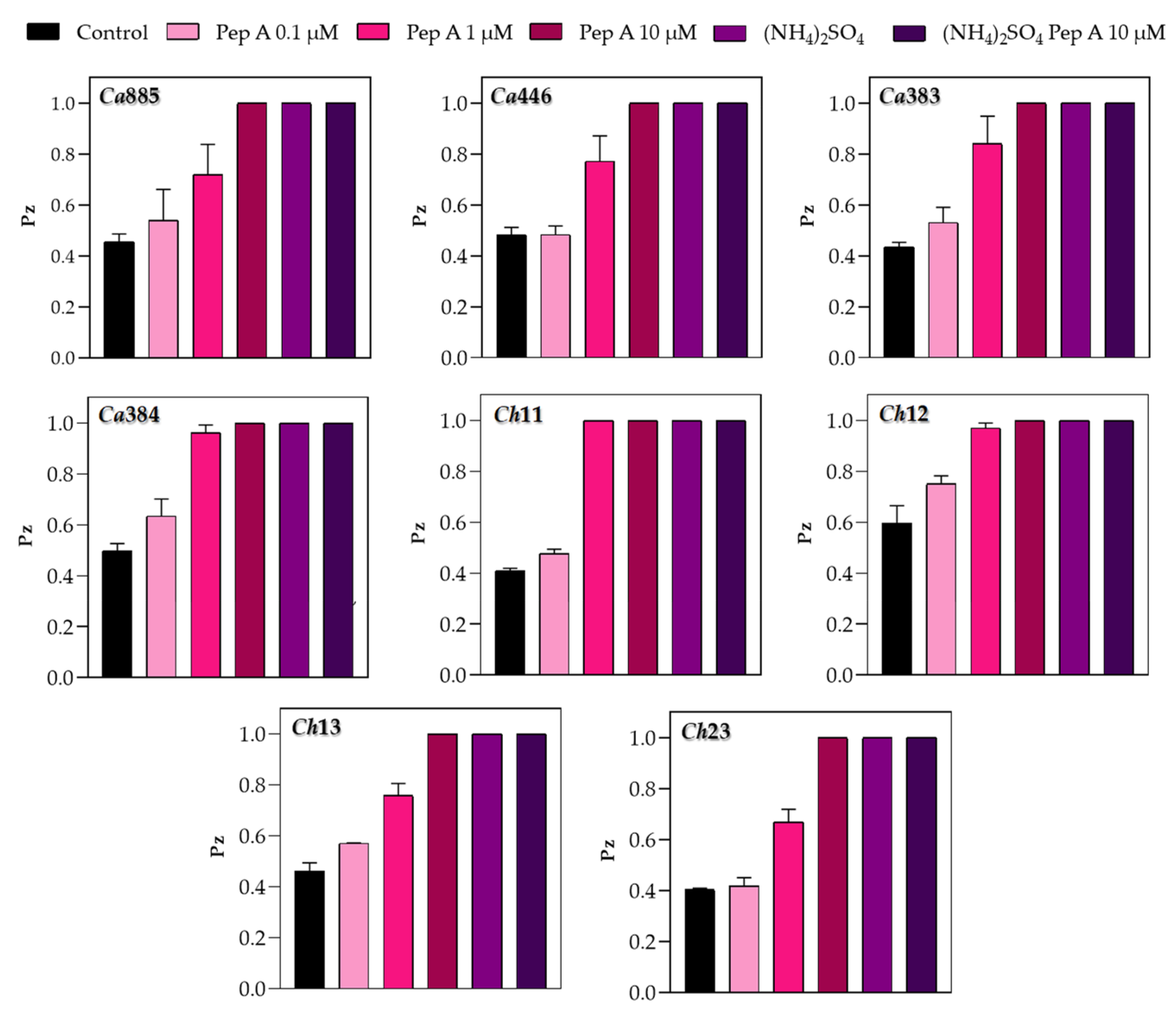
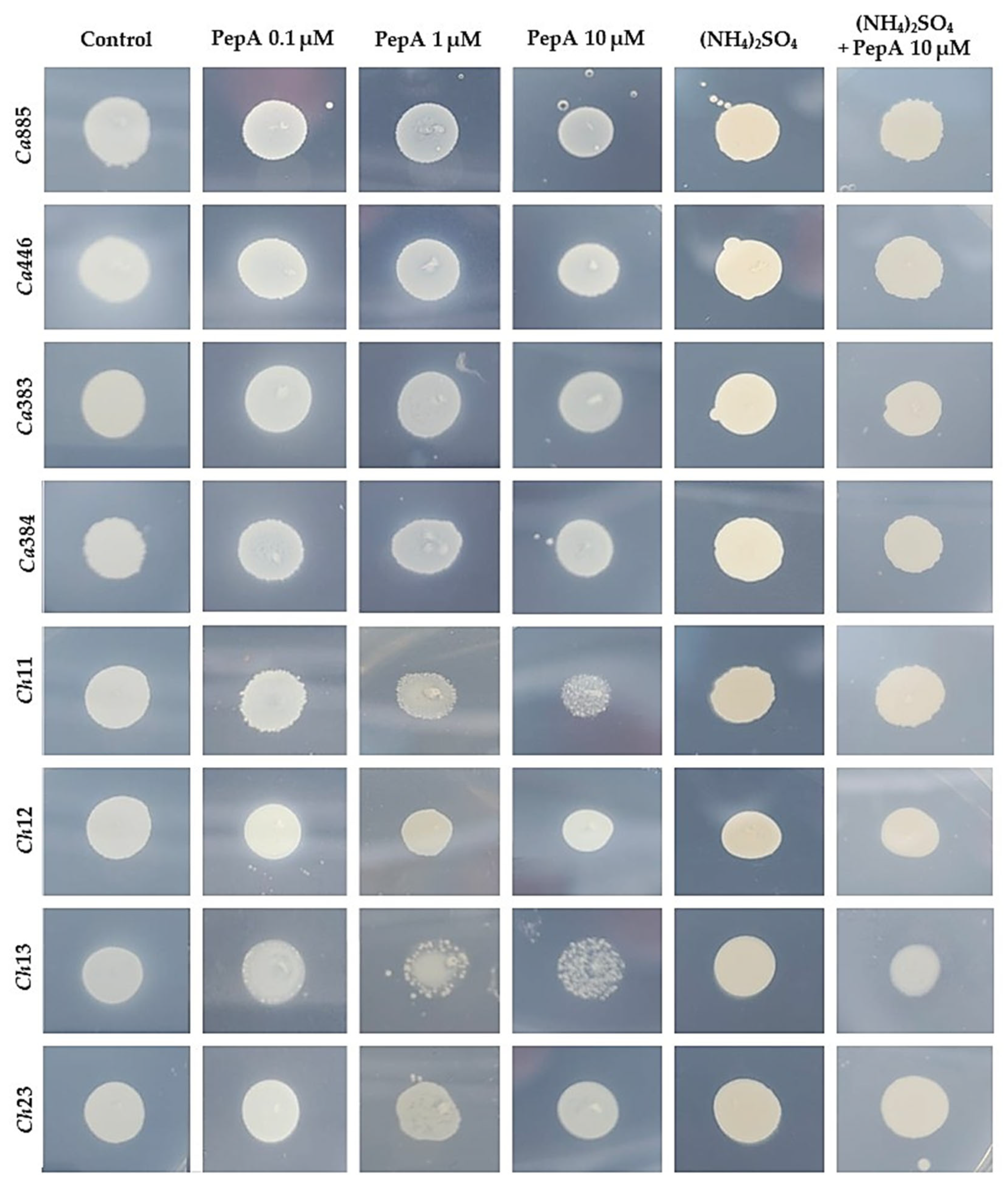
3.2. Inhibition of Sap Production by Aspartic Peptidase Inhibitor
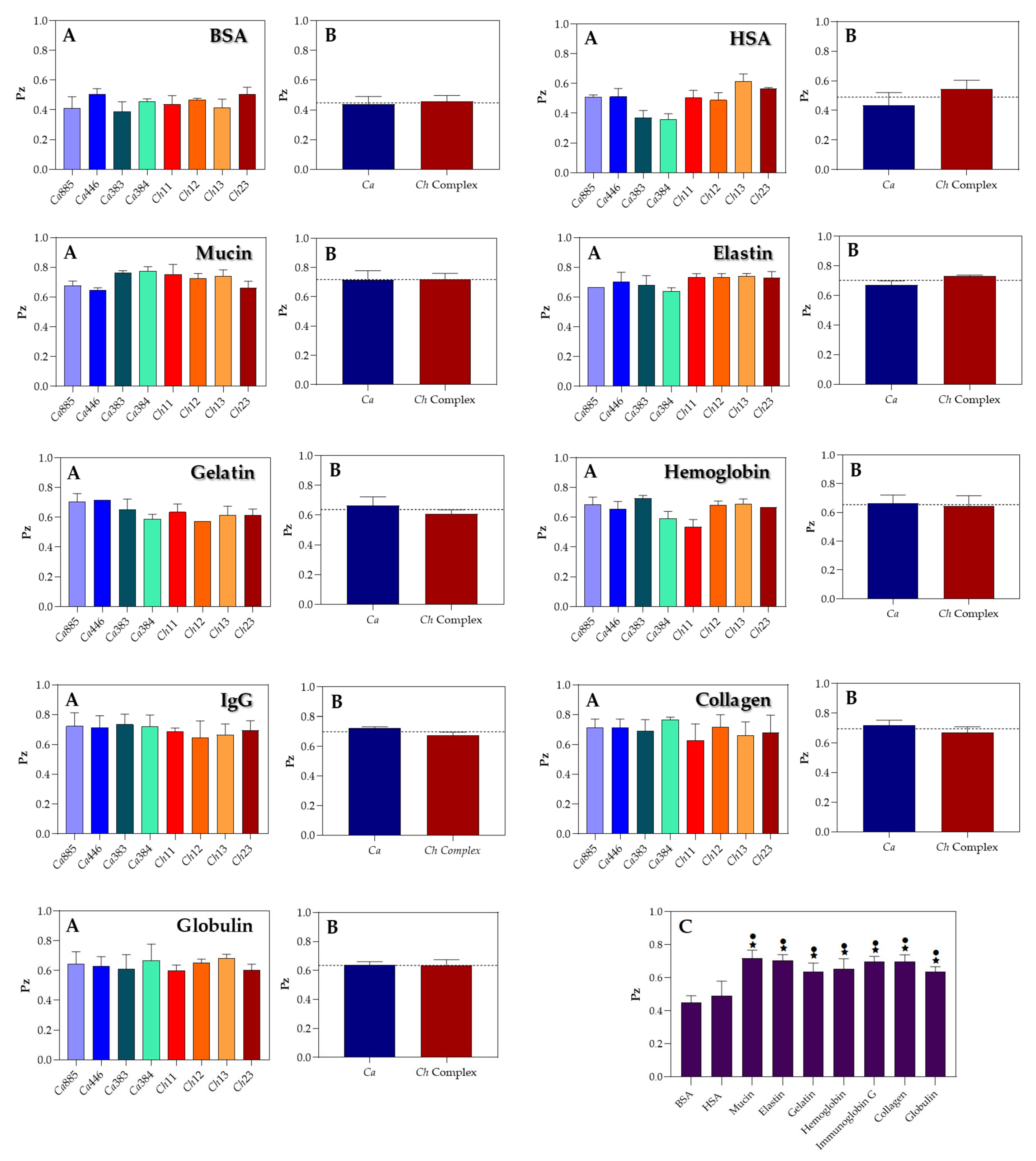
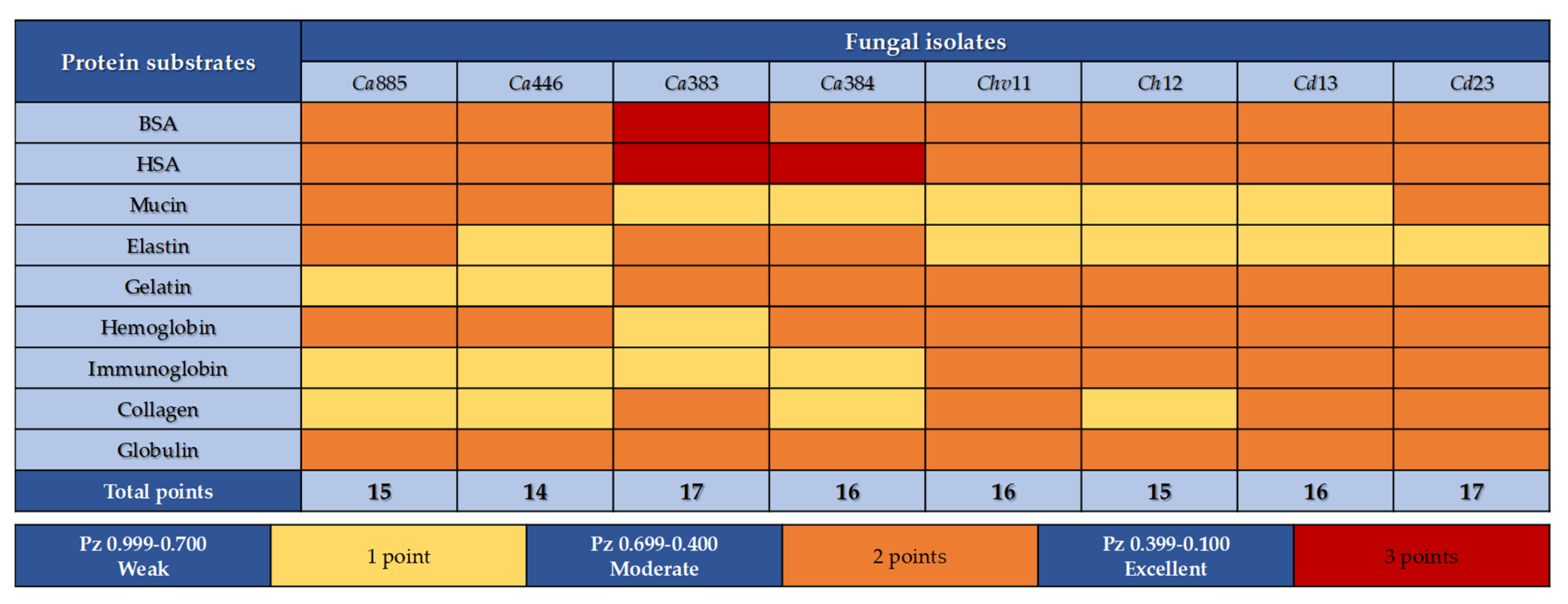
3.3. Modulation of Sap Production by Different Protein Substrates
3.4. Measurement of Sap Activity Via Fluorogenic Peptide-Based Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos-Pardo, A.; Castro-Álvarez, R.; Quindós, G.; Eraso, E.; Sevillano, E.; Kaberdin, V.R. Assessing pH-dependent activities of virulence factors secreted by Candida albicans. Microbiol. Open 2023, 12, e1342. [Google Scholar] [CrossRef]
- Harpf, V.; Rambach, G.; Würzner, R.; Lass-Flörl, C.; Speth, C. Candida and complement: New aspects in an old battle. Front. Immunol. 2020, 11, 1471. [Google Scholar] [CrossRef]
- Barbosa, P.F.; Gonçalves, D.S.; Ramos, L.S.; Mello, T.P.; Braga-Silva, L.A.; Pinto, M.R.; Taborda, C.P.; Branquinha, M.H.; Santos, A.L.S. Saps1-3 antigens in Candida albicans: Differential modulation following exposure to soluble proteins, mammalian cells, and infection in mice. Infect. Dis. Rep. 2024, 16, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Jain, K.; Chauhan, N. Candida auris genetics and emergence. Annu. Rev. Microbiol. 2023, 77, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.S.; Barbosa, P.F.; Lorentino, C.M.A.; Lima, J.C.; Braga, A.L.; Lima, R.V.; Giovanini, L.; Casemiro, A.L.; Siqueira, N.L.M.; Costa, S.C.; et al. The multidrug-resistant Candida auris, Candida haemulonii complex and phylogenetic related species: Insights into antifungal resistance mechanisms. Curr. Res. Microb. Sci. 2025, 8, 100354. [Google Scholar] [CrossRef] [PubMed]
- Françoise, U.; Desnos-Ollivier, M.; Le Govic, Y.; Sitbon, K.; Valentino, R.; Peugny, S.; Chouaki, T.; Mazars, E.; Paugam, A.; Nicolas, M.; et al. Candida haemulonii complex, an emerging threat from tropical regions? PLoS Negl. Trop. Dis. 2023, 17, e0011453. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Martínez-Álvarez, J.Á.; Chávez-Santiago, J.O.; Mora-Montes, H.M. Candida haemulonii complex and Candida auris: Biology, virulence factors, immune response, and multidrug resistance. Infect. Drug Resist. 2023, 16, 1455–1470. [Google Scholar] [CrossRef]
- Ramos, L.S.; Branquinha, M.H.; Santos, A.L.S. Different classes of hydrolytic enzymes produced by multidrug-resistant yeasts comprising the Candida haemulonii complex. Med. Mycol. 2017, 55, 228–232. [Google Scholar] [CrossRef]
- Bras, G.; Satala, D.; Juszczak, M.; Kulig, K.; Wronowska, E.; Bednarek, A.; Zawrotniak, M.; Rapala-Kozik, M.; Karkowska-Kuleta, J. Secreted aspartic proteinases: Key factors in Candida infections and host-pathogen interactions. Int. J. Mol. Sci. 2024, 25, 4775. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Ramirez, L.M.; Dias, L.d.S.; Rivas, L.A.; Ramos, L.S.; Santos, A.L.S.; Taborda, C.P.; Parra-Giraldo, C.M. pathogenicity levels of colombian strains of Candida auris and brazilian strains of Candida haemulonii species complex in both murine and Galleria mellonella experimental models. J. Fungi 2020, 6, 104. [Google Scholar] [CrossRef]
- Lohse, M.B.; Laurie, M.T.; Levan, S.; Ziv, N.; Ennis, C.L.; Nobile, C.J.; DeRisi, J.; Johnson, A.D. Broad susceptibility of Candida auris strains to 8-hydroxyquinolines and mechanisms of resistance. mBio 2023, 14, e0137623. [Google Scholar] [CrossRef]
- Ramos, L.S.; Figueiredo-Carvalho, M.H.G.; Silva, L.N.; Siqueira, N.L.M.; Lima, J.C.; Oliveira, S.S.; Almeida-Paes, R.; Zancopé-Oliveira, R.M.; Azevedo, F.S.; Ferreira, A.L.P.; et al. The threat called Candida haemulonii species complex in Rio de Janeiro State, Brazil: Focus on antifungal resistance and virulence attributes. J. Fungi 2022, 8, 574. [Google Scholar] [CrossRef]
- Ramos, L.S.; Oliveira, S.S.C.; Braga-Silva, L.A.; Branquinha, M.H.; Santos, A.L.S. Secreted aspartyl peptidases by the emerging, opportunistic and multidrug-resistant fungal pathogens comprising the Candida haemulonii complex. Fungal Biol. 2020, 124, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Rüchel, R.; Tegeler, R.; Trost, M. A comparison of secretory proteinases from different strains of Candida albicans. Sabouraudia 1982, 20, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia 1982, 20, 7–14. [Google Scholar] [CrossRef]
- Abi-Chacra, É.A.; Souza, L.O.; Cruz, L.P.; Braga-Silva, L.A.; Gonçalves, D.S.; Sodré, C.L.; Ribeiro, M.D.; Seabra, S.H.; Figueiredo-Carvalho, M.H.; Barbedo, L.S.; et al. Phenotypical properties associated with virulence from clinical isolates belonging to the Candida parapsilosis complex. FEMS Yeast Res. 2013, 13, 831–848. [Google Scholar] [CrossRef]
- Monod, M.; Togni, G.; Hube, B.; Sanglard, D. Multiplicity of genes encoding secreted aspartic proteinases in Candida species. Mol. Microbiol. 1994, 13, 357–368. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randal, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Martínez-Alarcón, D.; Saborowski, R.; Rojo-Arreola, L.; García-Carreño, F. Is digestive cathepsin D the rule in decapod crustaceans? Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 215, 31–38. [Google Scholar] [CrossRef]
- Lima, J.C.; Ramos, L.S.; Barbosa, P.F.; Barcellos, I.C.; Branquinha, M.H.; Dos Santos, A.L.S. Biofilm production by the multidrug-resistant fungus Candida haemulonii is affected by aspartic peptidase inhibitor. AIMS Microbiol. 2025, 11, 228–241. [Google Scholar] [CrossRef]
- Souto, X.M.; Ramos, L.S.; Branquinha, M.H.; Santos, A.L.S. Identification of cell-associated and secreted serine-type peptidases in multidrug-resistant emergent pathogens belonging to the Candida haemulonii complex. Folia Microbiol. 2019, 64, 245–255. [Google Scholar] [CrossRef]
- Bocheńska, O.; Rąpała-Kozik, M.; Wolak, N.; Braś, G.; Kozik, A.; Dubin, A.; Aoki, W.; Ueda, M.; Mak, P. Secreted aspartic peptidases of Candida albicans liberate bactericidal hemocidins from human hemoglobin. Peptides 2013, 48, 49–58. [Google Scholar] [CrossRef]
- Hube, B. Extracellular proteinases of human pathogenic fungi. Contrib. Microbiol. 2000, 5, 126–137. [Google Scholar] [CrossRef]
- Singh, D.K.; Németh, T.; Papp, A.; Tóth, R.; Lukácsi, S.; Heidingsfeld, O.; Dostal, J.; Vágvölgyi, C.; Bajtay, Z.; Józsi, M.; et al. Functional characterization of secreted aspartyl proteases in Candida parapsilosis. mSphere 2019, 4, e00484-19. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.B. Aspartic proteinases in disease: A structural perspective. Curr. Drug Targets 2002, 3, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Staib, F. Serum-proteins as nitrogen source for yeastlike fungi. Med. Mycol. 1966, 4, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Chibana, H.; Tanaka, K. Induction of extracellular proteinase in Candida albicans. J. Gen. Microbiol. 1993, 139 Pt 6, 1187–1193. [Google Scholar] [CrossRef][Green Version]
- Hube, B.; Monod, M.; Schofield, D.A.; Brown, A.J.; Gow, N.A. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol. Microbiol. 1994, 14, 87–99. [Google Scholar] [CrossRef]
- Monika, S.; Małgorzata, B.; Zbigniew, O. Contribution of aspartic proteases in Candida virulence. Protease inhibitors against Candida infections. Curr. Protein Pept. Sci. 2017, 18, 1050–1062. [Google Scholar] [CrossRef]
- Bing, J.; Wang, S.; Xu, H.; Fan, S.; Du, H.; Nobile, C.J.; Huang, G. A case of Candida auris candidemia in Xiamen, China, and a comparative analysis of clinical isolates in China. Mycology 2021, 13, 68–75. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, K.T.; Bahn, Y.S. Secreted aspartyl protease 3 regulated by the Ras/cAMP/PKA pathway promotes the virulence of Candida auris. Front. Cell. Infect. Microbiol. 2023, 13, 1257897. [Google Scholar] [CrossRef]
- White, T.C.; Agabian, N. Candida albicans secreted aspartyl proteinases: Isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. J. Bacteriol. 1995, 177, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, S.; Bing, J.; Liao, W.; Tao, L. Phenotypic switching and filamentation in Candida haemulonii, an emerging opportunistic pathogen of humans. Microbiol. Spectr. 2021, 9, e0077921. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Hube, B. Candida albicans secreted aspartyl proteinases. Curr. Top. Med. Mycol. 1996, 7, 55–69. [Google Scholar]
- Barelle, C.J.; Priest, C.L.; Maccallum, D.M.; Gow, N.A.; Odds, F.C.; Brown, A.J. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell. Microbiol. 2006, 8, 961–971. [Google Scholar] [CrossRef]
- Ries, L.N.A.; Beattie, S.; Cramer, R.A.; Goldman, G.H. Overview of carbon and nitrogen catabolite metabolism in the virulence of human pathogenic fungi. Mol. Microbiol. 2018, 107, 277–297. [Google Scholar] [CrossRef]
- Kumar, R.; Rojas, I.G.; Edgerton, M. Candida albicans Sap6 initiates oral mucosal inflammation via the protease activated receptor PAR2. Front. Immunol. 2022, 13, 912748. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Bochenska, O.; Zajac, D.; Karkowska-Kuleta, J.; Gogol, M.; Zawrotniak, M.; Kozik, A. Extracellular proteinases of Candida species pathogenic yeasts. Mol. Oral Microbiol. 2018, 33, 113–124. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Gupta, P. Secreted aspartyl proteases family: A perspective review on the regulation of fungal pathogenesis. Future Microbiol. 2023, 18, 295–309. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef]
- Braga-Silva, L.A.; Santos, A.L.S. Aspartic protease inhibitors as potential anti-Candida albicans drugs: Impacts on fungal biology, virulence and pathogenesis. Curr. Med. Chem. 2011, 18, 2401–2419. [Google Scholar] [CrossRef]
- Parra-Ortega, B.; Cruz-Torres, H.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Phylogeny and evolution of the aspartyl protease family from clinically relevant Candida species. Mem. Inst. Oswaldo Cruz 2009, 104, 505–512. [Google Scholar] [CrossRef]
- Fleck, C.B.; Schöbel, F.; Brock, M. Nutrient acquisition by pathogenic fungi: Nutrient availability, pathway regulation, and differences in substrate utilization. Int. J. Med. Microbiol. 2011, 301, 400–407. [Google Scholar] [CrossRef]
- Marzluf, G.A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 1997, 61, 17–32. [Google Scholar] [CrossRef] [PubMed]

| Candida Species | Isolate Codes | Origin | Country | Year of Collection | Isolation Site | Antifungal Susceptibility Profile | |
|---|---|---|---|---|---|---|---|
| FLC | AMB | ||||||
| C. auris | Ca885 | CIMCE | Colombia | 2016 | Blood | R | S |
| C. auris | Ca446 | UJ | Colombia | 2016 | Blood | R | S |
| C. auris | Ca383 | CDC | USA | 2012 | Blood | R | S |
| C. auris | Ca384 | CDC | USA | 2012 | Blood | R | S |
| C. haemulonii var. vulnera | Ch11 | INCA | Brazil | 2013 | Blood | R | R |
| C. haemulonii | Ch12 | INCA | Brazil | 2013 | Blood | R | R |
| C. duobushaemulonii | Ch13 | DASA | Brazil | 2012 | Blood | R | R |
| C. duobushaemulonii | Ch23 | DASA | Brazil | 2011 | Blood | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.C.; Barbosa, P.F.; Ramos, L.S.; Branquinha, M.H.; Santos, A.L.S. Impact of pH, Temperature and Exogenous Proteins on Aspartic Peptidase Secretion in Candida auris and the Candida haemulonii Species Complex. Pathogens 2025, 14, 873. https://doi.org/10.3390/pathogens14090873
Silva GC, Barbosa PF, Ramos LS, Branquinha MH, Santos ALS. Impact of pH, Temperature and Exogenous Proteins on Aspartic Peptidase Secretion in Candida auris and the Candida haemulonii Species Complex. Pathogens. 2025; 14(9):873. https://doi.org/10.3390/pathogens14090873
Chicago/Turabian StyleSilva, Gabriel C., Pedro F. Barbosa, Lívia S. Ramos, Marta H. Branquinha, and André L. S. Santos. 2025. "Impact of pH, Temperature and Exogenous Proteins on Aspartic Peptidase Secretion in Candida auris and the Candida haemulonii Species Complex" Pathogens 14, no. 9: 873. https://doi.org/10.3390/pathogens14090873
APA StyleSilva, G. C., Barbosa, P. F., Ramos, L. S., Branquinha, M. H., & Santos, A. L. S. (2025). Impact of pH, Temperature and Exogenous Proteins on Aspartic Peptidase Secretion in Candida auris and the Candida haemulonii Species Complex. Pathogens, 14(9), 873. https://doi.org/10.3390/pathogens14090873








