Dengue-Related Ocular Complications: Spectrum, Diagnosis, and Management
Abstract
1. Introduction
2. Classification and Clinical Course of Dengue Systemic Illness
3. Risk Factors for Dengue-Related Ocular Disease
4. Pathogenesis
5. Clinical Features of Dengue Eye Disease
5.1. Subconjunctival Hemorrhage
5.2. Uveal Inflammation
5.2.1. Dengue Uveitis
5.2.2. Choroiditis
5.2.3. Optic Neuritis
5.2.4. Henle Fiber Layer Hemorrhage
5.3. Retinal Vasculitis
5.4. Anterior Segment Implications
5.5. Maculopathy
5.6. Panophthalmitis
6. Investigations
6.1. Laboratory Diagnosis of Dengue
6.2. Amsler Grid Testing, Microperimetry and Visual Field Assessment
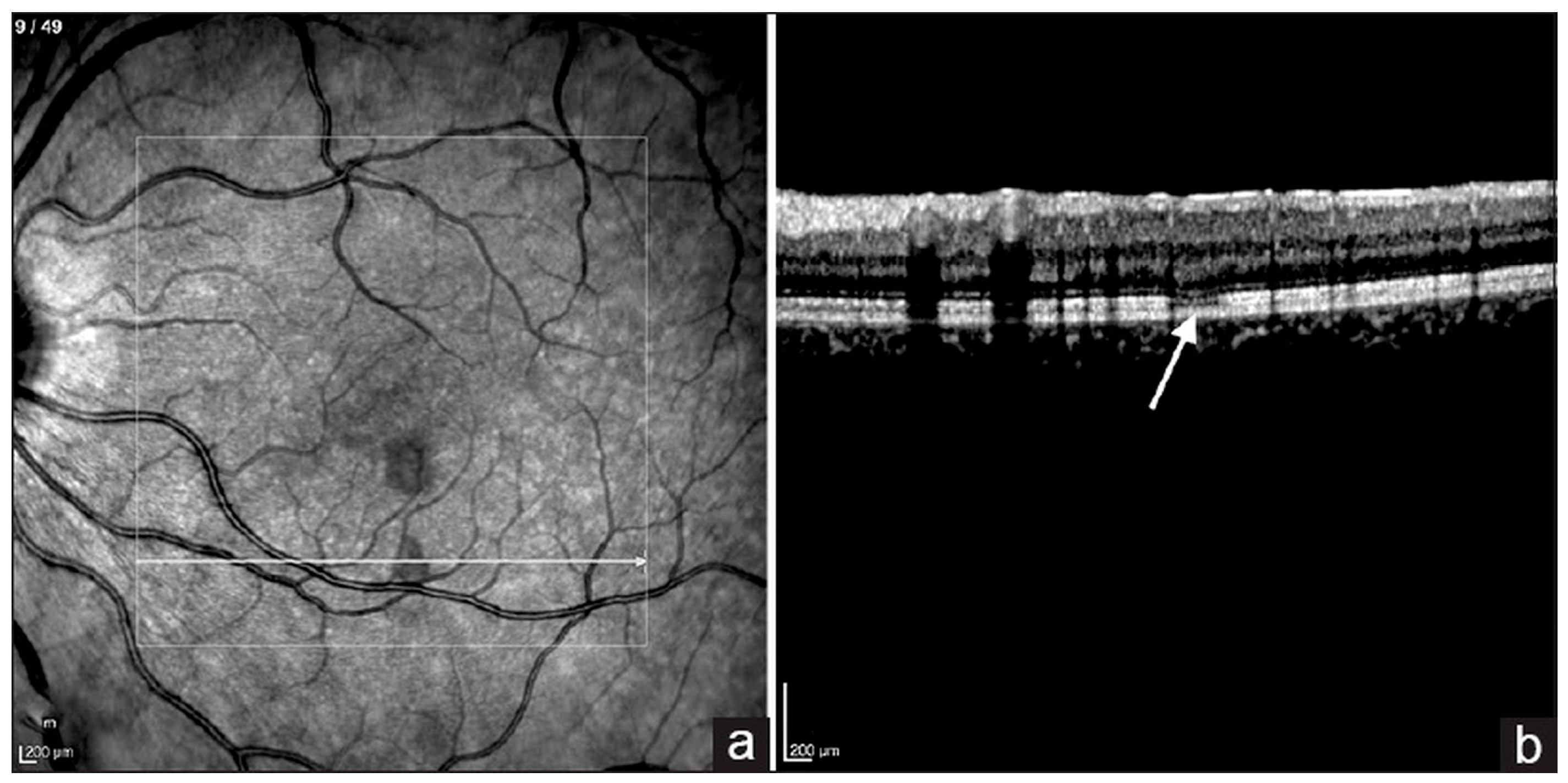
6.3. Optical Coherence Tomography (OCT)
6.4. Fundus Fluorescein Angiography (FFA)
6.5. Indocyanine Green Angiography (ICG-A)
6.6. Optical Coherence Tomography Angiography (OCTA)
7. Treatment
7.1. Vaccines and Prevention
7.2. Active Surveillance
7.3. Corticosteroid Therapy
7.4. Intravenous Immunoglobulin (IVIG)
7.5. Surgical Management
8. Prognosis
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cattarino, L.; Rodriguez-Barraquer, I.; Imai, N.; Cummings, D.A.T.; Ferguson, N.M. Mapping Global Variation in Dengue Transmission Intensity. Sci. Transl. Med. 2020, 12, eaax4144. [Google Scholar] [CrossRef]
- Osman, S.; Preet, R. Dengue, Chikungunya and Zika in GeoSentinel Surveillance of International Travellers: A Literature Review from 1995 to 2020. J. Travel Med. 2020, 27, taaa222. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A.; et al. The Global Burden of Dengue: An Analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef]
- Paz-Bailey, G.; Adams, L.E.; Deen, J.; Anderson, K.B.; Katzelnick, L.C. Dengue. Lancet 2024, 403, 667–682. [Google Scholar] [CrossRef]
- Dengue Viruses|Learn Science at Scitable. Available online: https://www.nature.com/scitable/topicpage/dengue-viruses-22400925/ (accessed on 26 May 2025).
- Wikramaratna, P.S.; Simmons, C.P.; Gupta, S.; Recker, M. The Effects of Tertiary and Quaternary Infections on the Epidemiology of Dengue. PLoS ONE 2010, 5, e12347. [Google Scholar] [CrossRef]
- CDC Clinical Testing Guidance for Dengue. Available online: https://www.cdc.gov/dengue/hcp/diagnosis-testing/index.html (accessed on 4 July 2025).
- World Health Organization (WHO). Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; WHO: Geneva, Switzerland, 2009; ISBN 978-92-4-154787-1. [Google Scholar]
- European Commission. Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the Communicable Diseases and Related Special Health Issues to be Covered by Epidemiological Surveillance as Well as Relevant Case Definitions. Official Journal of the European Union, L 170, 1–74. Annex II, Section 3.10 Dengue. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018D0945 (accessed on 27 August 2025).
- Chan, D.P.L.; Teoh, S.C.B.; Tan, C.S.H.; Nah, G.K.M.; Rajagopalan, R.; Prabhakaragupta, M.K.; Chee, C.K.L.; Lim, T.H.; Goh, K.Y. Ophthalmic Complications of Dengue. Emerg. Infect. Dis. 2006, 12, 285–289. [Google Scholar] [CrossRef]
- Agarwal, L.; Agrawal, N. Retinal Vasculitis with Macular Infarction: A Dengue-Related Ophthalmic Complication. Int. Med. Case Rep. J. 2020, 13, 363–366. [Google Scholar] [CrossRef]
- Chang, P.E.; Cheng, C.L.; Asok, K.; Fong, K.Y.; Chee, S.P.; Tan, C.K. Visual Disturbances in Dengue Fever: An Answer at Last. Singap. Med. J. 2007, 48, 71–73. [Google Scholar]
- Gupta, A.; Srinivasan, R.; Setia, S.; Soundravally, R.; Pandian, D.G. Uveitis Following Dengue Fever. Eye 2009, 23, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Arruda, M.P.; Lima, R.V.; Barbosa, L.I.T.; Hira, S.; Zinher, M.T.; Del Valle, G.S.; dos Anjos Filho, V.M.; Nogueira, H.S.; Muccioli, C.; Lima, L.H. Ocular Manifestations and OCT Findings in Dengue: A Single-Arm Meta-Analysis. Ophthalmology 2025, 132, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-Q.; Kuo, H.-T.; Hsu, A.Y.; Lin, C.-J.; Tien, P.-T.; Hsia, N.-Y.; Cheng, Y.-D.; Hsieh, Y.-W.; Huang, Y.-H.; Wang, S.-T.; et al. Risk of Uveitis in Dengue Fever Patients: A Population-Based Cohort Study in Taiwan. J. Med. Virol. 2024, 96, e70141. [Google Scholar] [CrossRef]
- Malavige, G.N.; Ogg, G.S. Pathogenesis of Vascular Leak in Dengue Virus Infection. Immunology 2017, 151, 261–269. [Google Scholar] [CrossRef]
- Yacoub, S.; Wertheim, H.; Simmons, C.P.; Screaton, G.; Wills, B. Microvascular and Endothelial Function for Risk Prediction in Dengue: An Observational Study. Lancet 2015, 385, S102. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.; Balmaseda, A.; Coelho, I.C.B.; Dimaano, E.; Hien, T.T.; Hung, N.T.; Jänisch, T.; Kroeger, A.; Lum, L.C.S.; Martinez, E.; et al. Multicentre Prospective Study on Dengue Classification in Four South-East Asian and Three Latin American Countries. Trop. Med. Int. Health 2011, 16, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Su, D.H.-W.; Bacsal, K.; Chee, S.-P.; Flores, J.V.P.; Lim, W.-K.; Cheng, B.C.-L.; Jap, A.H.-E.; Dengue Maculopathy Study Group. Prevalence of Dengue Maculopathy in Patients Hospitalized for Dengue Fever. Ophthalmology 2007, 114, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.W.W.; Mi, H.F.; Ho, S.L.; Teoh, S.C.B.; Agrawal, R. Ocular Autoimmune Systemic Inflammatory Infectious Study (OASIS)–Report 6: Dengue Uveitis at a Tertiary Eye Institution in Singapore. Ocul. Immunol. Inflamm. 2024, 32, 184–189. [Google Scholar] [CrossRef]
- Maan, V.; Guha, S.; Sapra, H.; Chauhan, L. Ocular and Adnexal Manifestations Post Dengue Hemorrhagic Fever. Indian J. Ophthalmol. 2024, 72, 1495–1500. [Google Scholar] [CrossRef]
- Vincent, M.; Paty, M.C.; Gerardin, P.; Balleydier, E.; Etienne, A.; Daoudi, J.; Thouillot, F.; Jaffar-Bandjee, M.-C.; Menudier, L.; Cousty, J.; et al. From Dengue Outbreaks to Endemicity: Reunion Island, France, 2018 to 2021. Eurosurveillance 2023, 28, 2200769. [Google Scholar] [CrossRef]
- Agarwal, A.; Aggarwal, K.; Dogra, M.; Kumar, A.; Akella, M.; Katoch, D.; Bansal, R.; Singh, R.; Gupta, V. Dengue-Induced Inflammatory, Ischemic Foveolitis and Outer Maculopathy: A Swept-Source Imaging Evaluation. Ophthalmol. Retin. 2019, 3, 170–177. [Google Scholar] [CrossRef]
- Teoh, S.C.; Chee, C.K.; Laude, A.; Goh, K.Y.; Barkham, T.; Ang, B.S.; Eye Institute Dengue-related Ophthalmic Complications Workgroup. Optical Coherence Tomography Patterns as Predictors of Visual Outcome in Dengue-Related Maculopathy. Retina 2010, 30, 390. [Google Scholar] [CrossRef]
- Chee, E.; Sims, J.L.; Jap, A.; Tan, B.H.; Oh, H.; Chee, S.-P. Comparison of Prevalence of Dengue Maculopathy during Two Epidemics with Differing Predominant Serotypes. Am. J. Ophthalmol. 2009, 148, 910–913. [Google Scholar] [CrossRef]
- Wagle, A.M.; Hegde, S.R.; Sanjay, S.; Au Eong, K.-G. Ophthalmic Manifestations in Seropositive Dengue Fever Patients during Epidemics Caused by Predominantly Different Dengue Serotypes. Adv. Ophthalmol. Pract. Res. 2022, 2, 100049. [Google Scholar] [CrossRef]
- Carr, J.M.; Ashander, L.M.; Calvert, J.K.; Ma, Y.; Aloia, A.; Bracho, G.G.; Chee, S.-P.; Appukuttan, B.; Smith, J.R. Molecular Responses of Human Retinal Cells to Infection with Dengue Virus. Mediat. Inflamm. 2017, 2017, 3164375. [Google Scholar] [CrossRef] [PubMed]
- Ashander, L.M.; Lumsden, A.L.; Dawson, A.C.; Ma, Y.; Ferreira, L.B.; Oliver, G.F.; Appukuttan, B.; Carr, J.M.; Smith, J.R. Infection of Human Retinal Pigment Epithelial Cells with Dengue Virus Strains Isolated during Outbreaks in Singapore. Microorganisms 2022, 10, 310. [Google Scholar] [CrossRef]
- Seet, R.C.S.; Quek, A.M.L.; Lim, E.C.H. Symptoms and Risk Factors of Ocular Complications Following Dengue Infection. J. Clin. Virol. 2007, 38, 101–105. [Google Scholar] [CrossRef]
- Noisakran, S.; Perng, G.C. Alternate Hypothesis on the Pathogenesis of Dengue Hemorrhagic Fever (DHF)/Dengue Shock Syndrome (DSS) in Dengue Virus Infection. Exp. Biol. Med. 2008, 233, 401–408. [Google Scholar] [CrossRef]
- Noisakran, S.; Chokephaibulkit, K.; Songprakhon, P.; Onlamoon, N.; Hsiao, H.-M.; Villinger, F.; Ansari, A.; Perng, G.C. A Re-Evaluation of the Mechanisms Leading to Dengue Hemorrhagic Fever. Ann. N. Y. Acad. Sci. 2009, 1171, E24–E35. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.; Gangaputra, S.; Kodati, S.; Melamud, A.; Sen, H.N. Multimodal Imaging in Dengue-Fever-Associated Maculopathy. Ocul. Immunol. Inflamm. 2018, 26, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Bacsal, K.E.; Chee, S.-P.; Cheng, C.-L.; Flores, J.V.P. Dengue-Associated Maculopathy. Arch. Ophthalmol. 2007, 125, 501–510. [Google Scholar] [CrossRef]
- Lim, W.-K.; Mathur, R.; Koh, A.; Yeoh, R.; Chee, S.-P. Ocular Manifestations of Dengue Fever. Ophthalmology 2004, 111, 2057–2064. [Google Scholar] [CrossRef]
- Bawankar, P.; Lahane, T.; Parekh, R.; Lahane, S.; Lahane, S.; Pathak, P.; Sonawane, S. An Unusual Occurrence of Stromal Keratitis in Dengue Fever. Indian J. Ophthalmol. 2018, 66, 1631. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A.; Mathew, A.; Rothman, A.L. Immune-Mediated Cytokine Storm and Its Role in Severe Dengue. Semin. Immunopathol. 2017, 39, 563–574. [Google Scholar] [CrossRef]
- Kapoor, H.K.; Bhai, S.; John, M.; Xavier, J. Ocular Manifestations of Dengue Fever in an East Indian Epidemic. Can. J. Ophthalmol. 2006, 41, 741–746. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathi, A.; Bhirud, A.; Agrawal, M.; Gupta, S.; Parihar, J. Ophthalmic Manifestations as the First Presenting Feature in Dengue Fever: A 10-Year Study. Rom. J. Ophthalmol. 2024, 68, 31–36. [Google Scholar] [CrossRef]
- Lin, C.-F.; Lei, H.-Y.; Shiau, A.-L.; Liu, C.-C.; Liu, H.-S.; Yeh, T.-M.; Chen, S.-H.; Lin, Y.-S. Antibodies from Dengue Patient Sera Cross-React with Endothelial Cells and Induce Damage. J. Med. Virol. 2003, 69, 82–90. [Google Scholar] [CrossRef]
- Tadkalkar, N.; Prasad, S.; Gangodkar, S.; Joshi, S.; Mahale, S.D. Dengue Virus NS1 Exposure Affects von Willebrand Factor Profile and Platelet Adhesion Properties of Cultured Vascular Endothelial Cells. Indian J. Hematol. Blood Transfus. 2019, 35, 502–506. [Google Scholar] [CrossRef]
- Xie Cen, A.; Ng, A.W.W.; Rojas-Carabali, W.; Cifuentes-González, C.; de-la-Torre, A.; Mahendradas, P.; Agrawal, R. Dengue Uveitis—A Major Review. Ocul. Immunol. Inflamm. 2023, 31, 1440–1453. [Google Scholar] [CrossRef]
- Teoh, S.C.B.; Chan, D.P.L.; Nah, G.K.M.; Rajagopalan, R.; Laude, A.; Ang, B.S.P.; Barkham, T.; Chee, C.K.L.; Lim, T.H.; Goh, K.Y. A Re-Look at Ocular Complications in Dengue Fever and Dengue Haemorrhagic Fever. Dengue Bull. 2006, 30, 184. [Google Scholar]
- Smith, J.R.; Todd, S.; Ashander, L.M.; Charitou, T.; Ma, Y.; Yeh, S.; Crozier, I.; Michael, M.Z.; Appukuttan, B.; Williams, K.A.; et al. Retinal Pigment Epithelial Cells Are a Potential Reservoir for Ebola Virus in the Human Eye. Transl. Vis. Sci. Technol. 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Tabbara, K. Dengue Retinochoroiditis. Ann. Saudi Med. 2012, 32, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Koundanya, V.V.; Chowdhary, N.; Agarwal, M.; Katre, P. Secondary Dengue Retinitis with Associated Occlusive Retinal Vasculitis. J. Ophthal Inflamm. Infect. 2019, 9, 7. [Google Scholar] [CrossRef]
- Hsiao, Y.-T.; Huang, H.-M. Bilateral Optic Neuritis After Dengue Infection Resulting in Legal Blindness in Both Eyes. J. Neuro-Ophthalmol. 2025, 45, e197–e199. [Google Scholar] [CrossRef]
- Boo, Y.; Lim, S.; Chin, P.; Hoo, F. Bilateral Optic Neuritis with Maculopathy: A Rare Manifestation of Dengue Fever. Malays. Fam. Physician 2017, 12, 32–34. [Google Scholar]
- Mahayana, I.T.; Anugrah, A.S.; Kartika, I.; Angsana, N.C.; Gani, T.T. Optic Neuritis as the Ocular Manifestation of Dengue Infection: A Case Report. Malays. J. Ophthalmol. 2021, 3, 46–51. [Google Scholar] [CrossRef]
- Ferreira, B.F.A.; Sakuno, G.; Kato, J.M.; Sato, R.H.; Ferreira, Á.F.; Missaka, R.F.B.G.; Nakashima, Y. Paracentral Acute Middle Maculopathy and Henle Fiber Layer Hemorrhage in Dengue Fever: Multimodal Imaging and Review of the Literature. Retin. Cases Brief Rep. 2024. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, G.C.; Ventura, C.V.; Mello Filho, P.A.d.A.; Maia, M.; Vianello, S.; Rodrigues, E.B. Arboviruses and the Eye. Int. J. Retin. Vitr. 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Vijitha, V.S.; Dave, T.V.; Murthy, S.I.; Ali, M.J.; Dave, V.P.; Pappuru, R.R.; Narayanan, R. Severe Ocular and Adnexal Complications in Dengue Hemorrhagic Fever: A Report of 29 Eyes. Indian J. Ophthalmol. 2021, 69, 617. [Google Scholar] [CrossRef] [PubMed]
- Kamoi, K.; Mochizuki, M.; Ohno-Matsui, K. Dengue Fever-Associated Necrotizing Scleritis. Medicine 2018, 97, e11875. [Google Scholar] [CrossRef]
- Al-Essa, A. Acute Angle-Closure Glaucoma as an Ocular Complication of Dengue Fever: A Comprehensive Review. Cureus 2025, 17, e82119. [Google Scholar] [CrossRef]
- Richier, Q.; Bataille, N.; Gauzëre, L.; Safla, I.; Villeroy, F.; Raffray, L. Dengue-Related Maculopathy. J. Travel Med. 2023, 30, taac106. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Ji, Y.; Ye, B.; Wen, F. Acute Macular Neuroretinopathy in Dengue Fever: Short-Term Prospectively Followed up Case Series. JAMA Ophthalmol. 2015, 133, 1329–1333. [Google Scholar] [CrossRef]
- Guardiola, G.A.; Villegas, V.M.; Cruz-Villegas, V.; Schwartz, S.G. Acute Macular Neuroretinopathy in Dengue Virus Serotype 1. Am. J. Ophthalmol. Case Rep. 2022, 25, 101250. [Google Scholar] [CrossRef]
- Wang, C.; Castillo, A.; Cortes-Bejarano, F.; Lopez, E.; de Souza, E.C.; Wu, L. An Update on the Ocular Manifestations of Dengue. Taiwan. J. Ophthalmol. 2024, 14, 540–547. [Google Scholar] [CrossRef]
- Mahendradas, P.; Acharya, I.; Rana, V.; Bansal, R.; Ben Amor, H.; Khairallah, M. Optical Coherence Tomography and Optical Coherence Tomography Angiography in Neglected Diseases. Ocul. Immunol. Inflamm. 2024, 32, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Shah, D.; Sharma, S.; Janani, M.K.; Kar, A.; Saurabh, K.; Roy, R.; Madhavan, H.N.R. Culture-Positiveunilateral Panophthalmitis in a Serology-Positive Case of Dengue Hemorrhagic Fever. Indian J. Ophthalmol. 2018, 66, 1017. [Google Scholar] [CrossRef]
- Janani, M.K.; Durgadevi, P.; Padmapriya, J.; Malathi, J.; Kulandai, L.T.; Rao Madhavan, H.N. First Report on Detection of Dengue Virus in the Donor Cornea. Cornea 2018, 37, 1586. [Google Scholar] [CrossRef] [PubMed]
- CDC. Molecular Tests for Dengue Virus. Available online: https://www.cdc.gov/dengue/hcp/diagnosis-testing/molecular-tests-for-dengue-virus.html (accessed on 24 August 2025).
- CDC. Serologic Tests for Dengue Virus. Available online: https://www.cdc.gov/dengue/hcp/diagnosis-testing/serologic-tests-for-dengue-virus.html (accessed on 4 July 2025).
- Changal, K.H.; Raina, A.H.; Raina, A.; Raina, M.; Bashir, R.; Latief, M.; Mir, T.; Changal, Q.H. Differentiating Secondary from Primary Dengue Using IgG to IgM Ratio in Early Dengue: An Observational Hospital Based Clinico-Serological Study from North India. BMC Infect. Dis. 2016, 16, 715. [Google Scholar] [CrossRef]
- Tan, M.H.; Tan, P.E.; Wong, E.N.; Chen, F.K. Structure and Function Correlation in a Patient with Dengue-Associated Maculopathy. Clin. Exp. Ophthalmol. 2014, 42, 194–196. [Google Scholar] [CrossRef]
- Zina, S.M.; Hoarau, G.; Labetoulle, M.; Khairallah, M.; Rousseau, A. Ocular Manifestations of Flavivirus Infections. Pathogens 2023, 12, 1457. [Google Scholar] [CrossRef] [PubMed]
- Tranos, P.; Karasavvidou, E.-M.; Gkorou, O.; Pavesio, C. Optical Coherence Tomography Angiography in Uveitis. J. Ophthalmic Inflamm. Infect. 2019, 9, 21. [Google Scholar] [CrossRef]
- Loh, B.-K.; Bacsal, K.; Chee, S.-P.; Cheng, B.C.-L.; Wong, D. Foveolitis Associated with Dengue Fever: A Case Series. Ophthalmologica 2008, 222, 317–320. [Google Scholar] [CrossRef]
- Aggarwal, K.; Agarwal, A.; Katoch, D.; Sharma, M.; Gupta, V. Optical Coherence Tomography Angiography Features of Acute Macular Neuroretinopathy in Dengue Fever. Indian J. Ophthalmol. 2017, 65, 1235. [Google Scholar] [CrossRef]
- Chlebicki, M.P.; Ang, B.; Barkham, T.; Laude, A. Retinal Hemorrhages in 4 Patients with Dengue Fever. Emerg. Infect. Dis. 2005, 11, 770. [Google Scholar] [CrossRef]
- Stewart, K.P.; Tawakol, J.B.; Khan, T.; Capriotti, J.A. Combination Immunotherapy in the Treatment of Chronic Bilateral Panuveitis and Uveitic Glaucoma during Acute Dengue Fever Infection in the Caribbean. Int. Med. Case Rep. J. 2015, 8, 151–153. [Google Scholar] [CrossRef]
- Nainiwal, S.; Garg, S.P.; Prakash, G.; Nainiwal, N. Bilateral Vitreous Haemorrhage Associated with Dengue Fever. Eye 2005, 19, 1012–1013. [Google Scholar] [CrossRef]
- Malek, M.I.A.; Niyonzima, J.C.; Pathan, M.A.H.K.; Rahman, M.M. Pars Plana Vitrectomy for a Sub-Internal Limiting Membrane Hemorrhage and Vitreous Hemorrhage Secondary to Dengue Fever: A Case Report. Cureus 2022, 14, e25916. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, S.K. Bilateral Ciliochoroidal Effusion with Secondary Angle Closure and Myopic Shift in Dengue Fever. Ocul. Immunol. Inflamm. 2023, 31, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Vitral, N.P.; Campos, W.R.; Oréfice, F.; de Moraes Figueiredo, L.T. Ocular Manifestations in Dengue Fever. Ocul. Immunol. Inflamm. 2004, 12, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.M.; Dutta Majumder, P.; Biswas, J. Dengue Associated Choroiditis: A Rare Entity. J. Ophthalmic Inflamm. Infect. 2017, 7, 14. [Google Scholar] [CrossRef][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Zou, Y.; Yang, M.; Zhang, J.; Ye, Z.; Zong, Y.; Ohno-Matsui, K.; Kamoi, K. Dengue-Related Ocular Complications: Spectrum, Diagnosis, and Management. Pathogens 2025, 14, 872. https://doi.org/10.3390/pathogens14090872
Deng J, Zou Y, Yang M, Zhang J, Ye Z, Zong Y, Ohno-Matsui K, Kamoi K. Dengue-Related Ocular Complications: Spectrum, Diagnosis, and Management. Pathogens. 2025; 14(9):872. https://doi.org/10.3390/pathogens14090872
Chicago/Turabian StyleDeng, Jiaxin, Yaru Zou, Mingming Yang, Jing Zhang, Zizhen Ye, Yuan Zong, Kyoko Ohno-Matsui, and Koju Kamoi. 2025. "Dengue-Related Ocular Complications: Spectrum, Diagnosis, and Management" Pathogens 14, no. 9: 872. https://doi.org/10.3390/pathogens14090872
APA StyleDeng, J., Zou, Y., Yang, M., Zhang, J., Ye, Z., Zong, Y., Ohno-Matsui, K., & Kamoi, K. (2025). Dengue-Related Ocular Complications: Spectrum, Diagnosis, and Management. Pathogens, 14(9), 872. https://doi.org/10.3390/pathogens14090872





