LMP7 as a Target for Coronavirus Therapy: Inhibition by Ixazomib and Interaction with SARS-CoV-2 Proteins Nsp13 and Nsp16
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Plasmid and Constructions
2.3. Generation of LMP7-Knockout Cells
2.4. Virus Infection
2.5. Cell Viability Assay
2.6. Immunofluorescence Staining and Fluorescence Microscopy
2.7. Co-Immunoprecipitation and Western Blot Analysis
2.8. Statistics
3. Results
3.1. Coronavirus Replication Inhibitors Selected from Screening Are LMP7 Inhibitors
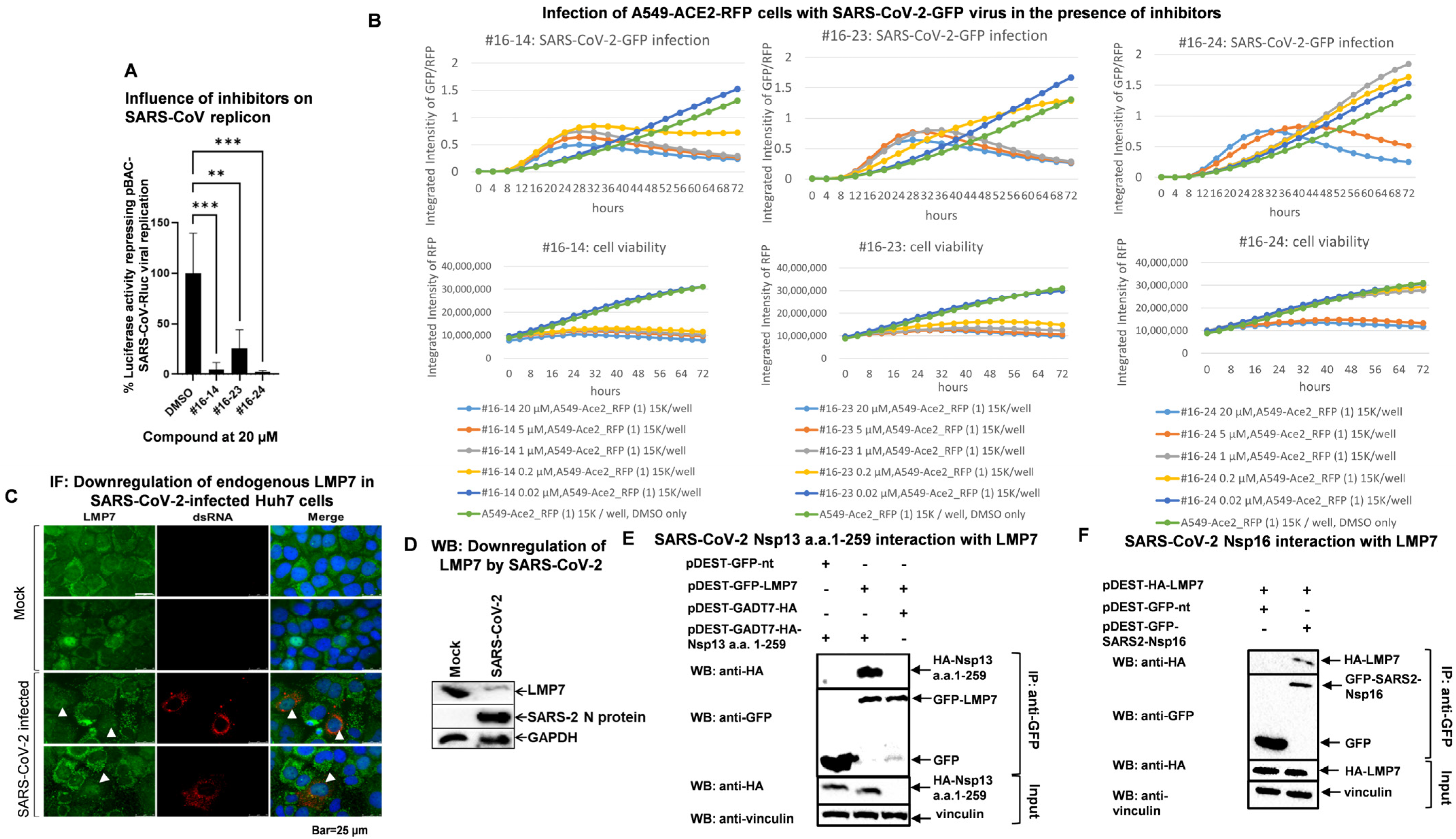
3.2. Candidate Compounds Inhibit Replication of SARS-CoV and SARS-CoV-2
3.3. SARS-CoV-2 Infection Reduces LMP7 Protein Levels, and SARS-CoV-2 Viral Proteins Target LMP7
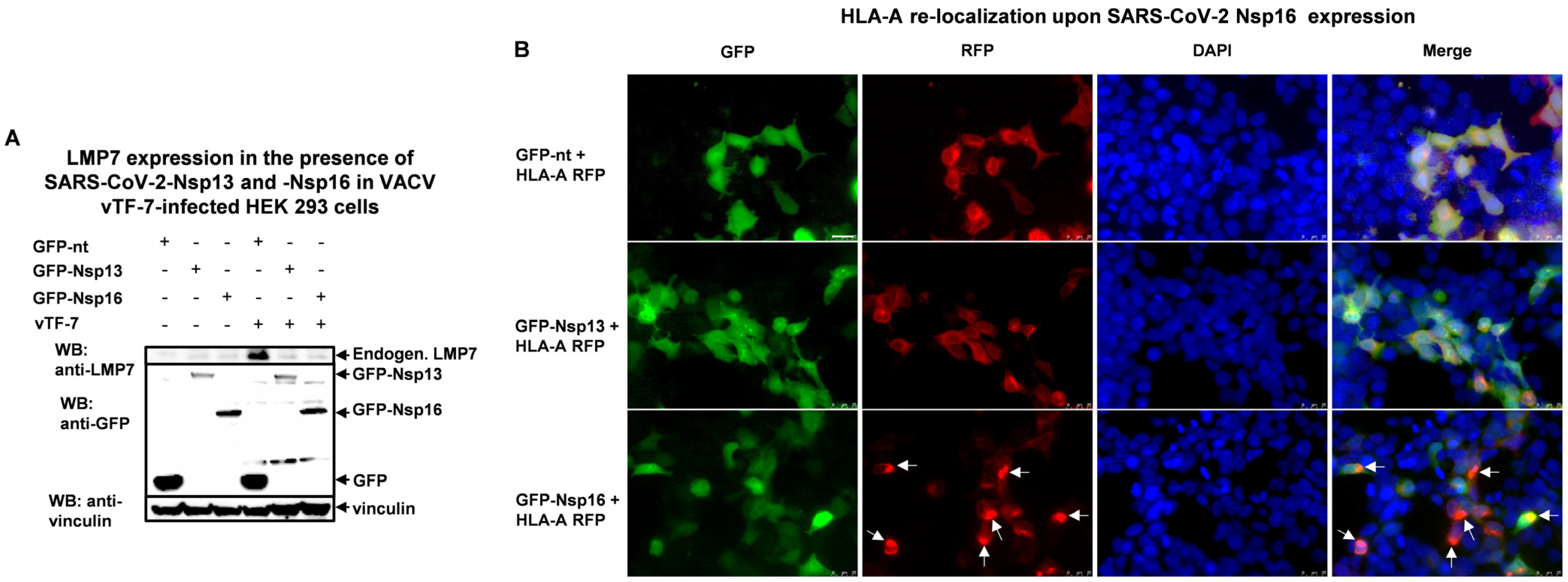
3.4. SARS-CoV-2 Nsp13 and Nsp16 Inhibit LMP7 Expression, and Nsp16 Disrupts LMP7-Mediated Antigen Presentation
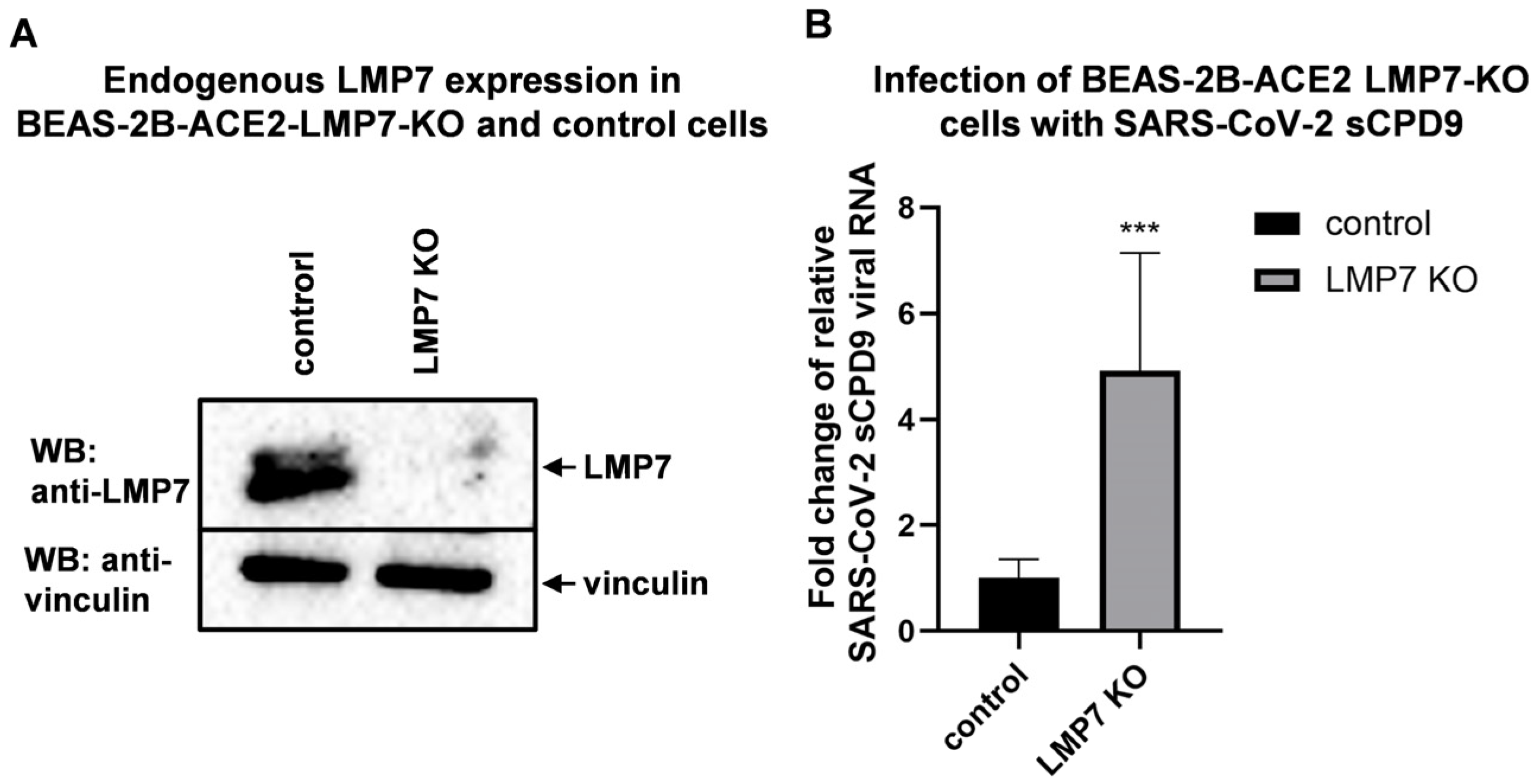
3.5. LMP7 Is an Antiviral Factor That Acts Against SARS-CoV-2
4. Discussion
Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Number of COVID-19 Deaths Reported to WHO (Cumulative Total). Available online: https://data.who.int/dashboards/covid19/deaths?n=c (accessed on 28 March 2025).
- World Health Organization (WHO). Middle East Respiratory Syndrome: Global Summary and Assessment of Risk; WHO Health Emergencies Programme (WHE): Geneva, Switzerland, 2022.
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Temmam, S.; Vongphayloth, K.; Baquero, E.; Munier, S.; Bonomi, M.; Regnault, B.; Douangboubpha, B.; Karami, Y.; Chretien, D.; Sanamxay, D.; et al. Bat coronaviruses related to SARS-CoV-2 and infectious for human cells. Nature 2022, 604, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Groettrup, M. On the Role of the Immunoproteasome in Protein Homeostasis. Cells 2021, 10, 3216. [Google Scholar] [CrossRef]
- Murata, S.; Takahama, Y.; Kasahara, M.; Tanaka, K. The imm unoproteasome and thymoproteasome: Functions, evolution and human disease. Nat. Immunol. 2018, 19, 923–931. [Google Scholar] [CrossRef]
- Groettrup, M.; Kirk, C.J.; Basler, M. Proteasomes in immune cells: More than peptide producers? Nat. Rev. Immunol. 2010, 10, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Niewerth, D.; van Meerloo, J.; Jansen, G.; Assaraf, Y.G.; Hendrickx, T.C.; Kirk, C.J.; Anderl, J.L.; Zweegman, S.; Kaspers, G.J.; Cloos, J. Anti-leukemic activity and mechanisms underlying resistance to the novel immunoproteasome inhibitor PR-924. Biochem. Pharmacol. 2014, 89, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wehenkel, M.; Ban, J.O.; Ho, Y.K.; Carmony, K.C.; Hong, J.T.; Kim, K.B. A selective inhibitor of the immunoproteasome subunit LMP2 induces apoptosis in PC-3 cells and suppresses tumour growth in nude mice. Br. J. Cancer 2012, 107, 53–62. [Google Scholar] [CrossRef]
- Raedler, L.A. Ninlaro (Ixazomib): First Oral Proteasome Inhibitor Approved for the Treatment of Patients with Relapsed or Refractory Multiple Myeloma. Am. Health Drug Benefits 2016, 9, 102–105. [Google Scholar]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Bergant, V.; Yamada, S.; Grass, V.; Tsukamoto, Y.; Lavacca, T.; Krey, K.; Mühlhofer, M.T.; Wittmann, S.; Ensser, A.; Herrmann, A.; et al. Attenuation of SARS-CoV-2 replication and associated inflammation by concomitant targeting of viral and host cap 2′-O-ribose methyltransferases. EMBO J. 2022, 41, e111608. [Google Scholar] [CrossRef]
- Mathieu, C.; Touret, F.; Jacquemin, C.; Janin, Y.L.; Nougairède, A.; Brailly, M.; Mazelier, M.; Décimo, D.; Vasseur, V.; Hans, A.; et al. A Bioluminescent 3CL(Pro) Activity Assay to Monitor SARS-CoV-2 Replication and Identify Inhibitors. Viruses 2021, 13, 1814. [Google Scholar] [CrossRef]
- Ma-Lauer, Y.; Li, P.; Niemeyer, D.; Richter, A.; Pusl, K.; von Brunn, B.; Ru, Y.; Xiang, C.; Schwinghammer, S.; Liu, J.; et al. Oxysterole-binding protein targeted by SARS-CoV-2 viral proteins regulates coronavirus replication. Front. Cell Infect. Microbiol. 2024, 14, 1383917. [Google Scholar] [CrossRef]
- Sanjana, N.E.; Shalem, O.; Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 2014, 11, 783–784. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Dykxhoorn, D.M.; Palliser, D.; Mizuno, H.; Yu, E.Y.; An, D.S.; Sabatini, D.M.; Chen, I.S.; Hahn, W.C.; Sharp, P.A.; et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. Rna 2003, 9, 493–501. [Google Scholar] [CrossRef]
- van den Worm, S.H.; Eriksson, K.K.; Zevenhoven, J.C.; Weber, F.; Züst, R.; Kuri, T.; Dijkman, R.; Chang, G.; Siddell, S.G.; Snijder, E.J.; et al. Reverse genetics of SARS-related coronavirus using vaccinia virus-based recombination. PLoS ONE 2012, 7, e32857. [Google Scholar] [CrossRef]
- Stukalov, A.; Girault, V.; Grass, V.; Karayel, O.; Bergant, V.; Urban, C.; Haas, D.A.; Huang, Y.; Oubraham, L.; Wang, A.; et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature 2021, 594, 246–252. [Google Scholar] [CrossRef]
- Kunec, D.; Osterrieder, N.; Trimpert, J. Synthetically recoded virus sCPD9—A tool to accelerate SARS-CoV-2 research under biosafety level 2 conditions. Comput. Struct. Biotechnol. J. 2022, 20, 4376–4380. [Google Scholar] [CrossRef]
- Nouailles, G.; Adler, J.M.; Pennitz, P.; Peidli, S.; Teixeira Alves, L.G.; Baumgardt, M.; Bushe, J.; Voss, A.; Langenhagen, A.; Langner, C.; et al. Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters. Nat. Microbiol. 2023, 8, 860–874. [Google Scholar] [CrossRef] [PubMed]
- von Brunn, A.; Teepe, C.; Simpson, J.C.; Pepperkok, R.; Friedel, C.C.; Zimmer, R.; Roberts, R.; Baric, R.; Haas, J. Analysis of intraviral protein-protein interactions of the SARS coronavirus ORFeome. PLoS ONE 2007, 2, e459. [Google Scholar] [CrossRef]
- Carbajo-Lozoya, J.; Ma-Lauer, Y.; Malešević, M.; Theuerkorn, M.; Kahlert, V.; Prell, E.; von Brunn, B.; Muth, D.; Baumert, T.F.; Drosten, C.; et al. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir. Virus Res. 2014, 184, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ma-Lauer, Y.; Zheng, Y.; Malesevic, M.; von Brunn, B.; Fischer, G.; von Brunn, A. Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication. Antivir. Res. 2020, 173, 104620. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.P.; Friese-Hamim, M.; Walter-Bausch, G.; Busch, M.; Gaus, S.; Musil, D.; Rohdich, F.; Zanelli, U.; Downey-Kopyscinski, S.L.; Mitsiades, C.S.; et al. M3258 Is a Selective Inhibitor of the Immunoproteasome Subunit LMP7 (β5i) Delivering Efficacy in Multiple Myeloma Models. Mol. Cancer Ther. 2021, 20, 1378–1387. [Google Scholar] [CrossRef]
- Kusov, Y.; Tan, J.; Alvarez, E.; Enjuanes, L.; Hilgenfeld, R. A G-quadruplex-binding macrodomain within the “SARS-unique domain” is essential for the activity of the SARS-coronavirus replication-transcription complex. Virology 2015, 484, 313–322. [Google Scholar] [CrossRef]
- Lei, J.; Ma-Lauer, Y.; Han, Y.; Thoms, M.; Buschauer, R.; Jores, J.; Thiel, V.; Beckmann, R.; Deng, W.; Leonhardt, H.; et al. The SARS-unique domain (SUD) of SARS-CoV and SARS-CoV-2 interacts with human Paip1 to enhance viral RNA translation. EMBO J. 2021, 40, e102277. [Google Scholar] [CrossRef]
- Bartel, S.; Doellinger, J.; Darsow, K.; Bourquain, D.; Buchholz, R.; Nitsche, A.; Lange, H.A. Proteome analysis of vaccinia virus IHD-W-infected HEK 293 cells with 2-dimensional gel electrophoresis and MALDI-PSD-TOF MS of on solid phase support N-terminally sulfonated peptides. Virol. J. 2011, 8, 380. [Google Scholar] [CrossRef]
- Leone, P.; Shin, E.C.; Perosa, F.; Vacca, A.; Dammacco, F.; Racanelli, V. MHC class I antigen processing and presenting machinery: Organization, function, and defects in tumor cells. J. Natl. Cancer Inst. 2013, 105, 1172–1187. [Google Scholar] [CrossRef]
- Dimasuay, K.G.; Schaunaman, N.; Berg, B.; Cervantes, D.; Kruger, E.; Heppner, F.L.; Ferrington, D.A.; Chu, H.W. Airway epithelial immunoproteasome subunit LMP7 protects against rhinovirus infection. Sci. Rep. 2022, 12, 14507. [Google Scholar] [CrossRef] [PubMed]
- Maelfait, J.; Roose, K.; Vereecke, L.; Mc Guire, C.; Sze, M.; Schuijs, M.J.; Willart, M.; Ibañez, L.I.; Hammad, H.; Lambrecht, B.N.; et al. A20 Deficiency in Lung Epithelial Cells Protects against Influenza A Virus Infection. PLoS Pathog. 2016, 12, e1005410. [Google Scholar] [CrossRef]
- Raaben, M.; Posthuma, C.C.; Verheije, M.H.; te Lintelo, E.G.; Kikkert, M.; Drijfhout, J.W.; Snijder, E.J.; Rottier, P.J.; de Haan, C.A. The ubiquitin-proteasome system plays an important role during various stages of the coronavirus infection cycle. J. Virol. 2010, 84, 7869–7879. [Google Scholar] [CrossRef]
- Longhitano, L.; Tibullo, D.; Giallongo, C.; Lazzarino, G.; Tartaglia, N.; Galimberti, S.; Li Volti, G.; Palumbo, G.A.; Liso, A. Proteasome Inhibitors as a Possible Therapy for SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 3622. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.Y.; Lai, M.M. The ubiquitin-proteasome system facilitates the transfer of murine coronavirus from endosome to cytoplasm during virus entry. J. Virol. 2005, 79, 644–648. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Moriyama, M.; Lucas, C.; Monteiro, V.S.; Iwasaki, A. Enhanced inhibition of MHC-I expression by SARS-CoV-2 Omicron subvariants. Proc. Natl. Acad. Sci. USA 2023, 120, e2221652120. [Google Scholar] [CrossRef] [PubMed]
- Arshad, N.; Laurent-Rolle, M.; Ahmed, W.S.; Hsu, J.C.; Mitchell, S.M.; Pawlak, J.; Sengupta, D.; Biswas, K.H.; Cresswell, P. SARS-CoV-2 accessory proteins ORF7a and ORF3a use distinct mechanisms to down-regulate MHC-I surface expression. Proc. Natl. Acad. Sci. USA 2023, 120, e2208525120. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of antigen processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef] [PubMed]
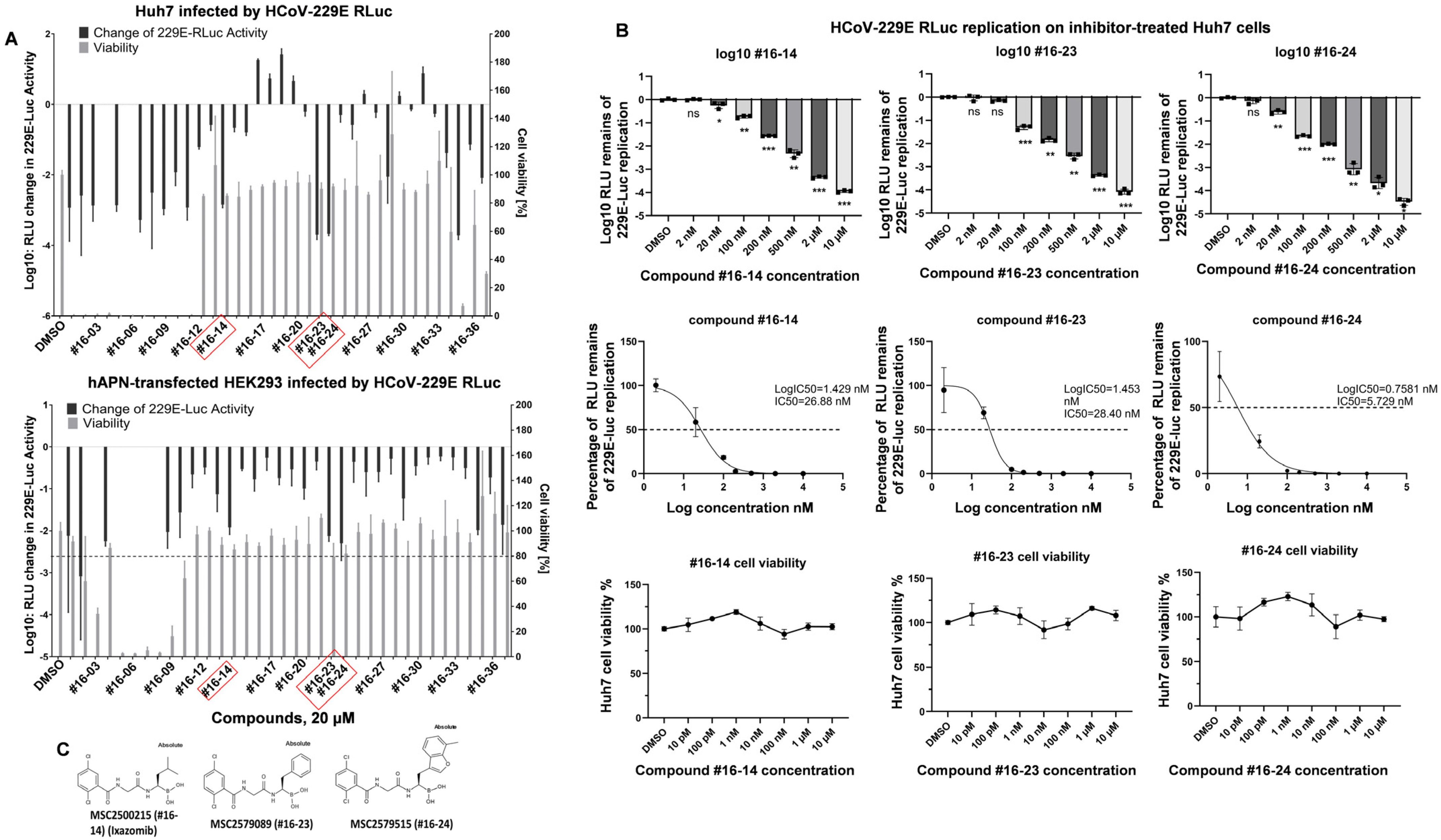
| Inhibitor ID | IC50 (nM) of LMP7 in Cell-Free Biochemical Assay | IC50 (nM) of 229E-Rluc Inhibition in Huh7 Cells |
|---|---|---|
| #16-14 | 2.88 | 26.88 |
| #16-23 | 1.1 | 28.40 |
| #16-24 | 0.3 | 5.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, Y.; Ma-Lauer, Y.; Xiang, C.; Li, P.; von Brunn, B.; Richter, A.; Drosten, C.; Pichlmair, A.; Pfefferle, S.; Klein, M.; et al. LMP7 as a Target for Coronavirus Therapy: Inhibition by Ixazomib and Interaction with SARS-CoV-2 Proteins Nsp13 and Nsp16. Pathogens 2025, 14, 871. https://doi.org/10.3390/pathogens14090871
Ru Y, Ma-Lauer Y, Xiang C, Li P, von Brunn B, Richter A, Drosten C, Pichlmair A, Pfefferle S, Klein M, et al. LMP7 as a Target for Coronavirus Therapy: Inhibition by Ixazomib and Interaction with SARS-CoV-2 Proteins Nsp13 and Nsp16. Pathogens. 2025; 14(9):871. https://doi.org/10.3390/pathogens14090871
Chicago/Turabian StyleRu, Yi, Yue Ma-Lauer, Chengyu Xiang, Pengyuan Li, Brigitte von Brunn, Anja Richter, Christian Drosten, Andreas Pichlmair, Susanne Pfefferle, Markus Klein, and et al. 2025. "LMP7 as a Target for Coronavirus Therapy: Inhibition by Ixazomib and Interaction with SARS-CoV-2 Proteins Nsp13 and Nsp16" Pathogens 14, no. 9: 871. https://doi.org/10.3390/pathogens14090871
APA StyleRu, Y., Ma-Lauer, Y., Xiang, C., Li, P., von Brunn, B., Richter, A., Drosten, C., Pichlmair, A., Pfefferle, S., Klein, M., Damoiseaux, R. D., Betz, U. A. K., & von Brunn, A. (2025). LMP7 as a Target for Coronavirus Therapy: Inhibition by Ixazomib and Interaction with SARS-CoV-2 Proteins Nsp13 and Nsp16. Pathogens, 14(9), 871. https://doi.org/10.3390/pathogens14090871






