Unravelling Anopheles Dynamics in a Malaria-Free Paraguay: Species Distributions, Bioclimatic Niches, and Implications for Resurgence Risks
Abstract
1. Introduction
2. Materials and Methods
2.1. Anopheles spp. Specimen Data
2.2. Geographical Distribution Modelling
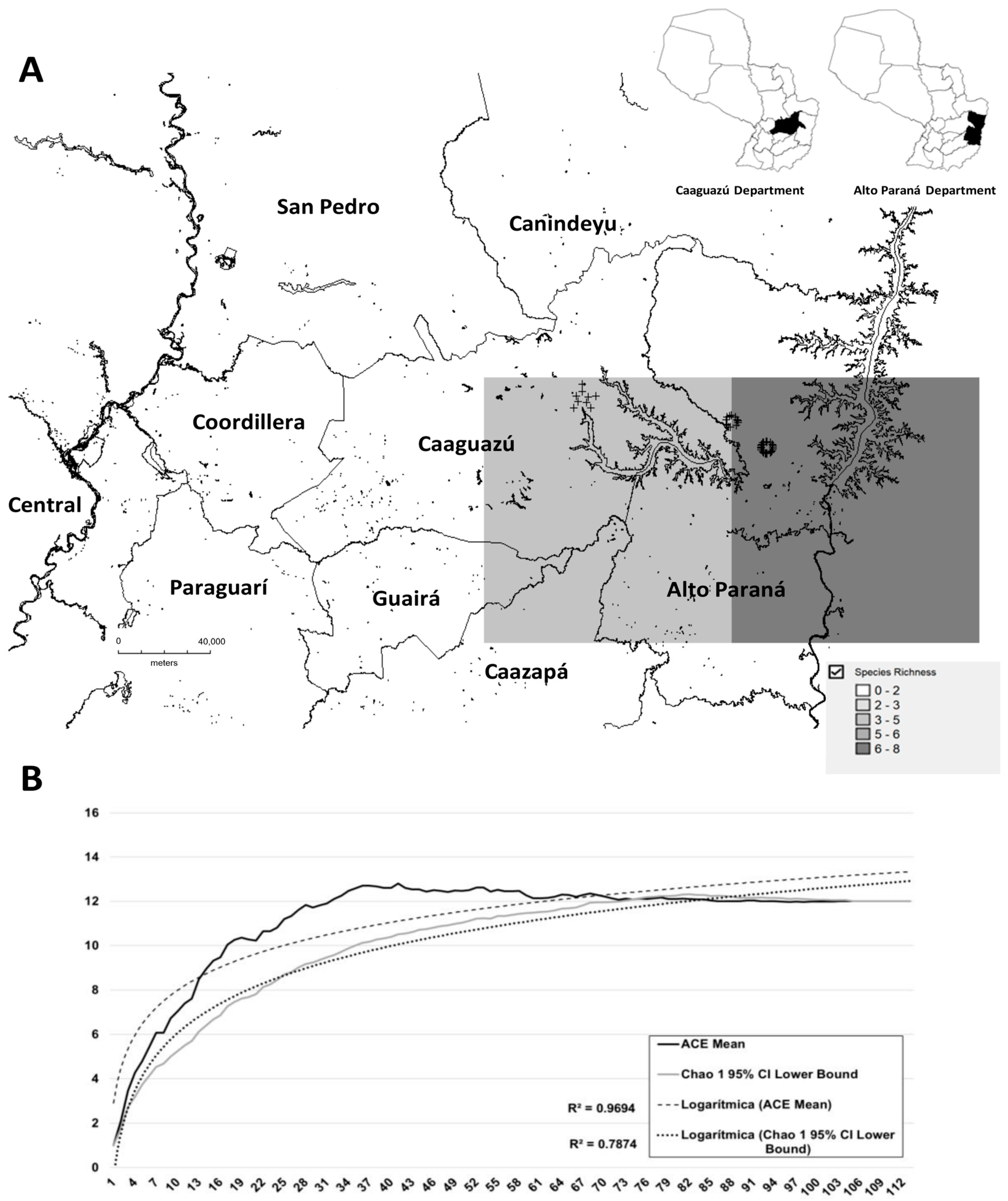
2.3. Species Richness
2.4. Ethical Considerations
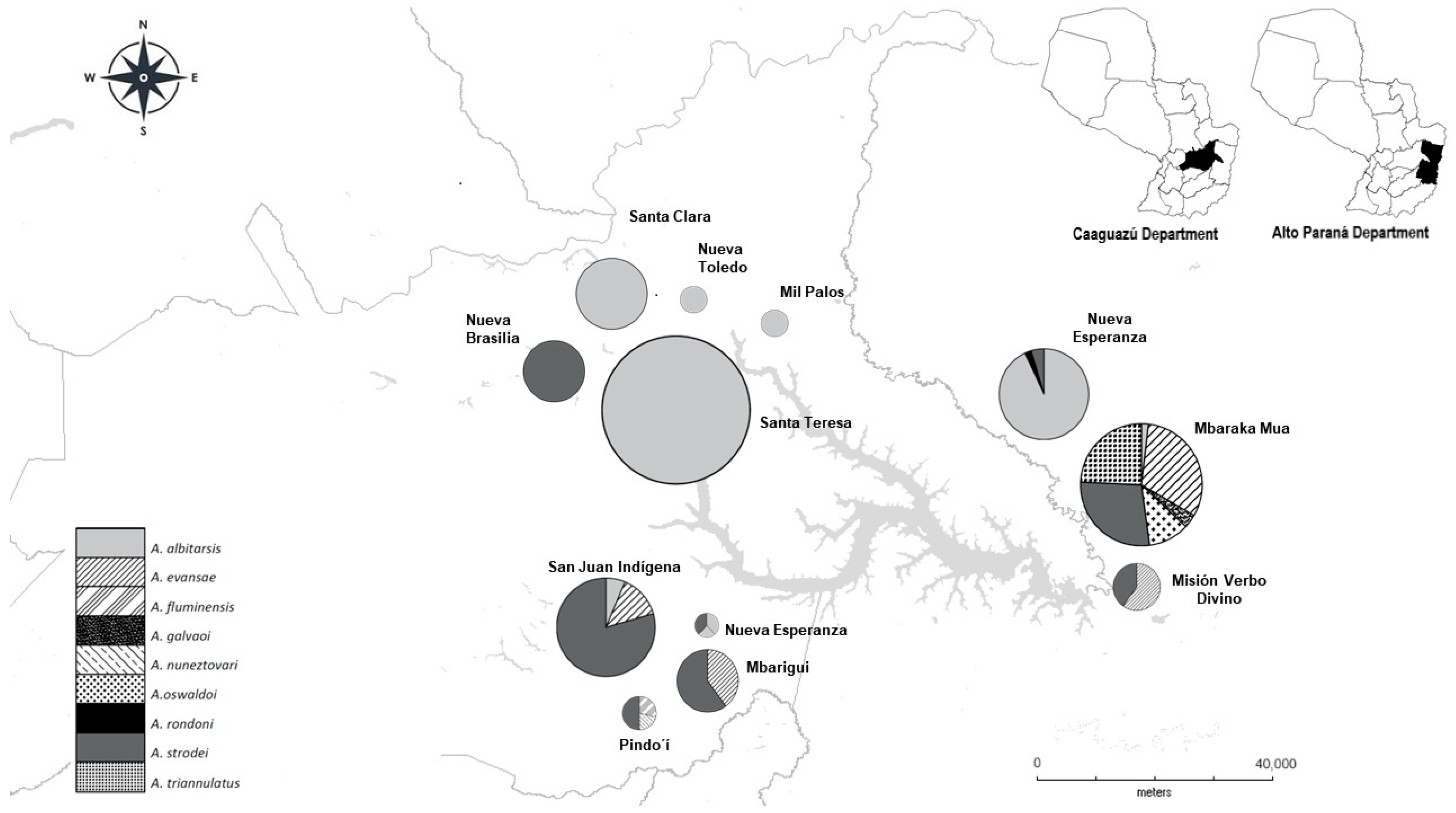
3. Results
Anopheles spp.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laporta, G.Z.; Linton, Y.M.; Wilkerson, R.C.; Bergo, E.S.; Nagaki, S.S.; Sant’Ana, D.C.; Sallum, M.A.M. Malaria vectors in South America: Current and future scenarios. Parasit Vectors 2015, 8, 416. [Google Scholar] [CrossRef]
- World Malaria Report 2024: Addressing Inequity in the Global Malaria Response; World Health Organization: Geneva, Switzerland, 2024.
- del Puerto, F.I.; Ozorio, M.; Trinidad, B.; Ferreira, E.; Russomando, G. Primer reporte de un caso importado de Malaria por Plasmodium ovale curtisi en Paraguay, confirmado por diagnóstico molecular. Mem. Inst. Investig. Cienc. Salud. 2015, 13, 76–82. [Google Scholar] [CrossRef]
- Torales, M.; Viveros de Franchi, C. Malaria en el Paraguay: Situación y riesgos del restablecimiento de la transmisión local. Rev. De Salud Publica Del Parag 2025, 15, 36–42. Available online: https://revistas.ins.gov.py/index.php/rspp/article/view/445 (accessed on 28 November 2024). [CrossRef]
- St. Laurent, B. Mosquito vector diversity and malaria transmission. Front. Malar. 2025, 3, 1600850. [Google Scholar] [CrossRef]
- Ministerio de Salud Pública y Bienestar Social (MSPBS); Servicio Nacional de Erradicación del Paludismo (SENEPA). Manual De Campo Para La Vigilancia Entomológica De Anopheles; MSPBS/SENEPA: Asunción, Paraguay, 2013. [Google Scholar]
- Viveros, C.; Ruiz Díaz, L.; Ozorio, M.; Martinez, N.; Torales, M. Epidemiología de la Malaria en Paraguay. Rev. De Salud Publica Del Parag. 2017, 7, 37–44. [Google Scholar] [CrossRef]
- Muñoz, M. El camino hacia la eliminación de la malaria en Paraguay. Mem. Inst. Investig. Cienc. Salud. 2017, 15, 3–5. Available online: https://revistascientificas.una.py/index.php/RIIC/article/view/1897 (accessed on 23 February 2025). [CrossRef]
- Agência Gov. Brasil Reduz 26% Dos Casos De Malária No Primeiro Trimestre De 2025; Governo do Brasil: Brasília, Brazil, 2025; Available online: https://agenciagov.ebc.com.br/noticias/202504/brasil-reduz-26-dos-casos-de-malaria-no-primeiro-trimestre-de-2025 (accessed on 6 August 2025).
- Babaie, J.; Barati, M.; Azizi, M.; Ephtekhari, A.; Sadat, S.J. A systematic evidence review of the effect of climate change on malaria in Iran. J. Parasit. Dis. 2018, 42, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Polgreen, P.M.; Polgreen, E.L. Infectious Diseases, Weather, and Climate. Clin. Infect. Dis. 2018, 66, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Climate and Infectious Diseases; CDC: Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/ncezid/topics-programs/climate-infectious-disease.html (accessed on 6 August 2025).
- del Puerto, F.; Ozorio, M.; Trinidad, B.; Martínez, N.; Torales, M.; Franco, L.; de Bilbao, N.V. Detection and characterization of Plasmodium spp. by semi-nested multiplex PCR both in mosquito vectors and in humans residing in historically endemic areas of Paraguay. Parasite Epidemiol. Control. 2020, 11, e00174. [Google Scholar] [CrossRef] [PubMed]
- del Puerto, F.; Pésole, D.; Molina, S.; Vera, K.; Arias, M.; Sosa, J.; Ortiz, M.L.; Fernández, J.; Garay, A. Vigilancia molecular de la malaria en potenciales reservorios silvestres que viven en una región históricamente endémica de Paraguay. Mem. Inst. Investig. Cienc. Salud 2018, 16, 30–34. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Mechanistic and correlative models of ecological niches. Eur. J. Ecol. 2015, 1, 28–38. [Google Scholar] [CrossRef]
- Muñoz, M.; Villarroel, J.; Scavuzzo, M.; Lanfri, M.; Cousiño, B.; Guido, C.; Russomando, G. Spatial-temporal analysis of malaria in endemic areas of Paraguay, 2002–2006. Acta Biol. Venez. 2011, 32, 17–21. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Manel, S.; Ceri Williams, H.; Ormerod, S.J. Evaluating presence-absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Colwell, R.K.; Elsensohn, J.E. EstimateS turns 20: Statistical estimation of species richness and shared species from samples, with non-parametric extrapolation. Ecography 2014, 37, 609–613. [Google Scholar] [CrossRef]
- Laxminarayan, R. Trans-boundary commons in infectious diseases. Oxf. Rev. Econ. Policy 2016, 32, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Guía De Vigilancia Para La Prevención Del Restablecimiento De La Malaria o Paludismo En Paraguay. El Ministerio de Salud Pública y Bienestar Social-Paraguay Asunción, Paraguay 2018. Available online: https://dgvs.mspbs.gov.py/files/malaria/Guia_ultima_de_vigilancia_malaria_paludismo.pdf (accessed on 28 November 2024).
- Almeida Ludueña, F.; Almirón, W.R.; Zapata, A. Culicidae (Diptera) del arco sur de la Laguna de Mar Chiquita (Córdoba, Argentina) y su importancia sanitaria. Rev. De La Soc. Entomológica Argent. 2004, 63, 25–28. [Google Scholar]
- Afrane, Y.A.; Githeko, A.K.; Yan, G. The ecology of Anopheles mosquitoes under climate change: Case studies from the effects of deforestation in East African highlands. Ann. N. Y. Acad. Sci. 2012, 1249, 204–210. [Google Scholar] [CrossRef] [PubMed]
- González Ayala, S.E.; Morales, M.A.; Enría, D.A. Reemergencia de la encefalitis equina del oeste (WEEV) en la Argentina: Una revisión de aspectos epidemiológicos, virológicos y clínicos de relevancia. Actual. En Sida E Infectología 2024, 32. Available online: https://revista.infectologia.info/index.php/revista/article/view/315 (accessed on 19 March 2025). [CrossRef]
- División de Vigilancia Sanitaria. Ministerio de Salud Pública y Bienestar Social. Boletín Epidemiológico; Semana 52. Asunción, Paraguay 2018. Available online: https://dgvs.mspbs.gov.py/files/boletines/SE52_2018_Boletin.pdf (accessed on 9 April 2024).
- Abiodun, G.J.; Maharaj, R.; Witbooi, P.; Okosun, K.O. Modelling the influence of temperature and rainfall on the population dynamics of Anopheles arabiensis. Malar. J. 2016, 15, 364. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Puerto, F.; Grissetti, M.; Ferreira, L.; Franco, L.; Herrera, L. Unravelling Anopheles Dynamics in a Malaria-Free Paraguay: Species Distributions, Bioclimatic Niches, and Implications for Resurgence Risks. Pathogens 2025, 14, 849. https://doi.org/10.3390/pathogens14090849
del Puerto F, Grissetti M, Ferreira L, Franco L, Herrera L. Unravelling Anopheles Dynamics in a Malaria-Free Paraguay: Species Distributions, Bioclimatic Niches, and Implications for Resurgence Risks. Pathogens. 2025; 14(9):849. https://doi.org/10.3390/pathogens14090849
Chicago/Turabian Styledel Puerto, Florencia, Mauricio Grissetti, Luis Ferreira, Luciano Franco, and Leidi Herrera. 2025. "Unravelling Anopheles Dynamics in a Malaria-Free Paraguay: Species Distributions, Bioclimatic Niches, and Implications for Resurgence Risks" Pathogens 14, no. 9: 849. https://doi.org/10.3390/pathogens14090849
APA Styledel Puerto, F., Grissetti, M., Ferreira, L., Franco, L., & Herrera, L. (2025). Unravelling Anopheles Dynamics in a Malaria-Free Paraguay: Species Distributions, Bioclimatic Niches, and Implications for Resurgence Risks. Pathogens, 14(9), 849. https://doi.org/10.3390/pathogens14090849





