Differential Exposure to Borrelia spp. and Spotted Fever Group Rickettsia in Serbia and North Macedonia: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Framework and Recruitment of Participants
2.2. Laboratory Analysis
2.3. Detection of Anti-Borrelia spp. IgG
2.4. Detection of Anti-SFGR IgG Antibodies
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics and Cohort Distribution
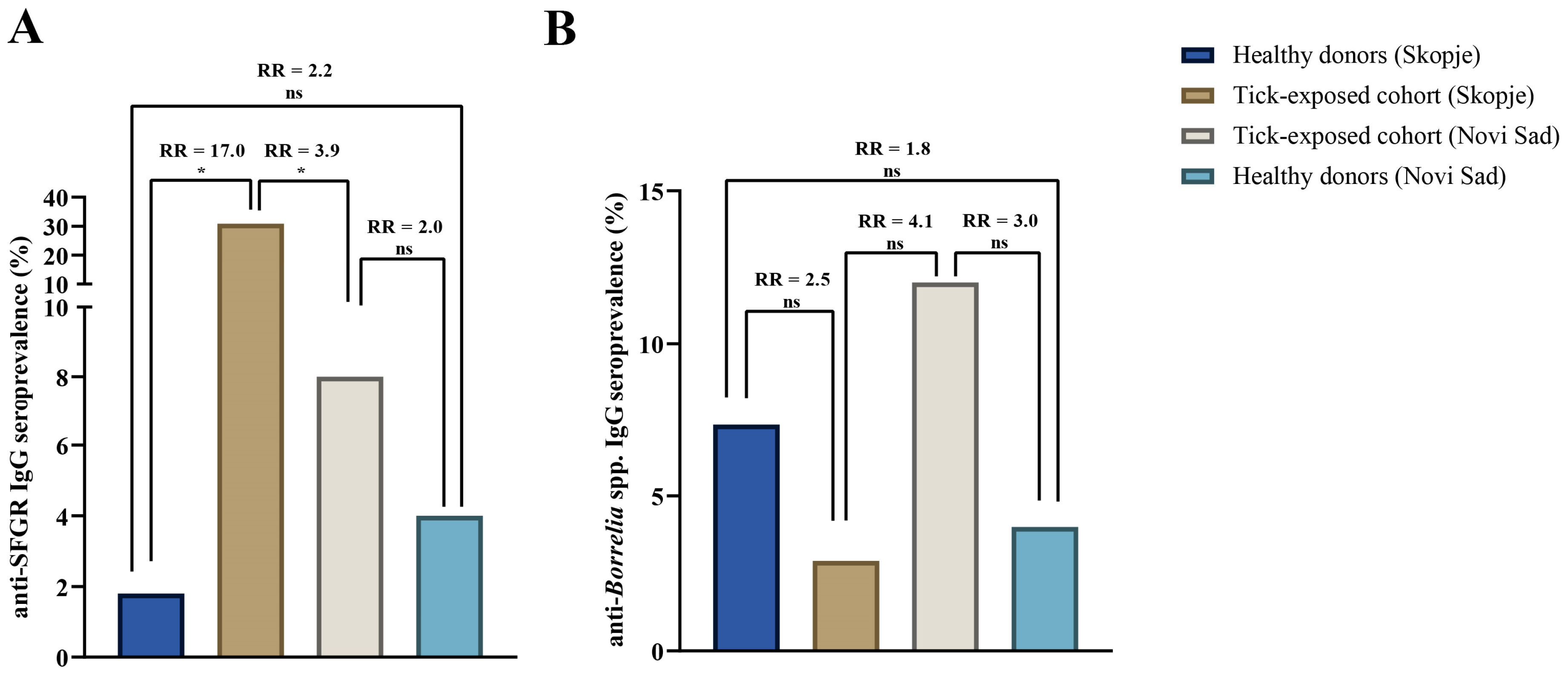
3.2. Comparative Analysis of Anti-SFGR IgG Seropositivity
3.3. Comparative Analysis of Anti-Borrelia IgG Seropositivity
3.4. Comparative Analysis of Dual Seropositivity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Antibody index |
| CIDS | Clinic for Infectious Diseases in Skopje |
| ELISA | Enzyme-linked immunosorbent assay |
| LB | Lyme borreliosis |
| MSF | Mediterranean spotted fever |
| O.D. | Optical density |
| PINS | Pasteur Institute in Novi Sad |
| RR | Relative risk |
| SFGR | Spotted fever group Rickettsia |
| TBDs | Tick-borne diseases |
References
- Banović, P.; Jakimovski, D.; Bogdan, I.; Simin, V.; Mijatović, D.; Bosilkovski, M.; Mateska, S.; Díaz-Sánchez, A.A.; Foucault-Simonin, A.; Zając, Z.; et al. Tick-borne diseases at the crossroads of the Middle East and Central Europe. Infect. Dis. Now. 2024, 54, 104959. [Google Scholar] [CrossRef]
- Taseva, E.; Christova, I.; Panayotova, E.; Ilieva, D.; Pavlova, V. Is there an outbreak of tick-borne encephalitis in Pernik District, Bulgaria? Four cases registered for a period of four years—Clinical manifestations and epidemiological relations. Probl. Infect. Parasit. Dis. 2021, 49, 19–25. [Google Scholar] [CrossRef]
- Banović, P.; Díaz-Sánchez, A.A.; Foucault-Simonin, A.; Mateos-Hernandez, L.; Wu-Chuang, A.; Galon, C.; Simin, V.; Mijatović, D.; Bogdan, I.; Corona-González, B.; et al. Emerging tick-borne spotted fever group rickettsioses in the Balkans. Infect. Genet. Evol. 2023, 107, 105400. [Google Scholar] [CrossRef] [PubMed]
- Sherifi, K.; Cadar, D.; Muji, S.; Robaj, A.; Ahmeti, S.; Jakupi, X.; Emmerich, P.; Krüger, A. Crimean-Congo hemorrhagic fever virus clades V and VI (Europe 1 and 2) in ticks in Kosovo, 2012. PLoS Negl. Trop. Dis. 2014, 8, e3168. [Google Scholar] [CrossRef]
- Banović, P.; Díaz-Sánchez, A.A.; Simin, V.; Foucault-Simonin, A.; Galon, C.; Wu-Chuang, A.; Mijatović, D.; Obregón, D.; Moutailler, S.; Cabezas-Cruz, A. Clinical aspects and detection of emerging rickettsial pathogens: A “One Health” approach study in Serbia, 2020. Front. Microbiol. 2022, 12, 797399. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Barbic, L.; Mrzljak, A.; Brnic, D.; Klobucar, A.; Ilic, M.; Janev-Holcer, N.; Bogdanic, M.; Jemersic, L.; Stevanovic, V.; et al. Emerging and neglected viruses of zoonotic importance in Croatia. Pathogens 2021, 10, 73. [Google Scholar] [CrossRef]
- Jakimovski, D.; Banović, P.; Spasovska, K.; Rangelov, G.; Cvetanovska, M.; Cana, F.; Simin, V.; Bogdan, I.; Mijatović, D.; Cvetkovikj, A.; et al. One Health investigation following a cluster of Crimean–Congo haemorrhagic fever, North Macedonia, July to November 2023. Euro Surveill. 2025, 30, 2400286. [Google Scholar] [CrossRef]
- Jakimovski, D.; Mateska, S.; Simin, V.; Bogdan, I.; Mijatović, D.; Estrada-Peña, A.; Mateos-Hernández, L.; Foucault-Simonin, A.; Moutailler, S.; Cabezas-Cruz, A.; et al. Mediterranean spotted fever-like illness caused by Rickettsia sibirica mongolitimonae, North Macedonia, June 2022. Euro Surveill. 2022, 27, 2200735. [Google Scholar] [CrossRef] [PubMed]
- Baltadzhiev, I.G.; Popivanova, N.I.; Stoilova, Y.M.; Kevorkian, A.K. Mediterranean spotted fever—Classification by disease course and criteria for determining the disease severity. Folia Medica 2012, 54, 53–61. [Google Scholar] [CrossRef]
- Baltadzhiev, I.; Kevorkyan, A.; Popivanova, N. Mediterranean spotted fever in child and adult patients: Investigation from an endemic region in Bulgaria. Cent. Eur. J. Public Health 2020, 28, 187–192. [Google Scholar] [CrossRef]
- Abdeljelil, M.; Sakly, H.; Kooli, I.; Marrakchi, W.; Aouam, A.; Loussaief, C.; Toumi, A.; Ben Brahim, H.; Chakroun, M. Mediterranean spotted fever as a cause of septic shock. IDCases 2019, 15, e00528. [Google Scholar] [CrossRef]
- Esmaeili, S.; Latifian, M.; Khalili, M.; Farrokhnia, M.; Stenos, J.; Shafiei, M.; Mostafavi, E. Fatal case of Mediterranean spotted fever associated with septic shock, Iran. Emerg. Infect. Dis. 2022, 28, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, A.; Skufca, J.; Vyse, A.; Pilz, A.; Begier, E.; Riera-Montes, M.; Gessner, B.D.; Stark, J.H. The landscape of Lyme borreliosis surveillance in Europe. Vector Borne Zoonotic Dis. 2023, 23, 142–155. [Google Scholar] [CrossRef]
- Burn, L.; Tran, T.M.P.; Pilz, A.; Vyse, A.; Fletcher, M.A.; Angulo, F.J.; Gessner, B.D.; Moïsi, J.C.; Jodar, L.; Stark, J.H. Incidence of Lyme borreliosis in Europe from national surveillance systems (2005–2020). Vector Borne Zoonotic Dis. 2023, 23, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Veliкj Stefanovska, V.; Zafirova Ivanovska, B.; Kocinski, D.; Grivchevska, M.; Sazdovska, S.; Radevska, A. Zoonoses in Republic of North Macedonia: Tularemia. Lyme Disease (1980–2023); Zoonoses in the Republic of North Macedonia; University “Ss. Cyril and Methodius University”, Faculty of Medicine; Institute of Epidemiology and Biostatistics with Medical Informatics: Skopje, North Macedonia, 2024; p. 54. [Google Scholar]
- Banović, P.; Díaz-Sánchez, A.A.; Galon, C.; Simin, V.; Mijatović, D.; Obregón, D.; Moutailler, S.; Cabezas-Cruz, A. Humans infested with Ixodes ricinus are exposed to a diverse array of tick-borne pathogens in Serbia. Ticks Tick-Borne Dis. 2020, 12, 101609. [Google Scholar] [CrossRef] [PubMed]
- Angulo, F.J.; Colby, E.; Lebech, A.-M.; Lindgren, P.-E.; Moniuszko-Malinowska, A.; Strle, F.; Olsen, J.; Brestrich, G.; Vyse, A.; Shafquat, M.; et al. Incidence of symptomatic Lyme borreliosis in nine European countries. Int. J. Infect. Dis. 2024, 149, 107242. [Google Scholar] [CrossRef]
- Jakimovski, D.; Mateska, S.; Dimitrova, E.; Bosilkovski, M.; Mijatović, D.; Simin, V.; Bogdan, I.; Grujić, J.; Budakov-Obradović, Z.; Meletis, E.; et al. Tick-borne encephalitis virus and Borrelia burgdorferi seroprevalence in Balkan tick-infested individuals: A two-centre study. Pathogens 2023, 12, 922. [Google Scholar] [CrossRef]
- Ngoc, K.; Trifonova, I.; Gladnishka, T.; Taseva, E.; Panayotova, E.; Vladimirova, I.; Ivanova, V.; Kuteva, E.; Christova, I. Serological assessment of Lyme borreliosis in Bulgaria: A nationwide study. Pathogens 2024, 13, 754. [Google Scholar] [CrossRef]
- Karageorgou, I.; Koutantou, M.; Papadogiannaki, I.; Voulgari-Kokota, A.; Makka, S.; Angelakis, E. Serological Evidence of possible Borrelia afzelii Lyme disease in Greece. New Microbes New Infect. 2022, 46, 100978. [Google Scholar] [CrossRef]
- Cheran, C.A.; Panciu, A.M.; Riciu, C.D.; Nedelcu, I.M.; Iacob, D.G.; Hristea, A. Identifying new areas of endemicity and risk factors for Rickettsia conorii subsp. conorii infection: Serosurvey in rural areas of Romania. Pathogens 2024, 13, 783. [Google Scholar] [CrossRef]
- Arz, C.; Król, N.; Imholt, C.; Jeske, K.; Rentería-Solís, Z.; Ulrich, R.G.; Jacob, J.; Pfeffer, M.; Obiegala, A. Spotted fever group rickettsiae in ticks and small mammals from grassland and forest habitats in central Germany. Pathogens 2023, 12, 933. [Google Scholar] [CrossRef]
- Dyczko, D.; Błażej, P.; Kiewra, D. The influence of forest habitat type on Ixodes ricinus infections with Rickettsia spp. in south-western Poland. Curr. Res. Parasitol. Vector-Borne Dis. 2024, 6, 100200. [Google Scholar] [CrossRef]
- Estrada-Pena, A.; Ayllon, N.; De La Fuente, J. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef]
- Baltadzhiev, I.; Popivanova, N.; Zaprianov, Z. Malignant forms of Mediterranean spotted fever: Risk factors for fatal outcomes. Braz. J. Infect. Dis. 2016, 20, 511–512. [Google Scholar] [CrossRef]
- Tsokana, C.N.; Kapna, I.; Valiakos, G. Current data on Rickettsia felis occurrence in vectors, human and animal hosts in Europe: A Scoping Review. Microorganisms 2022, 10, 2491. [Google Scholar] [CrossRef]
- Brown, L.D.; Macaluso, K.R. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr. Trop. Med. Rep. 2016, 3, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Sun, Y.-Q.; Chen, J.-J.; Teng, A.-Y.; Wang, T.; Li, H.; Hay, S.I.; Fang, L.-Q.; Yang, Y.; Liu, W. Mapping the global distribution of spotted fever group rickettsiae: A systematic review with modelling analysis. Lancet Digit. Health 2023, 5, e5–e15. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Bacellar, F.; Baranton, G.; Birtles, R.J.; Bjoërsdorff, A.; Blanco, J.R.; Caruso, G.; Cinco, M.; Fournier, P.E.; Francavilla, E.; et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 2004, 10, 1108–1132. [Google Scholar] [CrossRef]
- Malesios, C.; Kostoulas, P.; Dadousis, K.; Demiris, N. An early warning indicator for monitoring infectious animal diseases and its application in the case of a sheep pox epidemic. Stoch. Environ. Res. Risk Assess. 2017, 31, 329–337. [Google Scholar] [CrossRef]
- Meletis, E.; Poulakida, I.; Perlepe, G.; Katsea, A.; Pateras, K.; Boutlas, S.; Papadamou, G.; Gourgoulianis, K.; Kostoulas, P. Early Warning of Potential Epidemics: A pilot application of an early warning tool to data from the pulmonary clinic of the University Hospital of Thessaly, Greece. J. Infect. Public Health 2024, 17, 401–405. [Google Scholar] [CrossRef] [PubMed]

| Risk Group | Tick-Exposed Cohort | Healthy Donors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skopje | Novi Sad | Skopje | Novi Sad | |||||||
| n | (%) | n | (%) | p | n | (%) | n | (%) | p | |
| Gender | ||||||||||
| Male | 38 | (55.9) | 29 | (58) | 0.818 | 25 | (45.5) | 27 | (54) | 0.382 |
| Female | 30 | (44.1) | 21 | (42) | 30 | (54.5) | 23 | (46) | ||
| Total | 68 | (100) | 50 | (100) | n/a | 55 | (100) | 50 | (100) | n/a |
| Age | ||||||||||
| Young adults | 10 | (14.7) | 31 | (62) | 0.000 * 0.026 * 0.001 * | 27 | (49.1) | 22 | (44) | 0.601 |
| Middle-aged adults | 18 | (26.5) | 5 | (10) | 17 | (30.9) | 16 | (32) | 0.904 | |
| Older adults | 40 | (58.8) | 14 | (28) | 11 | (20) | 12 | (24) | 0.621 | |
| Total | 68 | (100) | 50 | (100) | n/a | 55 | (100) | 50 | (100) | n/a |
| Centre | Group | Sex | Samples Tested n (%) | Anti-SFGR IgG n (%) | p | Anti-Borrelia spp. IgG n (%) | p |
|---|---|---|---|---|---|---|---|
| CIDS | Tick-exposed | Male | 38 (55.9) | 13 (34.2) | 0.504 | 1 (2.6) | 1.000 |
| Female | 30 (44.1) | 8 (26.7) | 1 (3.3) | ||||
| Total | 68 (100) | 21 (30.9) | n/a | 2 (2.9) | n/a | ||
| Healthy donors | Male | 25 (45.5) | 0 (0.0) | 1.000 | 2 (8.0) | 1.000 | |
| Female | 30 (54.5) | 1 (3.3) | 2 (6.6) | ||||
| Total | 55 (100) | 1 (1.8) | n/a | 4 (7.3) | n/a | ||
| Total | 123 (100) | 22 (17.9) | n/a | 6 (4.9) | n/a | ||
| PINS | Tick-exposed | Male | 29 (58.0) | 2 (6.9) | 1.000 | 3 (10.3) | 0.686 |
| Female | 21 (42.0) | 2 (9.5) | 3 (14.3) | ||||
| Total | 50 (100) | 4 (8.0) | n/a | 6 (12.0) | n/a | ||
| Healthy donors | Male | 27 (54.0) | 1 (3.7) | 1.000 | 1 (3.7) | 1.000 | |
| Female | 23 (46.0) | 1 (4.3) | 1 (4.3) | ||||
| Total | 50 (100) | 2 (4.0) | n/a | 2 (4.0) | n/a | ||
| Total | 100 (100) | 6 (6.0) | n/a | 8 (8.0) | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakimovski, D.; Mateska, S.; Najdovska, M.; Stamenkovska, A.; Pavleva, V.; Bosilkovski, M.; Mijatović, D.; Simin, V.; Bogdan, I.; Grujić, J.; et al. Differential Exposure to Borrelia spp. and Spotted Fever Group Rickettsia in Serbia and North Macedonia: A Comparative Study. Pathogens 2025, 14, 814. https://doi.org/10.3390/pathogens14080814
Jakimovski D, Mateska S, Najdovska M, Stamenkovska A, Pavleva V, Bosilkovski M, Mijatović D, Simin V, Bogdan I, Grujić J, et al. Differential Exposure to Borrelia spp. and Spotted Fever Group Rickettsia in Serbia and North Macedonia: A Comparative Study. Pathogens. 2025; 14(8):814. https://doi.org/10.3390/pathogens14080814
Chicago/Turabian StyleJakimovski, Dejan, Sofija Mateska, Marija Najdovska, Angela Stamenkovska, Verica Pavleva, Mile Bosilkovski, Dragana Mijatović, Verica Simin, Ivana Bogdan, Jasmina Grujić, and et al. 2025. "Differential Exposure to Borrelia spp. and Spotted Fever Group Rickettsia in Serbia and North Macedonia: A Comparative Study" Pathogens 14, no. 8: 814. https://doi.org/10.3390/pathogens14080814
APA StyleJakimovski, D., Mateska, S., Najdovska, M., Stamenkovska, A., Pavleva, V., Bosilkovski, M., Mijatović, D., Simin, V., Bogdan, I., Grujić, J., Simeunović, M., Vranješ, M., Meletis, E., Kostoulas, P., Lioupi, O., & Banović, P. (2025). Differential Exposure to Borrelia spp. and Spotted Fever Group Rickettsia in Serbia and North Macedonia: A Comparative Study. Pathogens, 14(8), 814. https://doi.org/10.3390/pathogens14080814







