Perspectives on the History and Epidemiology of the Varicella Virus Vaccine and Future Challenges
Abstract
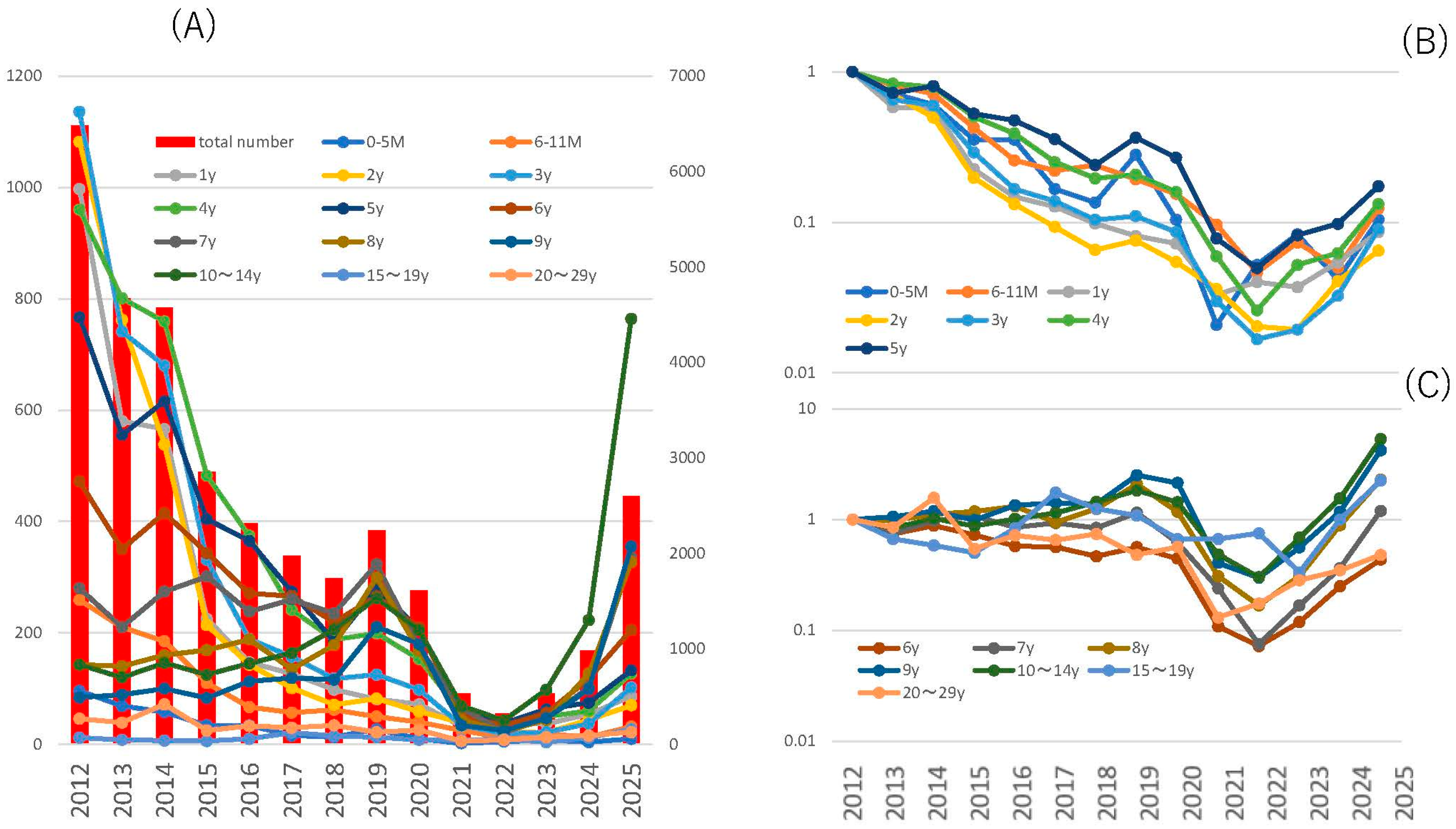
1. Introduction
2. Mechanism of Varicella Zoster Virus (VZV) Infection
3. Genes for Latent Infection
4. Immune Response to Varicella Zoster Virus (VZV)
5. Attenuated Live Vaccine Against Varicella Zoster Virus (VZV)
6. Are Varicella Vaccinees Infectious?
7. Efficacy of Attenuated Live Varicella Vaccine in Preventing Chickenpox
8. Childhood Shingles
9. Meningitis Due to Reactivation of Varicella Virus
10. Acute Retinal Necrosis Caused by VZV Attenuated Live Vaccine
11. Maintenance of Immunity to VZV
12. Problems and Future Challenges with Varicella Attenuated Live Vaccine
12.1. Breakthrough Infection
12.2. Persistence of Immunity
12.3. Trends in Shingles Caused by Vaccine Strains
12.4. Emergence of Mutant Vaccine Strains
13. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weller, T.H. Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster. Proc. Soc. Exp. Biol. Med. 1953, 83, 340–346. [Google Scholar] [CrossRef]
- Ligon, B.L. Thomas Huckle Weller MD: Nobel Laureate and research pioneer in poliomyelitis, varicella-zoster virus, cytomegalovirus, rubella, and other infectious diseases. Semin. Pediatr. Infect. Dis. 2002, 13, 55–63. [Google Scholar] [CrossRef]
- Cohen, R.; Ashman, M.; Taha, M.K.; Varon, E.; Angoulvant, F.; Levy, C.; Rybak, A.; Ouldali, N.; Guiso, N.; Grimprel, E. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now 2021, 51, 418–423. [Google Scholar] [CrossRef]
- Principi, N.; Autore, G.; Ramundo, G.; Esposito, S. Epidemiology of Respiratory Infections during the COVID-19 Pandemic. Viruses 2023, 15, 1160. [Google Scholar] [CrossRef]
- Yang, M.C.; Su, Y.T.; Chen, P.H.; Tsai, C.C.; Lin, T.I.; Wu, J.R. Changing patterns of infectious diseases in children during the COVID-19 pandemic. Front. Cell. Infect. Microbiol. 2023, 13, 1200617. [Google Scholar] [CrossRef]
- Nagasawa, M. Verification of Immune Debts in Children Caused by the COVID-19 Pandemic from an Epidemiological and Clinical Perspective. Immuno 2025, 5, 5. [Google Scholar] [CrossRef]
- Available online: https://survey.tmiph.metro.tokyo.lg.jp/epidinfo/epimenu.do (accessed on 8 June 2025).
- Soong, W.; Schultz, J.C.; Patera, A.C.; Sommer, M.H.; Cohen, J.I. Infection of human T lymphocytes with varicella-zoster virus: An analysis with viral mutants and clinical isolates. J. Virol. 2000, 74, 1864–1870. [Google Scholar] [CrossRef]
- Ku, C.C.; Padilla, J.A.; Grose, C.; Butcher, E.C.; Arvin, A.M. Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers. J. Virol. 2002, 76, 11425–11433. [Google Scholar] [CrossRef] [PubMed]
- Abendroth, A.; Morrow, G.; Cunningham, A.L.; Slobedman, B. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: Implications for virus dissemination in the host. J. Virol. 2001, 75, 6183–6192. [Google Scholar] [CrossRef]
- Ku, C.C.; Zerboni, L.; Ito, H.; Graham, B.S.; Wallace, M.; Arvin, A.M. Varicella-zoster virus transfer to skin by T Cells and modulation of viral replication by epidermal cell interferon-alpha. J. Exp. Med. 2004, 200, 917–925. [Google Scholar] [CrossRef]
- Levin, M.J.; Cai, G.Y.; Manchak, M.D.; Pizer, L.I. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J. Virol. 2003, 77, 6979–6987. [Google Scholar] [CrossRef]
- Annunziato, P.W.; Lungu, O.; Panagiotidis, C.; Zhang, J.H.; Silvers, D.N.; Gershon, A.A.; Silverstein, S.J. Varicella-zoster virus proteins in skin lesions: Implications for a novel role of ORF29p in chickenpox. J. Virol. 2000, 74, 2005–2010. [Google Scholar] [CrossRef]
- Nikkels, A.F.; Debrus, S.; Sadzot-Delvaux, C.; Piette, J.; Rentier, B.; Pierard, G.E. Localization of varicella-zoster virus nucleic acids and proteins in human skin. Neurology 1995, 45, S47–S49. [Google Scholar] [CrossRef]
- Weigle, K.A.; Grose, C. Common expression of varicella-zoster viral glycoprotein antigens in vitro and in chickenpox and zoster vesicles. J. Infect. Dis. 1983, 148, 630–638. [Google Scholar] [CrossRef]
- Cole, N.L.; Grose, C. Membrane fusion mediated by herpesvirus glycoproteins: The paradigm of varicella-zoster virus. Rev. Med. Virol. 2003, 13, 207–222. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhu, Z.; Gershon, A.A.; Gershon, M.D. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell 2004, 119, 915–926. [Google Scholar] [CrossRef]
- Li, Q.; Ali, M.A.; Cohen, J.I. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell 2006, 127, 305–316. [Google Scholar] [CrossRef]
- Suenaga, T.; Satoh, T.; Somboonthum, P.; Kawaguchi, Y.; Mori, Y.; Arase, H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. USA 2010, 107, 866–871. [Google Scholar] [CrossRef]
- Quarles, R.H. A hypothesis about the relationship of myelin-associated glycoprotein’s function in myelinated axons to its capacity to inhibit neurite outgrowth. Neurochem. Res. 2009, 34, 79–86. [Google Scholar] [CrossRef]
- Cohrs, R.J.; Barbour, M.; Gilden, D.H. Varicella-zoster virus (VZV) transcription during latency in human ganglia: Detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J. Virol. 1996, 70, 2789–2796. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Grinfeld, E.; Bell, J.E. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J. Virol. 2000, 74, 11893–11898. [Google Scholar] [CrossRef]
- Cohen, J.I.; Krogmann, T.; Ross, J.P.; Pesnicak, L.; Prikhod’ko, E.A. Varicella-zoster virus ORF4 latency-associated protein is important for establishment of latency. J. Virol. 2005, 79, 6969–6975. [Google Scholar] [CrossRef]
- Xia, D.; Srinivas, S.; Sato, H.; Pesnicak, L.; Straus, S.E.; Cohen, J.I. Varicella-zoster virus open reading frame 21, which is expressed during latency, is essential for virus replication but dispensable for establishment of latency. J. Virol. 2003, 77, 1211–1218. [Google Scholar] [CrossRef]
- Cohen, J.I.; Krogmann, T.; Pesnicak, L.; Ali, M.A. Absence or overexpression of the Varicella-Zoster Virus (VZV) ORF29 latency-associated protein impairs late gene expression and reduces VZV latency in a rodent model. J. Virol. 2007, 81, 1586–1591. [Google Scholar] [CrossRef]
- Cohen, J.I.; Krogmann, T.; Bontems, S.; Sadzot-Delvaux, C.; Pesnicak, L. Regions of the varicella-zoster virus open reading frame 63 latency-associated protein important for replication in vitro are also critical for efficient establishment of latency. J. Virol. 2005, 79, 5069–5077. [Google Scholar] [CrossRef]
- Depledge, D.P.; Ouwendijk, W.J.D.; Sadaoka, T.; Braspenning, S.E.; Mori, Y.; Cohrs, R.J.; Verjans, G.; Breuer, J. A spliced latency-associated VZV transcript maps antisense to the viral transactivator gene 61. Nat. Commun. 2018, 9, 1167. [Google Scholar] [CrossRef]
- Balachandra, K.; Thawaranantha, D.; Ayuthaya, P.I.; Bhumisawasdi, J.; Shiraki, K.; Yamanishi, K. Effects of human alpha, beta and gamma interferons on varicella zoster virus in vitro. Southeast Asian J. Trop. Med. Public Health 1994, 25, 252–257. [Google Scholar]
- Desloges, N.; Rahaus, M.; Wolff, M.H. Role of the protein kinase PKR in the inhibition of varicella-zoster virus replication by beta interferon and gamma interferon. J. Gen. Virol. 2005, 86, 1–6. [Google Scholar] [CrossRef]
- Arvin, A.M.; Kushner, J.H.; Feldman, S.; Baehner, R.L.; Hammond, D.; Merigan, T.C. Human leukocyte interferon for the treatment of varicella in children with cancer. N. Engl. J. Med. 1982, 306, 761–765. [Google Scholar] [CrossRef]
- Jarosinski, K.W.; Carpenter, J.E.; Buckingham, E.M.; Jackson, W.; Knudtson, K.; Moffat, J.F.; Kita, H.; Grose, C. Cellular Stress Response to Varicella-Zoster Virus Infection of Human Skin Includes Highly Elevated Interleukin-6 Expression. Open Forum Infect. Dis. 2018, 5, ofy118. [Google Scholar] [CrossRef]
- Arvin, A.M.; Koropchak, C.M.; Williams, B.R.; Grumet, F.C.; Foung, S.K. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J. Infect. Dis. 1986, 154, 422–429. [Google Scholar] [CrossRef]
- Kumagai, T.; Chiba, Y.; Wataya, Y.; Hanazono, H.; Chiba, S.; Nakao, T. Development and characteristics of the cellular immune response to infection with varicella-zoster virus. J. Infect. Dis. 1980, 141, 7–13. [Google Scholar] [CrossRef]
- Burke, B.L.; Steele, R.W.; Beard, O.W.; Wood, J.S.; Cain, T.D.; Marmer, D.J. Immune responses to varicella-zoster in the aged. Arch. Intern. Med. 1982, 142, 291–293. [Google Scholar] [CrossRef]
- Oxman, M.N.; Levin, M.J. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J. Infect. Dis. 2008, 197 (Suppl. S2), S228–S236. [Google Scholar] [CrossRef]
- Takahashi, M.; Otsuka, T.; Okuno, Y.; Asano, Y.; Yazaki, T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet 1974, 2, 1288–1290. [Google Scholar] [CrossRef]
- Gomi, Y.; Sunamachi, H.; Mori, Y.; Nagaike, K.; Takahashi, M.; Yamanishi, K. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J. Virol. 2002, 76, 11447–11459. [Google Scholar] [CrossRef]
- Depledge, D.P.; Yamanishi, K.; Gomi, Y.; Gershon, A.A.; Breuer, J. Deep Sequencing of Distinct Preparations of the Live Attenuated Varicella-Zoster Virus Vaccine Reveals a Conserved Core of Attenuating Single-Nucleotide Polymorphisms. J. Virol. 2016, 90, 8698–8704. [Google Scholar] [CrossRef]
- Gutzeit, C.; Raftery, M.J.; Peiser, M.; Tischer, K.B.; Ulrich, M.; Eberhardt, M.; Stockfleth, E.; Giese, T.; Sauerbrei, A.; Morita, C.T.; et al. Identification of an important immunological difference between virulent varicella-zoster virus and its avirulent vaccine: Viral disruption of dendritic cell instruction. J. Immunol. 2010, 185, 488–497. [Google Scholar] [CrossRef]
- Moffat, J.F.; Stein, M.D.; Kaneshima, H.; Arvin, A.M. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 1995, 69, 5236–5242. [Google Scholar] [CrossRef]
- Moffat, J.F.; Zerboni, L.; Kinchington, P.R.; Grose, C.; Kaneshima, H.; Arvin, A.M. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 1998, 72, 965–974. [Google Scholar] [CrossRef]
- Gomi, Y.; Imagawa, T.; Takahashi, M.; Yamanishi, K. Oka varicella vaccine is distinguishable from its parental virus in DNA sequence of open reading frame 62 and its transactivation activity. J. Med. Virol. 2000, 61, 497–503. [Google Scholar] [CrossRef]
- Zerboni, L.; Hinchliffe, S.; Sommer, M.H.; Ito, H.; Besser, J.; Stamatis, S.; Cheng, J.; Distefano, D.; Kraiouchkine, N.; Shaw, A.; et al. Analysis of varicella zoster virus attenuation by evaluation of chimeric parent Oka/vaccine Oka recombinant viruses in skin xenografts in the SCIDhu mouse model. Virology 2005, 332, 337–346. [Google Scholar] [CrossRef]
- Weibel, R.E.; Neff, B.J.; Kuter, B.J.; Guess, H.A.; Rothenberger, C.A.; Fitzgerald, A.J.; Connor, K.A.; McLean, A.A.; Hilleman, M.R.; Buynak, E.B.; et al. Live attenuated varicella virus vaccine. Efficacy trial in healthy children. N. Engl. J. Med. 1984, 310, 1409–1415. [Google Scholar] [CrossRef]
- Marin, M.; Leung, J.; Gershon, A.A. Transmission of Vaccine-Strain Varicella-Zoster Virus: A Systematic Review. Pediatrics 2019, 144, e20191305. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A.; LaRussa, P.; Steinberg, S. The varicella vaccine. Clinical trials in immunocompromised individuals. Infect. Dis. Clin. N. Am. 1996, 10, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Willis, E.D.; Woodward, M.; Brown, E.; Popmihajlov, Z.; Saddier, P.; Annunziato, P.W.; Halsey, N.A.; Gershon, A.A. Herpes zoster vaccine live: A 10 year review of post-marketing safety experience. Vaccine 2017, 35, 7231–7239. [Google Scholar] [CrossRef]
- Bollaerts, K.; Riera-Montes, M.; Heininger, U.; Hens, N.; Souverain, A.; Verstraeten, T.; Hartwig, S. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: Deriving incidence from seroprevalence data. Epidemiol. Infect. 2017, 145, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Haruyama, C.; Ohba, H.; Wada, A.; Takeuchi, Y. A seroepidemiological study of varicella. Kansenshogaku zasshi J. Jpn. Assoc. Infect. Dis. 1987, 61, 783–788. [Google Scholar] [CrossRef]
- Varis, T.; Vesikari, T. Efficacy of high-titer live attenuated varicella vaccine in healthy young children. J. Infect. Dis. 1996, 174 (Suppl. S3), S330–S334. [Google Scholar] [CrossRef]
- Gershon, A.A.; Steinberg, S.P.; LaRussa, P.; Ferrara, A.; Hammerschlag, M.; Gelb, L. Immunization of healthy adults with live attenuated varicella vaccine. J. Infect. Dis. 1988, 158, 132–137. [Google Scholar] [CrossRef]
- Shapiro, E.D.; Vazquez, M.; Esposito, D.; Holabird, N.; Steinberg, S.P.; Dziura, J.; LaRussa, P.S.; Gershon, A.A. Effectiveness of 2 doses of varicella vaccine in children. J. Infect. Dis. 2011, 203, 312–315. [Google Scholar] [CrossRef]
- Gershon, A.A.; Gershon, M.D.; Shapiro, E.D. Live Attenuated Varicella Vaccine: Prevention of Varicella and of Zoster. J. Infect. Dis. 2021, 224, S387–S397. [Google Scholar] [CrossRef] [PubMed]
- Uda, K.; Okubo, Y.; Tsuge, M.; Tsukahara, H.; Miyairi, I. Impacts of routine varicella vaccination program and COVID-19 pandemic on varicella and herpes zoster incidence and health resource use among children in Japan. Vaccine 2023, 41, 4958–4966. [Google Scholar] [CrossRef]
- Guess, H.A.; Broughton, D.D.; Melton, L.J., 3rd; Kurland, L.T. Epidemiology of herpes zoster in children and adolescents: A population-based study. Pediatrics 1985, 76, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Yabuuchi, H.; Takahashi, M.; Ogra, P.L. Increased incidence of herpes zoster in normal children infected with varicella zoster virus during infancy: Community-based follow-up study. J. Pediatr. 1986, 108, 372–377. [Google Scholar] [CrossRef]
- Terada, K.; Kawano, S.; Yoshihiro, K.; Miyashima, H.; Morita, T. Characteristics of herpes zoster in otherwise normal children. Pediatr. Infect. Dis. J. 1993, 12, 960–961. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chao, Y.H.; Wu, K.H.; Yen, T.Y.; Hsu, Y.L.; Hsieh, T.H.; Wei, H.M.; Wu, J.L.; Muo, C.H.; Hwang, K.P.; et al. Increased risk of herpes zoster in children with cancer: A nationwide population-based cohort study. Medicine 2016, 95, e4037. [Google Scholar] [CrossRef]
- Leung, A.K.; Robson, W.L.; Leong, A.G. Herpes zoster in childhood. J. Pediatr. Health Care 2006, 20, 300–303. [Google Scholar] [CrossRef]
- Weinmann, S.; Chun, C.; Schmid, D.S.; Roberts, M.; Vandermeer, M.; Riedlinger, K.; Bialek, S.R.; Marin, M. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J. Infect. Dis. 2013, 208, 1859–1868. [Google Scholar] [CrossRef]
- Harpaz, R.; Leung, J.W. The Epidemiology of Herpes Zoster in the United States During the Era of Varicella and Herpes Zoster Vaccines: Changing Patterns Among Children. Clin. Infect. Dis. 2019, 69, 345–347. [Google Scholar] [CrossRef]
- Terada, K.; Wakabayashi, S.; Ono, S.; Tanaka, Y.; Kato, A.; Teranishi, H.; Miyata, I.; Ogita, S.; Oishi, T.; Ohno, N.; et al. Characteristics of zoster in otherwise healthy children—A comparative study between 55 zoster patients between 1990 and 2000 (11 years) and 56 from 2001 to 2017 (17 years). Pediatr. Infect. Immun. 2019, 31, 3–6. [Google Scholar]
- Ozaki, T.; Masuda, S.; Asano, Y.; Kondo, K.; Namazue, J.; Yamanishi, K. Investigation of varicella-zoster virus DNA by the polymerase chain reaction in healthy children with varicella vaccination. J. Med. Virol. 1994, 42, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.S.; Hung, C.J.; Lue, K.H. Herpes Zoster in an Immunocompetent Child without a History of Varicella. Pediatr. Rep. 2021, 13, 162–167. [Google Scholar] [CrossRef]
- Ishikawa, H.; Tamai, K.; Mibou, K.; Tunoda, T.; Sawamura, D.; Umeki, K.; Sugawara, T.; Yajima, H.; Sasaki, C.; Kumano, T.; et al. A multicenter, joint annual statistical analysis of herpes zoster (April 2000–March 2001). Jpn. J. Dermatol. 2003, 113, 1229–1239. [Google Scholar] [CrossRef]
- Liang, M.G.; Heidelberg, K.A.; Jacobson, R.M.; McEvoy, M.T. Herpes zoster after varicella immunization. J. Am. Acad. Dermatol. 1998, 38, 761–763. [Google Scholar] [CrossRef]
- Uebe, B.; Sauerbrei, A.; Burdach, S.; Horneff, G. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child. Eur. J. Pediatr. 2002, 161, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Kim, V.; Lavi, S.; Ford-Jones, E.L.; Tipples, G.; Scolnik, D.; Tellier, R. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr. Infect. Dis. J. 2008, 27, 847–848. [Google Scholar] [CrossRef]
- Iwasaki, S.; Motokura, K.; Honda, Y.; Mikami, M.; Hata, D.; Hata, A. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: A case report. J. Clin. Virol. 2016, 85, 44–47. [Google Scholar] [CrossRef]
- Dreyer, S.; Hemarajata, P.; Hogeling, M.; Henderson, G.P. Pediatric vaccine-strain herpes zoster: A case series. Pediatr. Dermatol. 2017, 34, 665–667. [Google Scholar] [CrossRef]
- Moodley, A.; Swanson, J.; Grose, C.; Bonthius, D.J. Severe Herpes Zoster Following Varicella Vaccination in Immunocompetent Young Children. J. Child Neurol. 2019, 34, 184–188. [Google Scholar] [CrossRef]
- Takahashi, M.; Baba, K.; Horiuchi, K.; Kamiya, H.; Asano, Y. A live varicella vaccine. Adv. Exp. Med. Biol. 1990, 278, 49–58. [Google Scholar] [CrossRef]
- Hardy, I.; Gershon, A.A.; Steinberg, S.P.; LaRussa, P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N. Engl. J. Med. 1991, 325, 1545–1550. [Google Scholar] [CrossRef]
- Galea, S.A.; Sweet, A.; Beninger, P.; Steinberg, S.P.; Larussa, P.S.; Gershon, A.A.; Sharrar, R.G. The safety profile of varicella vaccine: A 10-year review. J. Infect. Dis. 2008, 197 (Suppl. S2), S165–S169. [Google Scholar] [CrossRef]
- Goulleret, N.; Mauvisseau, E.; Essevaz-Roulet, M.; Quinlivan, M.; Breuer, J. Safety profile of live varicella virus vaccine (Oka/Merck): Five-year results of the European Varicella Zoster Virus Identification Program (EU VZVIP). Vaccine 2010, 28, 5878–5882. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Ando, Y.; Nakagawa, T.; Gomi, Y. Safety profile of the varicella vaccine (Oka vaccine strain) based on reported cases from 2005 to 2015 in Japan. Vaccine 2016, 34, 4943–4947. [Google Scholar] [CrossRef]
- Petursson, G.; Helgason, S.; Gudmundsson, S.; Sigurdsson, J.A. Herpes zoster in children and adolescents. Pediatr. Infect. Dis. J. 1998, 17, 905–908. [Google Scholar] [CrossRef]
- Amaral, V.; Shi, J.Z.; Tsang, A.M.; Chiu, S.S. Primary varicella zoster infection compared to varicella vaccine reactivation associated meningitis in immunocompetent children. J. Paediatr. Child Health 2021, 57, 19–25. [Google Scholar] [CrossRef]
- Barry, R.; Prentice, M.; Costello, D.; O’Mahony, O.; DeGascun, C.; Felsenstein, S. Varicella Zoster Reactivation Causing Aseptic Meningitis in Healthy Adolescents: A Case Series And Review of the Literature. Pediatr. Infect. Dis. J. 2020, 39, e278–e282. [Google Scholar] [CrossRef]
- Kawamura, Y.; Suzuki, D.; Kono, T.; Miura, H.; Kozawa, K.; Mizuno, H.; Yoshikawa, T. A Case of Aseptic Meningitis Without Skin Rash Caused by Oka Varicella Vaccine. Pediatr. Infect. Dis. J. 2022, 41, 78–79. [Google Scholar] [CrossRef]
- Bierbaum, S.; Fischer, V.; Briedigkeit, L.; Werner, C.; Hengel, H.; Huzly, D. Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy. Vaccines 2023, 11, 309. [Google Scholar] [CrossRef]
- Ramachandran, P.S.; Wilson, M.R.; Catho, G.; Blanchard-Rohner, G.; Schiess, N.; Cohrs, R.J.; Boutolleau, D.; Burrel, S.; Yoshikawa, T.; Wapniarski, A.; et al. Meningitis Caused by the Live Varicella Vaccine Virus: Metagenomic Next Generation Sequencing, Immunology Exome Sequencing and Cytokine Multiplex Profiling. Viruses 2021, 13, 2286. [Google Scholar] [CrossRef]
- Heusel, E.H.; Grose, C. Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus. Viruses 2020, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Dahl, K.M.; Weinberg, A.; Giller, R.; Patel, A.; Krause, P.R. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus, in an immunosuppressed child. J. Infect. Dis. 2003, 188, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Chouliaras, G.; Spoulou, V.; Quinlivan, M.; Breuer, J.; Theodoridou, M. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child. Pediatrics 2010, 125, e969–e972. [Google Scholar] [CrossRef] [PubMed]
- Chaves, S.S.; Haber, P.; Walton, K.; Wise, R.P.; Izurieta, H.S.; Schmid, D.S.; Seward, J.F. Safety of Varicella Vaccine after Licensure in the United States: Experience from Reports to the Vaccine Adverse Event Reporting System, 1995–2005. J. Infect. Dis. 2008, 197, S170–S177. [Google Scholar] [CrossRef]
- Han, J.-Y.; Hanson, D.C.; Way, S.S. Herpes Zoster and Meningitis due to Reactivation of Varicella Vaccine Virus in An Immunocompetent Child. Pediatr. Infect. Dis. J. 2011, 30, 266–268. [Google Scholar] [CrossRef]
- Levin, M.J.; DeBiasi, R.L.; Bostik, V.; Schmid, D.S. Herpes Zoster with Skin Lesions and Meningitis Caused by 2 Different Genotypes of the Oka Varicella-Zoster Virus Vaccine. J. Infect. Dis. 2008, 198, 1444–1447. [Google Scholar] [CrossRef]
- Iyer, S.; Mittal, M.K.; Hodinka, R.L. Herpes Zoster and Meningitis Resulting from Reactivation of Varicella Vaccine Virus in an Immunocompetent Child. Ann. Emerg. Med. 2009, 53, 792–795. [Google Scholar] [CrossRef]
- Pahud, B.A.; Glaser, C.A.; Dekker, C.L.; Arvin, A.M.; Schmid, D.S. Varicella Zoster Disease of the Central Nervous System: Epidemiological, Clinical, and Laboratory Features 10 Years after the Introduction of the Varicella Vaccine. J. Infect. Dis. 2011, 203, 316–323. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Kung, E.; Madhavan, V.; James, A. A Case of Herpes Zoster and Meningitis in a Twice-Vaccinated Healthy Adolescent. J. Pediatr. Infect. Dis. 2017, 12, 142–144. [Google Scholar] [CrossRef]
- Harrington, W.E.; Mató, S.; Burroughs, L.; Carpenter, P.A.; Gershon, A.; Schmid, D.S.; Englund, J.A. Vaccine Oka Varicella Meningitis in Two Adolescents. Pediatrics 2019, 144, e20191522. [Google Scholar] [CrossRef]
- Ramachandran, V.; Elliott, S.C.; Rogers, K.L.; Cohrs, R.J.; Weinberger, M.; Jackson, W.; Carpenter, J.E.; Grose, C.; Bonthius, D.J. Varicella Vaccine Meningitis as a Complication of Herpes Zoster in Twice-Immunized Immunocompetent Adolescents. J. Child Neurol. 2020, 35, 889–895. [Google Scholar] [CrossRef]
- Rand, K.; He, K.; Bard, J.D.; Braskett, M.; Mohandas, S.; Burnham, C.-A.D. The Brief Case: Vaccine strain herpes zoster ophthalmicus and meningoencephalitis in an immunocompetent child. J. Clin. Microbiol. 2023, 61, e0071422. [Google Scholar] [CrossRef]
- Daouk, S.K.; Kamau, E.; Adachi, K.; Aldrovandi, G.M. Zoster Meningitis in an Immunocompetent Child after COVID-19 Vaccination, California, USA. Emerg. Infect. Dis. 2022, 28, 1523–1524. [Google Scholar] [CrossRef]
- Kincaid, A.E. The Role of the Nasal Cavity in the Pathogenesis of Prion Diseases. Viruses 2021, 13, 2287. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.N. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am. J. Ophthalmol. 1994, 117, 663–667. [Google Scholar] [CrossRef]
- Oxman, M.N.; Levin, M.J.; Johnson, G.R.; Schmader, K.E.; Straus, S.E.; Gelb, L.D.; Arbeit, R.D.; Simberkoff, M.S.; Gershon, A.A.; Davis, L.E.; et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005, 352, 2271–2284. [Google Scholar] [CrossRef] [PubMed]
- Heath, G.; Depledge, D.P.; Brown, J.R.; Hale, A.D.; Tutil, H.; Williams, R.; Breuer, J. Acute Retinal Necrosis Caused by the Zoster Vaccine Virus. Clin. Infect. Dis. 2017, 65, 2122–2125. [Google Scholar] [CrossRef]
- Hayat, U.; Afroz, S. Generalized Rash and Bilateral Retinal Necrosis in an Adult Healthcare Worker after Post-Exposure Herpes Zoster Vaccination: A Rare Case Report. Kans. J. Med. 2020, 13, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://id-info.jihs.go.jp/surveillance/nesvpd/graph/YearComparison/vzv2024/2024/20250602154218.html (accessed on 8 June 2025).
- Available online: https://www.mhlw.go.jp/topics/bcg/other/5.html (accessed on 8 June 2025).
- Gershon, A.A.; LaRussa, P.; Steinberg, S.; Mervish, N.; Lo, S.H.; Meier, P. The protective effect of immunologic boosting against zoster: An analysis in leukemic children who were vaccinated against chickenpox. J. Infect. Dis. 1996, 173, 450–453. [Google Scholar] [CrossRef]
- Hope-Simpson, R.E. The Nature of Herpes Zoster: A Long-Term Study and A New Hypothesis. Proc. R. Soc. Med. 1965, 58, 9–20. [Google Scholar] [CrossRef]
- Mehta, S.K.; Cohrs, R.J.; Forghani, B.; Zerbe, G.; Gilden, D.H.; Pierson, D.L. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J. Med. Virol. 2004, 72, 174–179. [Google Scholar] [CrossRef] [PubMed]
- White, C.J. Clinical trials of varicella vaccine in healthy children. Infect. Dis. Clin. N. Am. 1996, 10, 595–608. [Google Scholar] [CrossRef]
- Gaillat, J.; Gajdos, V.; Launay, O.; Malvy, D.; Demoures, B.; Lewden, L.; Pinchinat, S.; Derrough, T.; Sana, C.; Caulin, E.; et al. Does monastic life predispose to the risk of Saint Anthony’s fire (herpes zoster)? Clin. Infect. Dis. 2011, 53, 405–410. [Google Scholar] [CrossRef]
- Brisson, M.; Gay, N.J.; Edmunds, W.J.; Andrews, N.J. Exposure to varicella boosts immunity to herpes-zoster: Implications for mass vaccination against chickenpox. Vaccine 2002, 20, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, R. Do varicella vaccination programs change the epidemiology of herpes zoster? A comprehensive review, with focus on the United States. Expert Rev. Vaccines 2019, 18, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Zussman, J.; Young, L. Zoster vaccine live for the prevention of shingles in the elderly patient. Clin. Interv. Aging 2008, 3, 241–250. [Google Scholar] [CrossRef]
- Mbinta, J.F.; Nguyen, B.P.; Awuni, P.M.A.; Paynter, J.; Simpson, C.R. Post-licensure zoster vaccine effectiveness against herpes zoster and postherpetic neuralgia in older adults: A systematic review and meta-analysis. Lancet Healthy Longev. 2022, 3, e263–e275. [Google Scholar] [CrossRef]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Díez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barberà, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef]
- Racine, É.; Gilca, V.; Amini, R.; Tunis, M.; Ismail, S.; Sauvageau, C. A systematic literature review of the recombinant subunit herpes zoster vaccine use in immunocompromised 18–49 year old patients. Vaccine 2020, 38, 6205–6214. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, T.; Zhao, W.; Shao, A.; Zhao, H.; Ma, W.; Gong, Y.; Zeng, X.; Weng, C.; Bu, L.; et al. Herpes zoster mRNA vaccine induces superior vaccine immunity over licensed vaccine in mice and rhesus macaques. Emerg. Microbes Infect. 2024, 13, 2309985. [Google Scholar] [CrossRef]
- Scheifele, D.W.; Halperin, S.A.; Diaz-Mitoma, F. Three-year follow-up of protection rates in children given varicella vaccine. Can. J. Infect. Dis. Med. Microbiol. 2002, 13, 382–386. [Google Scholar] [CrossRef]
- Vessey, S.J.; Chan, C.Y.; Kuter, B.J.; Kaplan, K.M.; Waters, M.; Kutzler, D.P.; Carfagno, P.A.; Sadoff, J.C.; Heyse, J.F.; Matthews, H.; et al. Childhood vaccination against varicella: Persistence of antibody, duration of protection, and vaccine efficacy. J. Pediatr. 2001, 139, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Takayama, N.; Minamitani, M.; Takayama, M. High incidence of breakthrough varicella observed in healthy Japanese children immunized with live attenuated varicella vaccine (Oka strain). Acta Paediatr. Jpn. Overseas Ed. 1997, 39, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Kuter, B.; Matthews, H.; Shinefield, H.; Black, S.; Dennehy, P.; Watson, B.; Reisinger, K.; Kim, L.L.; Lupinacci, L.; Hartzel, J.; et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr. Infect. Dis. J. 2004, 23, 132–137. [Google Scholar] [CrossRef]
- Cohen, J.I. Varicella-zoster vaccine virus: Evolution in action. Proc. Natl. Acad. Sci. USA 2007, 104, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, M.L.; Gershon, A.A.; Al Bassam, M.M.; Steinberg, S.P.; LaRussa, P.; Nichols, R.A.; Breuer, J. Natural selection for rash-forming genotypes of the varicella-zoster vaccine virus detected within immunized human hosts. Proc. Natl. Acad. Sci. USA 2007, 104, 208–212. [Google Scholar] [CrossRef]

| No | Age | Age at Vaccine | Varicella History | Interval | Region | Strain | Virus Isolation | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 y 7 m | 1 y 3 m | yes (7 m) | 4 m | L C6-7 | vaccine | skin | J. Am. Acad. Dermatol. 1998; 38, 761–763 [66]. |
| 2 | 2 y 3 m | 11 m | no | 1 y 4 m | R C6-8 | vaccine | skin | Eur. J. Pediatr. 2002; 161, 442–444 [67]. |
| 3 | 2 y 4 m | 1 y 1 m | N/A | 1 y 3 m | L chest-upper limb | vaccine | skin | Pediatr. Infect. Dis. J. 2008; 27, 847–848 [68]. |
| 4 | 2 y | 1 y 5 m | no | 7 m | L V1-2 | vaccine | skin | J. Clin. Virol. 2016; 85, 44–47 [69] |
| 5 | 3 y | 1 y | N/A | 2 y | R L2 | vaccine | skin | Pediatr. Dermatol. 2017; 34, 665–667 [70]. |
| 6 | 2 y | 1 y | no | 1 y | L L4 | vaccine | skin | Pediatr. Dermatol. 2017; 34, 665–668 [70]. |
| 7 | 3 y 3 m | 1 y 8 m | no | 1 y 7 m | L L4-S1 | variant of vaccine | skin | J. Child Neurol. 2019; 34, 184–188 [71] |
| No | Age at 1st Vaccine | Age at 2nd Vaccine | Age at Meningitis | Site of Singles | Immunocompromised | References |
|---|---|---|---|---|---|---|
| 1 | 1 y | none | 1.3 y | Lumbar | yes | J Infect Dis. 2003; 188: 954–9 [84]. |
| 2 | 1.8 y | none | 3 y | Trigeminal | no | Pediatrics. 2010; 125: e969–72 [85]. |
| 3 | 1.3 y | none | 4 y | Cervical | no | J Infect Dis. 2008; 197 (Suppl 2): S170–7 [86]. |
| 4 | 2.4 y | none | 4 y | Cervical | yes | J Infect Dis. 2008; 197 (Suppl 2): S170–7 [86]. |
| 5 | 1 y | none | 7 y | Cervical | no | Pediatr. Infect. Dis. J. 2011; 30, 266–268 [87]. |
| 6 | 1 y | none | 8 y | Cervical | no | J Infect Dis. 2008; 198: 1444–7 [88]. |
| 7 | 1 y | none | 9 y | Cervical | no | Ann Emerg Med. 2009; 53: 792–5 [89]. |
| 8 | 1 y | none | 12 y | Cervical | no | J Infect Dis. 2011; 203: 316–23 [90]. |
| 9 | 1.5 y | 12 y | 14 y | Thoracic | no | J. Pediatr. Infect. Dis. 2017; 12, 142–144 [91]. |
| 10 | 1 y | 4 y | 15 y | Lumbar | no | Pediatrics 2019; 144, e20191522 [92]. |
| 11 | 1 y | 10 y | 16 y | Thoracic | yes | Pediatrics 2019; 144, e20191522 [92]. |
| 12 | 1 y | 5 y | 14 y | Lumbar | no | J. Child Neurol. 2020; 35(13): 889-895 [93]. |
| 13 | yes | yes | 11 y | Trigeminal | no | J Clin Microbiol. 2023; 61(8): e0071422 [94]. |
| 14 | 1 y | 1.5 y | 12 y | Lumbar | no | Emerg Infect Dis. 2022; 28(7): 1523–1524 [95]. |
| 15 | 1 y | 5 y | 14 y | Yes (unknown) | no | Viruses 2021; 13(11):2287 [96]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagasawa, M. Perspectives on the History and Epidemiology of the Varicella Virus Vaccine and Future Challenges. Pathogens 2025, 14, 813. https://doi.org/10.3390/pathogens14080813
Nagasawa M. Perspectives on the History and Epidemiology of the Varicella Virus Vaccine and Future Challenges. Pathogens. 2025; 14(8):813. https://doi.org/10.3390/pathogens14080813
Chicago/Turabian StyleNagasawa, Masayuki. 2025. "Perspectives on the History and Epidemiology of the Varicella Virus Vaccine and Future Challenges" Pathogens 14, no. 8: 813. https://doi.org/10.3390/pathogens14080813
APA StyleNagasawa, M. (2025). Perspectives on the History and Epidemiology of the Varicella Virus Vaccine and Future Challenges. Pathogens, 14(8), 813. https://doi.org/10.3390/pathogens14080813






