Candidemia: An Update on Epidemiology, Risk Factors, Diagnosis, Susceptibility, and Treatment
Abstract
1. Introduction
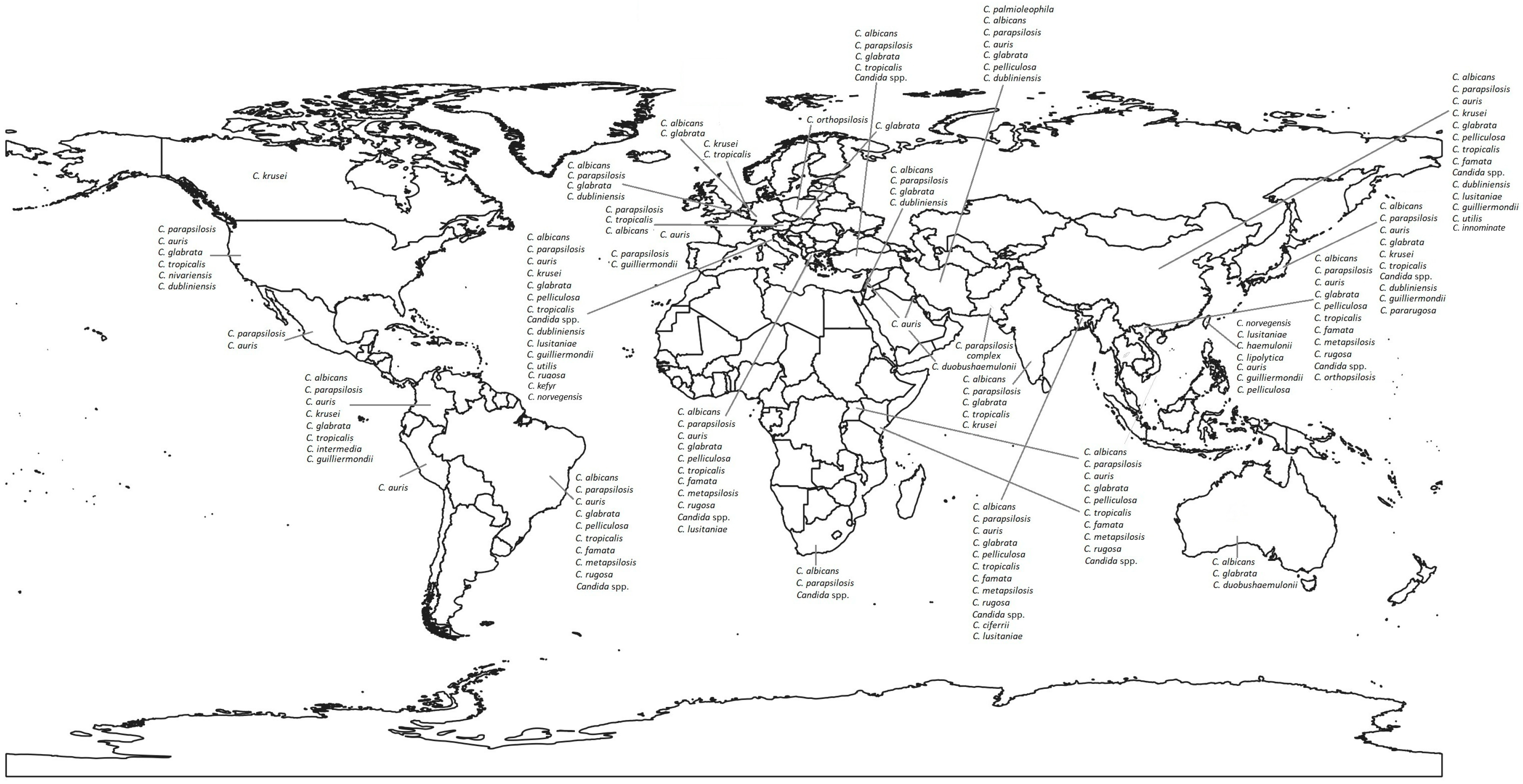
2. Main Etiological Agents of Candidemia
3. Candida spp. Susceptibility to Antifungals
3.1. Asia
3.2. Europe
3.3. America
3.4. Africa
3.5. Oceania
4. Mechanisms of Antifungal Resistance
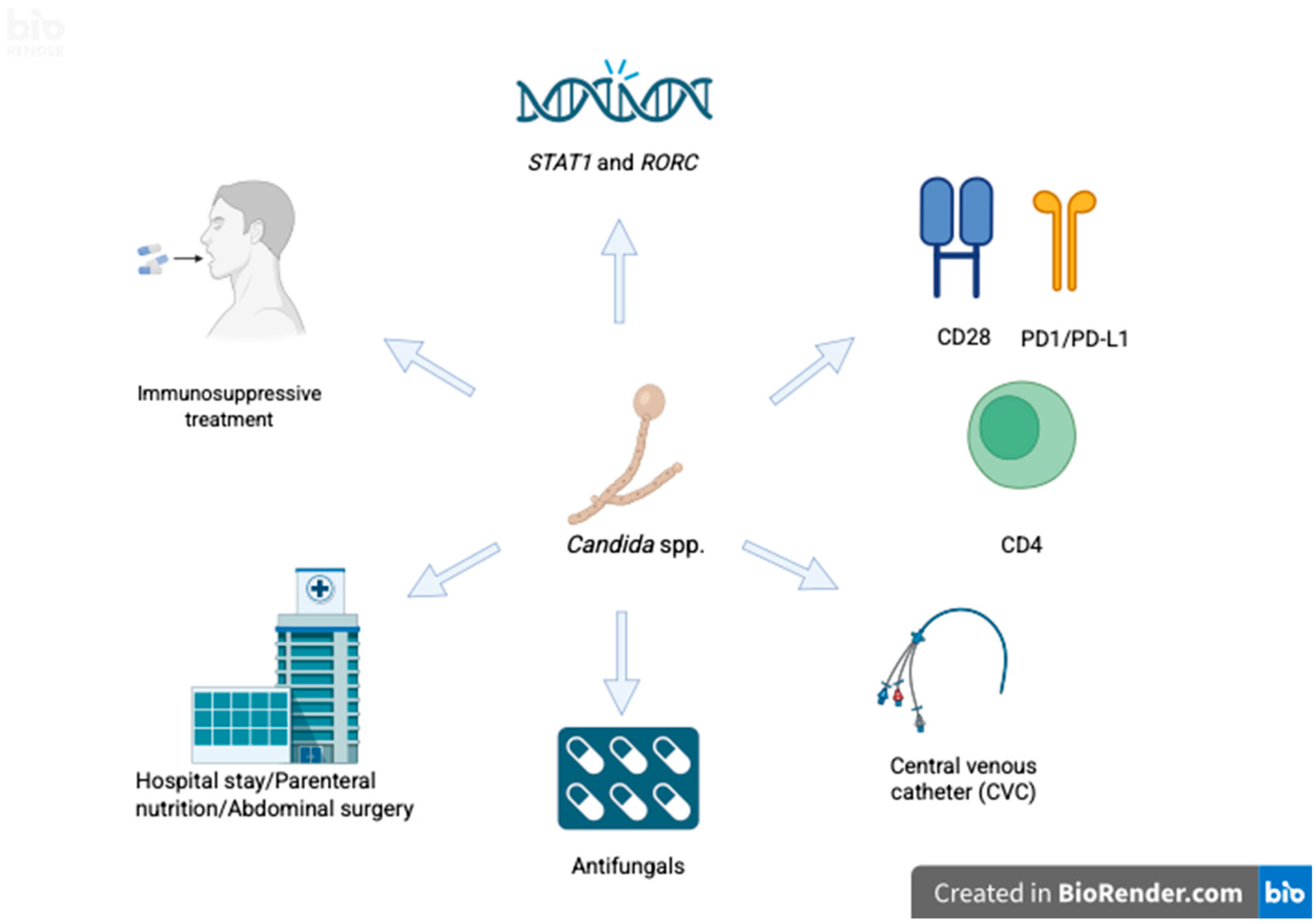
5. Risk Factors for Candidemia
5.1. Host-Related Factors
5.1.1. Immunosuppression and Microbiota
5.1.2. Chronic Diseases and Age
5.2. Factors Related to Hospital Care/Intensive Care Unit
5.2.1. Health Care
5.2.2. Use of Broad-Spectrum Antibiotics
5.2.3. Central Venous Catheter
5.2.4. Total Parenteral Nutrition
5.2.5. Development of Biofilms
5.2.6. Abdominal Surgery
6. Diagnosis of Candidemia
6.1. Blood Cultures and Histopathology
6.2. Molecular-Based Techniques
6.2.1. Polymerase Chain Reaction (PCR)
6.2.2. Next-Generation Sequencing
6.2.3. T2 Candida
6.2.4. New Molecular Tools
6.2.5. Matrix-Assisted Laser Desorption–Ionization Time-of-Flight (MALDI-TOF)
6.3. Non-Culture-Based Methods
6.3.1. 1,3 Beta-D-Glucano
6.3.2. Biosensors for the Identification of Candida spp.
7. Treatment of Candidemia
7.1. Antifungal Treatment
7.2. Profilaxis
7.3. Newborn Patients Weighing < 1000 g
7.4. Patients with Prolonged Neutropenia
7.5. Critically Ill Patients with Abdominal Surgery
7.6. Anticipatory Therapy
7.7. Therapy
7.8. Monitoring
7.9. New Antifungal Drugs
7.10. Other Considerations
7.11. Removal of Vascular Access in Patients Without Neutropenia
7.12. Ophthalmological Evaluation
7.13. Assessment by an Infectious Disease/Microbiology Specialist
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mora Carpio, A.L.; Climaco, A. Candidemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R.; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Prim. 2024, 10, 20. [Google Scholar] [CrossRef]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef]
- Uraguchi, K.; Makino, T.; Kariya, S.; Noda, Y.; Marunaka, H.; Doi, A.; Kozakura, K.; Takao, S.; Ando, M. Candidemia in patients with head and neck cancer: Mortality and a novel risk factor. Support. Care Cancer 2022, 30, 5921–5930. [Google Scholar] [CrossRef] [PubMed]
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Tejera, E.; Machado, A. Prevalence of biofilms in Candida spp. bloodstream infections: A meta-analysis. PLoS ONE 2022, 17, e0263522. [Google Scholar] [CrossRef] [PubMed]
- Harchand, R.; Spruijtenburg, B.; Meijer, E.F.J.; de Groot, T.; Rudramurthy, S.M.; Meis, J.F. Candida vulturna, the Next Fungal Menace? A Narrative Review. Mycoses 2025, 68, e70070. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Camacho, P.M.; Vargas-Moran, C.; Torres-Canchala, L.; Ariza-Insignares, C.; Sandoval-Calle, L.M.; Gómez-Hernández, I.E.; Solís-Núñez, P.; Cedeño-Castaño, J.V.; Aguilar-González, A.M.; Patiño-Niño, J.A. Epidemiological characteristics of pediatric patients with invasive candidiasis in an intensive care unit in southwestern Colombia. Biomedica 2025, 45, 151–164. [Google Scholar] [CrossRef]
- Cook, A.; Ferreras-Antolin, L.; Adhisivam, B.; Ballot, D.; Berkley, J.A.; Bernaschi, P.; Carvalheriro, C.G.; Chaikittisuk, N.; Chen, Y.; Chibabhai, V. Neonatal invasive candidiasis in low- and middle-income countries: Data from the NeoOBS study. Med. Mycol. 2023, 61, myad010. [Google Scholar] [CrossRef]
- Brescini, L.; Mazzanti, S.; Orsetti, E.; Morroni, G.; Masucci, A.; Pocognoli, A.; Barchiesi, F. Species distribution and antifungal susceptibilities of bloodstream Candida isolates: A nine-years single center survey. J. Chemother. 2020, 32, 244–250. [Google Scholar] [CrossRef]
- Posteraro, B.; De Carolis, E.; Criscuolo, M.; Ballanti, S.; De Angelis, G.; Del Principe, M.I.; Delia, M.; Fracchiolla, N.; Marchesi, F.; Nadali, G. Candidaemia in haematological malignancy patients from a SEIFEM study: Epidemiological patterns according to antifungal prophylaxis. Mycoses 2020, 63, 900–910. [Google Scholar] [CrossRef]
- Egger, M.; Salmanton-García, J.; Barac, A.; Gangneux, J.P.; Guegan, H.; Arsic-Arsenijevic, V.; Matos, T.; Tomazin, R.; Klimko, N.; Bassetti, M.; et al. ECMM Candida III Study Group Predictors for Prolonged Hospital Stay Solely to Complete Intravenous Antifungal Treatment in Patients with Candidemia: Results from the ECMM Candida III multinational European observational cohort study. Mycopathologia 2023, 188, 983–994. [Google Scholar] [CrossRef]
- Liu, T.; Sun, S.; Zhu, X.; Wu, H.; Sun, Z.; Peng, S. Epidemiology, clinical characteristics, and outcome in candidemia: A retrospective five-year analysis from two tertiary general hospitals. BMC Infect. Dis. 2025, 25, 512. [Google Scholar] [CrossRef]
- Zhang, W.; Song, X.; Wu, H.; Zheng, R. Epidemiology, species distribution, and predictive factors for mortality of candidemia in adult surgical patients. BMC Infect. Dis. 2020, 20, 506. [Google Scholar] [CrossRef]
- Kajihara, T.; Yahara, K.; Nagi, M.; Kitamura, N.; Hirabayashi, A.; Hosaka, Y.; Abe, M.; Miyazaki, Y.; Sugai, M. Distribution, trends, and antifungal susceptibility of Candida species causing candidemia in Japan, 2010–2019: A retrospective observational study based on national surveillance data. Med. Mycol. 2022, 60, myac071. [Google Scholar] [CrossRef] [PubMed]
- Doğan, Ö.; Yeşilkaya, A.; Menekşe, Ş.; Güler, Ö.; Karakoç, Ç.; Çınar, G.; Kapmaz, M.; Aydin, M.; Keske, S.; Sahin, S.; et al. Effect of initial antifungal therapy on mortality among patients with bloodstream infections with different Candida species and resistance to antifungal agents: A multicentre observational study by the Turkish Fungal Infections Study Group. Int. J. Antimicrob. Agents 2020, 56, 105992. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Yazdanpanah, S.; Bakhtiari, M.; Fang, W.; Pan, W.; Mahmoudi, S.; Pakshir, K.; Daneshina, F.; Boekhout, T.; Ilkit, M.; et al. Epidemiology of candidemia in Shiraz, southern Iran: A prospective multicenter study (2016–2018). Med. Mycol. 2021, 59, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Szekely, J.; Rakchang, W.; Rattanaphan, P.; Kositpantawong, N. Fluconazole and echinocandin resistance of Candida species in invasive candidiasis at a university hospital during pre-COVID-19 and the COVID-19 outbreak. Epidemiol. Infect. 2023, 151, e146. [Google Scholar] [CrossRef]
- Pappas, P.G.; Vazquez, J.A.; Oren, I.; Rahav, G.; Aoun, M.; Bulpa, P.; Ben-Ami, R.; Ferrer, R.; Mccarty, T.; Thompson, G.R.; et al. Clinical safety and efficacy of novel antifungal, fosmanogepix, for the treatment of candidaemia: Results from a Phase 2 trial. J. Antimicrob. Chemother. 2023, 78, 2471–2480. [Google Scholar] [CrossRef]
- Shuping, L.; Mpembe, R.; Mhlanga, M.; Naicker, S.D.; Maphanga, T.G.; Tsotetsi, E.; Wadula, J.; Velaphi, S.; Nakwa, F.; Chibabhai, V. Epidemiology of culture-confirmed candidemia among hospitalized children in South Africa, 2012–2017. Pediatr. Infect. Dis. J. 2021, 40, 730–737. [Google Scholar] [CrossRef]
- Huang, Y.S.; Wang, F.D.; Chen, Y.C.; Huang, Y.T.; Hsieh, M.H.; Hii, I.M.; Lee, Y.L.; Ho, M.W.; Liu, C.E.; Chen, Y.H.; et al. High rates of misidentification of uncommon Candida species causing bloodstream infections using conventional phenotypic methods. J. Formos. Med. Assoc. 2021, 120, 1179–1187. [Google Scholar] [CrossRef]
- Mirza, A.; Senol, E.; Kalkanci, A. Epidemiology and risk factors of candidemia among hospitalized patients in a Turkish tertiary care hospital. Clin. Lab. 2022, 68. [Google Scholar] [CrossRef] [PubMed]
- Sathi, F.A.; Alam, M.M.; Haque, N.; Khan, M.J.A.; Mamun, A.A.; Hossain, T.; Chowdhury, C.S.; Islam, M.N.; Ferdaus, S.J.; Sultana, M. An alarming rise of candidemia caused by non-albicans Candida species in intensive care unit in Mymensingh, Bangladesh. Mymensingh Med. 2024, 33, 671–676. [Google Scholar]
- Corzo-Leon, D.E.; Peacock, M.; Rodriguez-Zulueta, P.; Salazar-Tamayo, G.J.; MacCallum, D.M. General hospital outbreak of invasive candidiasis due to azole-resistant Candida parapsilosis associated with an Erg11 Y132F mutation. Med. Mycol. 2021, 59, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Kerai, A.; Doshi, S.; Laleker, A.; Majumdar, A. Fungal foe and mechanical hearts: A retrospective case series on Candida auris bloodstream infection with left ventricular assist devices. Open Forum Infect. Dis. 2024, 11, ofae286. [Google Scholar] [CrossRef] [PubMed]
- Cabanilla, M.G.; Villalobos, N. A successful daptomycin and micafungin dosing strategy in veno-venous ECMO and continuous renal replacement. J. Clin. Pharm. Ther. 2022, 47, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Bartolo, C.; Hall, V.; Friedman, N.D.; Lanyon, C.; Fuller, A.; Morrissey, C.O.; Athan, E. Bittersweet: Infective complications of drug-induced glycosuria in patients with diabetes mellitus on SGLT2-inhibitors: Two case reports. BMC Infect. Dis. 2021, 21, 284. [Google Scholar] [CrossRef]
- Rahimli, L.; Salmanton-García, J.; Kasper, P.; Simon, M.; Cornely, O.A.; Stemler, J. Necrotizing pancreatitis with invasive candidiasis and candidemia due to Candida albicans and pan-echinocandin-resistant Candidaglabrata. Med. Mycol. Case Rep. 2024, 43, 100636. [Google Scholar] [CrossRef]
- Rodríguez de la Garza, P.; Cruz-de la Cruz, C.; Bejarano, J.I.C.; Romo, A.E.L.; Delgado, J.V.; Ramos, B.A.; Martínez Neira, M.N.; Siller Rodríguez, D.; Sánchez Rodríguez, H.M.; Rangel Selvera, O.A. A multicentric outbreak of Candida auris in Mexico: 2020 to 2023. Am. J. Infect. Control 2024, 52, 1384–1389. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Pappas, P.G.; Boffard, K.; Paruk, F.; Bien, P.A.; Tawadrous, M.; Ople, E.; Wedel, P.; Oborska, I.; Hodges, M.R. Clinical Efficacy and safety of a novel antifungal, fosmanogepix, in patients with candidemia caused by Candida auris: Results from a phase 2 trial. Antimicrob. Agents Chemother. 2023, 67, e0141922. [Google Scholar] [CrossRef]
- Randazza, O.; Erickson, K.; Denmeade, T.; Luther, V.; Palavecino, E.; Beardsley, J. Treatment of Candida nivariensis blood stream infection with oral isavuconazole. Cureus 2022, 14, e32137. [Google Scholar] [CrossRef]
- Griffith, E.M.; Marsalisi, C.; Verdecia, J.; Buchanan, S.R.; Goulart, M.A. Recurrent fungemia due to Candida auris. Cureus 2024, 16, e62478. [Google Scholar] [CrossRef]
- Lingas, E.; Lucio Paredes, M.D.M.; Jahan, M.; Bathina, P.; Dahdouh, M. A Case of Candida auris candidemia in an immunocompetent traumatic brain injury patient post ventriculoperitoneal shunt and peripherally inserted central catheter line. Cureus 2020, 12, e8850. [Google Scholar] [CrossRef]
- Xie, O.; Streitberg, R.; Hughes, C.; Stuart, R.; Graham, M. Candida duobushaemulonii sepsis and Candida auris co-isolation following hospitalisation in Vietnam. Pathology 2020, 52, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Katsiari, M.; Nikolaou, C.; Palla, E.; Theodoridou, K.; Tsakris, A.; Vrioni, G. Impact of Candida auris on critically ill patients: A three-year observational study in a Greek intensive care unit. Pathogens 2025, 14, 328. [Google Scholar] [CrossRef]
- Rimoldi, S.G.; Nodari, R.; Rizzo, A.; Tamoni, A.; Longobardi, C.; Pagani, C.; Grosso, S.; Salari., F.; Galimberti, L.; Olivieri, P. First imported case of Candida auris infection in Milan, Italy: Genomic characterisation. Infection 2024, 52, 1633–1638. [Google Scholar] [CrossRef]
- Stolfa, S.; Caggiano, G.; Ronga, L.; Dalfino, L.; Centrone, F.; Sallustio, A.; Sacco, D.; Mosca, A.; Stufano, M.; Saracino, A.; et al. First case of Candida auris Sepsis in Southern Italy: Antifungal susceptibility and genomic characterisation of a difficult-to-treat emerging yeast. Microorganisms 2024, 12, 1962. [Google Scholar] [CrossRef]
- Reque, J.; Arlandis, R.; Panizo, N.; Pascual, M.J.; Perez-Alba, A. Candida auris invasive infection after kidney transplantation. Case Rep. Nephrol. 2022, 2022, 6007607. [Google Scholar] [CrossRef] [PubMed]
- Apsemidou, A.; Füller, M.A.; Idelevich, E.A.; Kurzai, O.; Tragiannidis, A.; Groll, A.H. Candida lusitaniae breakthrough fungemia in an immuno-compromised adolescent: Case report and review of the literature. J. Fungi 2020, 6, 380. [Google Scholar] [CrossRef]
- Mnichowska-Polanowska, M.; Adamowicz, M.; Wojciechowska-Koszko, I.; Kisiel, A.; Wojciuk, B.; Jarosz, K.; Dolegowska, B. Molecular investigation of the fatal bloodstream Candida orthopsilosis infection case following gastrectomy. Int. J. Mol. Sci. 2023, 24, 6541. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Lu, P.L.; Tang, H.J.; Huang, C.H.; Hung, W.C.; Tseng, Y.T.; Lee, K.-M.; Lin, S.-Y. The first invasive Candida auris infection in Taiwan. Emer. Microbes Infect. 2022, 11, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Matono, T.; Suzuki, S.; Yoshino, S.; Alshahni, M.M.; Komori, A.; Makimura, K. The first case of clade I Candida auris candidemia in a patient with COVID-19 in Japan. J. Infect. Chemother. 2023, 29, 713–717. [Google Scholar] [CrossRef]
- Murata, S.; Mimura, K.; Kawamura, T.; Saito, H.; Ohno, H.; Tsujii, E.; Shinohara, T.; Miyazaki, Y.; Ohki, T. Bloodstream infection caused by Wickerhamiella pararugosa in a patient with intestinal obstruction: A case report. J. Infect. Chemother Off. J. Jpn. Soc. Chemother. 2024, 30, 942–945. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Han, R.; Ding, C.; Shou, H.; Duan, X.; Zhang, S. A candidemia case caused by a novel drug-resistant Candida auris with the Y132F mutation in Erg11 in Mainland China. Infect. Drug Resist. 2023, 16, 3065–3072. [Google Scholar] [CrossRef]
- Aboutalebian, S.; Nikmanesh, B.; Mohammadpour, M.; Charsizadeh, A.; Mirhend, H. Candida palmioleophila candidemia and bacterial co-infection in a 3-month-old infant with biliary atresia. Front. Cell Infect. Microbiol. 2023, 13, 1277607. [Google Scholar] [CrossRef]
- Awada, B.; Alam, W.; Chalfoun, M.; Araj, G.; Bizri, A.R. COVID-19 and Candida duobushaemulonii superinfection: A case report. J. Mycol. Med. 2021, 31, 101168. [Google Scholar] [CrossRef]
- Alashqar, M.B.; Alabdan, L.; Khan, M.; Almakadma, A.H.; Almustanyir, S. A case report of a Candida auris infection in Saudi Arabia. Cureus 2021, 13, e15240. [Google Scholar] [CrossRef] [PubMed]
- Mirhendi, H.; Charsizadeh, A.; Aboutalebian, S.; Mohammadpour, M.; Nikmanesh, B.; de Groot, T.; Meis, J.F.; Badali, H. South Asian (Clade I) Candida auris meningitis in a paediatric patient in Iran with a review of the literature. Mycoses 2022, 65, 134–139. [Google Scholar] [CrossRef]
- Mallick, D.C.; Kaushik, N.; Goyal, L.; Mallick, L.; Singh, P. A Comprehensive Review of Candidemia and Invasive Candidiasis in Adults: Focus on the Emerging Multidrug-Resistant Fungus Candida auris. Diseases 2025, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, C.; Yang, Y.; Ding, Y.; Ye, C.; Tang, M.; Liu, J.; Zeng, Z. Epidemiology, antifungal susceptibility, risk factors, and mortality of persistent candidemia in adult patients in China: A 6-year multicenter retrospective study. BMC Infect. Dis. 2023, 23, 369. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Ding, Y.; Tian, G.; Yang, K.; Deng, J.; Li, G.; Liu, J. A seven-year surveillance study of the epidemiology, antifungal susceptibility, risk factors and mortality of candidaemia among paediatric and adult inpatients in a tertiary teaching hospital in China. Antimicrob. Resist. Infect. Control 2020, 9, 133. [Google Scholar] [CrossRef]
- Zeeshan, M.; Memon, S.; Malick, A.; Naqvi, S.F.; Farooqi, J.; Ghanchi, N.K.; Jabeen, K. Fluconazole-resistant Candida parapsilosis complex candidemia and analysis of mutations in the ERG11 gene from Pakistan. Mycoses 2024, 67, e13677. [Google Scholar] [CrossRef]
- Salmanton-García, J.; Cornely, O.A.; Stemler, J.; Barać, A.; Steinmann, J.; Siváková, A.; Akalin, E.H.; Arikan-Akdagli, S.; Loughlin, L.; Toscano, C.; et al. Attributable mortality of candidemia—Results from the ECMM Candida III multinational European Observational Cohort Study. J. Infect. 2024, 89, 106229. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Mokaddas, E.; Al-Banwan, K.; Alfouzan, W.; Al-Obaid, I.; Al-Obaid, K.; Asadzadeh, M.; Jeragh, A.; et al. Changing trends in epidemiology and antifungal susceptibility patterns of six bloodstream Candida species isolates over a 12-year period in Kuwait. PLoS ONE 2019, 14, e0216250. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20 (Suppl. 6), 5–10. [Google Scholar] [CrossRef] [PubMed]
- Lamba, M.; Sharma, D.; Sharma, R.; Vyas, A.; Mamoria, V. To study the profile of Candida isolates and antifungal susceptibility pattern of neonatal sepsis in a tertiary care hospital of North India. J. Matern. Fetal Neonatal Med. 2021, 34, 2655–2659. [Google Scholar] [CrossRef]
- Subba, S.H.; Sharma, T.D.; Dopthapa, Y.P.; Bhutia, U.G.N.; Sharma, R. Candida glabrata meningitis in a patient with newly diagnosed acquired immunodeficiency syndrome from Sikkim, India. Curr. Med. Mycol. 2024, 10, e2024.345242.1542. [Google Scholar] [CrossRef]
- Najafzadeh, M.J.; Shaban, T.; Zarrinfar, H.; Sedaghat, A.; Hosseinikargar, N.; Berenji, F.; Jalali, M.; Lackner, M.; James, J.E.; Iikit, M. COVID-19 associated candidemia: From a shift in fungal epidemiology to a rise in azole drug resistance. Med. Mycol. 2024, 62, myae031. [Google Scholar] [CrossRef]
- Arastehfar, A.; Hilmioğlu-Polat, S.; Daneshnia, F.; Hafez, A.; Salehi, M.; Polat, F.; Yasar, M.; Arslan, N.; Hosbul, T.; Unal, N.; et al. Recent increase in the prevalence of fluconazole-non-susceptible Candida tropicalis blood isolates in Turkey: Clinical implication of azole-non-susceptible and fluconazole tolerant phenotypes and genotyping. Front. Microbiol. 2020, 11, 587278. [Google Scholar] [CrossRef] [PubMed]
- Ponta, G.; Morena, V.; Strano, M.; Molteni, C.; Pontiggia, S.; Cavalli, E.M.; Grancini, A.; Mauri, C.; Castagna, A.; Galanti, A.; et al. Safety of rezafungin as a long-term treatment option in two patients with complicated fungal infections: Two cases from Lecco Hospital (Italy). Antimicrob. Agents Chemother. 2024, 68, e0075024. [Google Scholar] [CrossRef]
- Trovato, L.; Bongiorno, D.; Calvo, M.; Migliorisi, G.; Boraccino, A.; Musso, N.; Oliveri, S.; Stefani, S.; Scalia, G. Resistance to echinocandins complicates a case of Candida albicans bloodstream infection: A case report. J. Fungi 2021, 7, 405. [Google Scholar] [CrossRef]
- Teixeira da Silva, F.; Cardoso, F.S.; Esteves, A.; Carvalho, J.; Maia, R. Relapsing Candida parapsilosis endocarditis with septic embolization: A case report. Cureus 2021, 13, e13159. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Hafez, A.; Khodavaisy, S.; Najafzadeh, M.J.; Charsizadeh, A.; Zarrinfar, H.; Salehi, M.; Shahrabadi, Z.Z.; Sasani, E. Antifungal susceptibility, genotyping, resistance mechanism, and clinical profile of Candida tropicalis blood isolates. Med. Mycol. 2020, 58, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.; Oravec, T.; Nishi, C.; Eckbo, E.; Marcon, K.; Wright, A.; Mah, A.; White, J.; Nevill, T.; Belga, S. Severe hypercalcemia as a result of disseminated Candida krusei infection. Int. J. Infect. Dis. 2024, 140, 110–112. [Google Scholar] [CrossRef]
- Prabhudas-Strycker, K.K.; Butt, S.; Reddy, M.T. Candida tropicalis endocarditis successfully treated with AngioVac and micafungin followed by long-term isavuconazole suppression. IDCases 2020, 21, e00889. [Google Scholar] [CrossRef]
- Paucar-Miranda, C.J.; Sandoval-Ahumada, R.E.; López-Martínez, R.L.; Terrel-Gutierrez, L.; Zurita-Macalapu, S.; Urcia-Ausejo, F.; Flores-León, D.; Gavilan-Chávez, R.; Quispe-Pardo, Z.; Yagui-Moscoso, M. Primer reporte de Candida auris en Perú. An. Fac. Med. 2021, 82, 56–61. [Google Scholar] [CrossRef]
- Li, Y.; Hind, C.; Furner-Pardoe, J.; Sutton, J.M.; Rahman, K.M. Understanding the mechanisms of resistance to azole antifungals in Candida species. JAC-Antimicrob. Resist. 2025, 7, dlaf106. [Google Scholar] [CrossRef] [PubMed]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Anfotericin B and other polyenes: Discovery, clinical use, mode of action and drug rsistence. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 539. [Google Scholar] [CrossRef]
- Czajka, K.M.; Venkataraman, K.; Brabant-Kirwan, D.; Santi, S.A.; Verschoor, C.; Appanna, V.D.; Singh, R.; Saunders, D.P.; Tharmalingam, S. Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species. Cells 2023, 12, 2655. [Google Scholar] [CrossRef]
- Wurster, S.; Albert, N.D.; Kontoyiannis, D.P. Candida auris Bloodstream Infection Induces Upregulation of the PD-1/PD-L1 Immune Checkpoint Pathway in an Immunocompetent Mouse Model. mSphere 2022, 7, e00817–e00821. [Google Scholar] [CrossRef] [PubMed]
- Pathakumari, B.; Liu, W.; Wang, Q.; Kong, X.; Liang, G.; Chokkakula, S.; Pathakamuri, V.; Nunna, V. Comparative Evaluation of Candida Species-Specific T-Cell Immune Response in Human Peripheral Blood Mononuclear Cells. Biomedicines 2024, 12, 1487. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.; Lambourne, J.; Porter, S.; Hodgson, T. Chronic mucocutaneous candidiasis due to gain-of-function mutation in STAT1. Oral Dis. 2019, 25, 684–692. [Google Scholar] [CrossRef]
- Okada, S.; Markle, J.G.; Deenick, E.K.; Mele, F.; Averbuch, D.; Lagos, M. Impairment of immunity to Candida and Mycobacterium in humans with bi allelic RORC mutations. Science 2015, 349, 606–613. [Google Scholar] [CrossRef]
- Smeekens, S.P.; Plantinga, T.S.; van de Veerdonk, F.L.; Heinhuis, B.; Hoischen, A.; Joosten, L.A. STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS ONE 2011, 6, e29248. [Google Scholar] [CrossRef]
- Zaongo, S.D.; Jing, O.; Stéphane, I.; Xin, Z.; Vijay, H.; Hongjuan, C.; Routy, J.-P.; Chen, Y. Candida albicans can foster gut dysbiosis and systemic inflammation during HIV infection. Gut Microbes 2023, 15, 2167171. [Google Scholar] [CrossRef]
- Savage, H.P.; Bays, D.J.; Tiffany, C.R.; Gonzalez, M.A.F.; Bejarano, E.J.; Carvalho, T.P.; Luo, Z.; Masson, H.L.P.; Nguyen, H.; Santos, R.L.; et al. Epithelial hypoxia maintains colonization resistance against Candida albicans. Cell Host Microbe 2024, 32, 1103–1113.e1106. [Google Scholar] [CrossRef] [PubMed]
- Guinan, J.; Wang, S.; Hazbun, T.R.; Yadav, H.; Thangamani, S. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci. Rep. 2019, 9, 8872. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, H.; Lin, Y.; Lin, J.; Li, C.; Kuang, Y.; Zhou, W.; Huang, B.; Wang, P. Antibiotic-induced depletion of Clostridium species increases the risk of secondary fungal infections in preterm infants. Front. Cell. Infect. Microbiol. 2022, 12, 981823. [Google Scholar] [CrossRef]
- Bromuro, C.; Posteraro, B.; Murri, R.; Fantoni, M.; Tumbarello, M.; Sanguinetti, M.; Dattilo, R.; Cauda, R.; Cassone, A.; Torosantucci, A. Identification of two anti Candida antibodies associated with the survival of patients with candidemia. mBio 2024, 15, e02769-23. [Google Scholar] [CrossRef]
- Sari, N.; Yanik Yalçin, T.; Erol, Ç.; Kurt Azap, Ö.; Arslan, H.; Karakaya, E.; Sezgin, A.; Haberal, M. Evaluation of Candidemia in Solid-Organ Transplant Recipients. Exp. Clin. Transplant. 2024, 22, 160–166. [Google Scholar] [CrossRef]
- Marinelli, T.; Pennington, K.M.; Hamandi, B.; Donahoe, L.; Rotstein, C.; Martinu, T.; Husain, S. Epidemiology of candidemia in lung transplant recipients and risk factors for candidemia in the early posttransplant period in the absence of universal antifungal prophylaxis. Transpl. Infect. Dis. 2022, 24, e13812. [Google Scholar] [CrossRef]
- Puel, A. Human inborn errors of immunity underlying superficial or invasive candidiasis. Hum. Genet. 2020, 139, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Silvester, E.J.; Watanabe, M.M.Y.; Pittet, L.F.; Boast, A.; Bryant, P.A.; Haeusler, G.M.; Daley, A.J.; Curtis, N.; Gwee, A. Candidemia in Children: A 16-year Longitudinal Epidemiologic Study. Pediatr. Infect. Dis. J. 2021, 40, 537–543. [Google Scholar] [CrossRef]
- Keighley, C.L.; Pope, A.; Marriott, D.J.E.; Chapman, B.; Bak, N.; Daveson, K. Risk factors for candidaemia: A prospective multi-centre case-control study. Mycoses 2021, 64, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Candida sp. Infections in Patients with Diabetes Mellitus. J. Clin. Med. 2019, 8, 76. [Google Scholar] [CrossRef]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The Impact of Immune System Aging on Infectious Diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef]
- Hesstvedt, L.; Gaustad, P.; Müller, F.; Torp Andersen, C.; Brunborg, C.; Mylvaganam, H.; leiva, R.A.; Berdal, J.E.; Ranheim, T.E.; Johnsen, B.O.; et al. The impact of age on risk assessment, therapeutic practice and outcome in candidemia. Infect. Dis. 2019, 51, 425–434. [Google Scholar] [CrossRef]
- Nascimento, T.; Inácio, J.; Guerreiro, D.; Patrício, P.; Proença, L.; Toscano, C.; Diaz, P.; Barroso, H. Insights into Candida Colonization in Intensive Care Unit Patients: A Prospective Multicenter Study. J. Fungi 2024, 10, 378. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Giacobbe, D.R.; Trucchi, C.; Ansaldi, F.; Antonelli, M.; Adamkova, V.; Alicino, C.; Almyroudi, M.-P.; Atchade, E.; et al. Risk Factors for Intra-Abdominal Candidiasis in Intensive Care Units: Results from EUCANDICU Study. Infect. Dis. Ther. 2022, 11, 827–840. [Google Scholar] [CrossRef]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients: A Systematic Review and Meta-analysis. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.; Turner, N.A.; Yarrington, M.E.; Shaefer Spires, S.; Rebekah, W.M.; Alexander, B.D.; Park, L.P.; Johnson, M.D. Cumulative Antibiotic Exposure and Risk for Candidemia. Open Forum Infect. Dis. 2022, 9, ofac492.099. [Google Scholar] [CrossRef]
- Jung, P.; Mischo, C.E.; Gunaratnam, G.; Spengler, C.; Becker, S.L.; Hube, B.; Jacobs, K.; Bischoff, M. Candida albicans adhesion to central venous catheters: Impact of blood plasma-driven germ tube formation and pathogen-derived adhesins. Virulence 2020, 11, 1453–1465. [Google Scholar] [CrossRef]
- Huang, L.; Li, S.; Jiang, R.; Lei, S.; Wu, J.; Huang, L.; Zhu, M. Glucocorticoid use and parenteral nutrition are risk factors for catheter-related Candida bloodstream infection: A retrospective study. Asian Biomed. (Res. Rev. News) 2024, 18, 109–115. [Google Scholar] [CrossRef]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm Formation in Medically Important Candida Species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Venditti, M. Candidiasis invasiva en cirugía. In Infecciones en Cirugía. Actualizaciones en Cirugía; Bartoli, S., Cortese, F., Sartelli, M., Sganga, G., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Meng, Y.; Kang, M.; Li, D.; Wang, T.; Kuang, Z.; Ma, Y. Performance of a new Candida anti-mannan IgM and IgG assays in the diagnosis of candidemia. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e25. [Google Scholar] [CrossRef] [PubMed]
- Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef]
- Montagna, M.T.; Coretti, C.; Rella, A.; Barbuti, G.; Manca, F.; Montagna, O.; Laforgia, N.; Caggiano, G. The role of procalcitonin in neonatal intensive care unit patients with candidemia. Folia Microbiol. 2013, 58, 27–31. [Google Scholar] [CrossRef][Green Version]
- Cortegiani, A.; Misseri, G.; Ippolito, M.; Bassetti, M.; Giarratano, A.; Martin-Loeches, I.; Einav, S. Procalcitonin levels in candidemia versus bacteremia: A systematic review. Crit. Care 2019, 23, 190. [Google Scholar] [CrossRef]
- Chen, B.; Li, W.; Pang, Y.; Zhang, N.; Bian, S.; Liu, C.; Yang, Z.; Jiang, Y.; Li, R.; Xie, Y.; et al. Rapid detection of fungi from blood samples of patients with candidemia using modified calcofluor white stain. J. Microbiol. Methods 2021, 184, 106202. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kanaujia, R.; Singh, S.; Kajal, K.; Jayashree, M.; Peter, N.J.; Verma, S.; Gupta, M.; Ray, P.; Ghosh, A.; et al. Clinical utility of time to positivity of blood cultures in cases of fungaemia: A prospective study. Indian. J. Med. Microbiol. 2023, 43, 85–89. [Google Scholar] [CrossRef]
- Keighley, C.; Pope, A.L.; Marriott, D.; Chen, S.C.; Slavin, M.A.; Australia and New Zealand Mycoses Interest Group. Time-to-positivity in bloodstream infection for Candida species as a prognostic marker for mortality. Med. Mycol. 2023, 61, myad028. [Google Scholar] [CrossRef]
- Bonfim-Mendonça, P.d.S.; Fiorini, A.; Shinobu-Mesquita, C.S.; Baeza, L.C.; Fernandez, M.A.; Svidzinski, T.I. Molecular typing of Candida albicans isolates from hospitalized patients. Rev. Inst. Med. Trop. 2013, 55, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Camp, I.; Spettel, K.; Willinger, B. Molecular Methods for the Diagnosis of Invasive Candidiasis. J. Fungi 2020, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Mesini, A.; Mikulska, M.; Giacobbe, D.R.; Del Puente, F.; Gandolfo, N.; Codda, G.; Orsi, A.; Tassinari, F.; Beltramini, S.; Marchese, A.; et al. Changing epidemiology of candidaemia: Increase in fluconazole-resistant Candida parapsilosis. Mycoses 2020, 63, 361–368. [Google Scholar] [CrossRef]
- Daneshnia, F.; Hilmioğlu-Polat, S.; Ilkit, M.; Fuentes, D.; Lombardi, L.; Binder, U.; Scheler, J.; Hagen, F.; Mansour, M.K.; Butler, G.; et al. Whole-genome sequencing confirms a persistent candidaemia clonal outbreak due to multidrug-resistant Candida parapsilosis. J. Antimicrob. Chemother. 2023, 78, 1488–1494. [Google Scholar] [CrossRef]
- Monday, L.M.; Parraga Acosta, T.; Alangaden, G. T2Candida for the Diagnosis and Management of Invasive Candida Infections. J. Fungi 2021, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Huh, J.W.; Choi, S.H.; Sung, H.; Kim, M.N. Clinical evaluation of the T2Candida assay for the rapid diagnosis of candidemia. Diagn. Microbiol. Infect. Dis. 2024, 110, 116406. [Google Scholar] [CrossRef]
- Krifors, A.; Ullberg, M.; Castegren, M.; Petersson, J.; Sparrelid, E.; Hammarström, H.; Sjolin, J.; Ozenci, V.; Blennow, O. T2Candida Assay in the Diagnosis of Intraabdominal Candidiasis: A Prospective Multicenter Study. J. Fungi 2022, 8, 86. [Google Scholar] [CrossRef]
- Camp, I.; Füszl, A.; Selitsch, B.; Kröckel, I.; Kovac, K.; Wahrmann, M.; Steinlechner, B.; Weber, J.; Schellongowski, P.; Zauner, C.; et al. Is the T2MR Candida Panel a suitable alternative to the SeptiFast for the rapid diagnosis of candidemia in routine clinical practice? Clin. Microbiol. Infect. 2024, 30, 816–821. [Google Scholar] [CrossRef]
- Park, B.; Oh, E.H.; Won, E.J.; Kang, J.; Jin, D.; Yoo, C.; Park, J.; Sung, H.; Kim, M.-N. Detection of clinically relevant Candida species from positive blood cultures using a novel sample-to-answer molecular assay. Sci. Rep. 2025, 15, 5417. [Google Scholar] [CrossRef] [PubMed]
- Balaji, L.; Manoharan, H.; Prabhakaran, N.; Manivannan, N. Time-to-Positivity for Candida in Bloodstream Infections: Prognostic Implications for Mortality. Cureus 2024, 16, e66364. [Google Scholar] [CrossRef]
- Aziz, H.S.A.; Ismail, D.K.; Mohammed, N.S.A.; Elgendy, M.O.; Bassiouny, D.M. Distribution and antifungal susceptibility profiles of Candida species isolated from candidemia patients admitted to Egyptian tertiary hospitals: A cross-sectional study. BMC Infect. Dis. 2024, 24, 1177. [Google Scholar] [CrossRef]
- de Souza, Â.C.; Pereira, D.C.; Barth, P.O.; Roesch, E.W.; Lutz, L.; Aquino, V.R.; Goldani, L.Z. Rapid identification, fluconazole and micafungin susceptibility testing of Candida species from blood culture by a short incubation method. Diagn. Microbiol. Infect. Dis. 2024, 109, 116271. [Google Scholar] [CrossRef] [PubMed]
- Meawed, T.E.; AlNakeera, A.M.; Attia, O.; Hassan, N.A.M.; Anis, R.H. Candida auris central line-associated blood stream infection in critically ill patients: The worst end of a bad scenario. Int. Microbiol. 2025, 28, 377–383. [Google Scholar] [CrossRef]
- Peter Donnelly, J.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Dobiáš, R.; Káňová, M.; Petejová, N.; Pisti, Š.K.; Bocek, R.; Krejčí, E.; Struzkoca, H.; Cachová, M.; Tomasková, H.; Hamal, P.; et al. Combined Use of Presepsin and (1, 3)-β-D-glucan as Biomarkers for Diagnosing Candida Sepsis and Monitoring the Effectiveness of Treatment in Critically Ill Patients. J. Fungi 2022, 8, 308. [Google Scholar] [CrossRef]
- Zacharioudakis, I.M.; Zervou, F.N.; Marsh, K.; Siegfried, J.; Yang, J.; Decano, A.; Dubrovskaya, Y.; Mazo, D.; Aguero-Rosenfeld, M. Utility of incorporation of beta-D-glucan and T2Candida testing for diagnosis and treatment of candidemia. Diagn. Microbiol. Infect. Dis. 2024, 108, 116107. [Google Scholar] [CrossRef]
- Kinet-Poleur, A.; Deckers, C.; Saad Albichr, I.; Bogaerts, P.; Honoré, P.M.; Bulpa, P.; Ausselet, N.; Foret, F.; Kidd, F.; Huang, T.-D.; et al. Evaluation of Serum Biomarkers for Improved Diagnosis of Candidemia. J. Fungi 2025, 11, 224. [Google Scholar] [CrossRef]
- Lorenzo-Villegas, D.L.; Gohil, N.V.; Lamo, P.; Gurajala, S.; Bagiu, I.C.; Vulcanescu, D.D.; Horhat, F.G.; Sorop, V.B.; Diaconu, M.; Sorop, M.I.; et al. Innovative Biosensing Approaches for Swift Identification of Candida Species, Intrusive Pathogenic Organisms. Life 2023, 13, 2099. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef]
- Aydin, S.; Derin, O.; Sahin, M.; Dinleyici, R.; Yilmaz, M.; Ceylan, B.; Tosun, A.I.; Ozturk, R.; Mert, A. Epidemiology of Nosocomial Candidemia, Mortality, and Antifungal Resistance: 7-Year Experience in Turkey. Jpn. J. Infect. Dis. 2022, 75, 597–603. [Google Scholar] [CrossRef]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.A.; Chang, C.-H.; Webster, K.M. Epidemiology and outcomes of candidemia in 2019 patients: Data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 2009, 48, 1695–1703. [Google Scholar] [CrossRef]
- Fang, X.; Su, C.; Luo, Y.; Pan, K.; Lin, J.; Song, Y.; Huang, Y.; Hu, X.; Shen, Z. Risk factors associated with short-term mortality in patients with candidemia and the predictive value of serum cytokine level. Cytokine 2025, 185, 156803. [Google Scholar] [CrossRef]
- Liu, F.; Shao, X.; Dong, Z.; Tang, K.; Zhong, L.; Xu, W.; Chen, Z.; Zheng, C.; Zhang, K.; Cai, J.; et al. Clinical characteristics and prognostic risk factors of candidemia in non-neutropenic patients: A retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1389–1394. [Google Scholar] [CrossRef]
- Suh, J.W.; Kim, M.J.; Kim, J.H. Risk factors of septic shock development and thirty-day mortality with a predictive model in adult candidemia patients in intensive care units. Infect. Dis. 2021, 53, 908–919. [Google Scholar] [CrossRef]
- Kutlu, M.; Sayın-Kutlu, S.; Alp-Çavuş, S.; Öztürk, Ş.B.; Taşbakan, M.; Özhak, B.; Kaya, O.; Kutsoylu, O.E.; Senol-Akar, S.; Turhan, O.; et al. Mortality-associated factors of candidemia: A multi-center prospective cohort in Turkey. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Salmanton-García, J.; Egger, M.; Gangneux, J.P.; Bicanic, T.; Arikan-Akdagli, S.; Alastruey-Izquierdo, A.; Klimko, N.; Barac, A.; Ozenci, V.; et al. Guideline adherence and survival of patients with candidaemia in Europe: Results from the ECMM Candida III multinational European observational cohort study. Lancet Infect. Dis. 2023, 23, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Goyal, R.; Dutta, S.; Joshi, P.; Sanyal, K. Candida albicans: Insights into the Biology and Experimental Innovations of a Commonly Isolated Human Fungal Pathogen. ACS Infect. Dis. 2025, 11, 1780–1815. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Bicanic, T. Drug Resistance and Novel Therapeutic Approaches in Invasive Candidiasis. Front. Cell. Infect. Microbiol. 2021, 11, 759408. [Google Scholar] [CrossRef]
- Kollef, M.; Micek, S.; Hampton, N.; Doherty, J.A.; Kumar, A. Septic shock attributed to Candida infection: Importance of empiric therapy and source control. Clin. Infect. Dis. 2012, 54, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Rouzé, A.; Loridant, S.; Poissy, J.; Dervaux, B.; Sendid, B.; Cornu, M.; Nseir, S.; Study Group S-TAFE. Biomarker-based strategy for early discontinuation of empirical antifungal treatment in critically ill patients: A randomized controlled trial. Intensive Care Med. 2017, 43, 1668–1677. [Google Scholar] [CrossRef]
- Paiva, J.A.; Charles, P.E. Biomarker-guided antifungal therapy in patients with suspected invasive candidiasis: Ready for prime time? Intensive Care Med. 2017, 43, 1889–1891. [Google Scholar] [CrossRef][Green Version]
- Healy, C.M.; Baker, C.J.; Zaccaria, E.; Campbell, J.R. Impact of fluconazole prophylaxis on incidence and outcome of invasive candidiasis in a neonatal intensive care unit. J. Pediatr. 2005, 147, 166–171. [Google Scholar] [CrossRef]
- Kaufman, D.; Boyle, R.; Hazen, K.C.; Patrie, J.T.; Robinson, M.; Grossman, L.B. Twice weekly fluconazole prophylaxis for prevention of invasive Candida infection in high-risk infants of <1000 grams birth weight. J. Pediatr. 2005, 147, 172–179. [Google Scholar] [CrossRef]
- Cokro, F.; Yolanda, L.; Caroline, S.; Studi Farmasi, P.; Kedokteran dan Ilmu Kesehatan, F. Fluconazole Effectiveness in Preventing Invasive Fungal Infection in Very Low Birth Weight Infants: Systematic Review and Meta-analysis (Efektivitas Flukonazol dalam Mencegah Infeksi Jamur Invasif pada Bayi dengan Berat Lahir Sangat Rendah: Tinjauan Sistematis dan Meta-analisis). J. Ilmu Kefarmasian Indonesia. 2022, 20, 14–22. [Google Scholar]
- Lona-Reyes, J.C.; Gómez-Ruiz, L.M.; Cordero-Zamora, A.; Cortés-González, S.I.; Quiles-Corona, M.; Pérez-Ramírez, R.O.; Pinto-Macedo, H. Incidence and factors associated with invasive candidiasis in a neonatal intensive care unit in Mexico. An. Pediatr. (Engl. Ed.) 2022, 97, 79–86. [Google Scholar] [CrossRef]
- Estagnasié, C.; Mohr, C.; Kamus, L.; Zunic, P.; Chirpaz, E.; Moiton, M.P.; Lagrange-Xelot, M. Invasive fungal infections in patients with haematological malignancies at the University Hospital of Reunion Island (2018–2022): An observational study. Med. Mycol. 2024, 62, myae102. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.-T.; et al. Posaconazole vs. Fluconazole or Itraconazole Prophylaxis in Patients with Neutropenia. N. Engl. J. Med. 2007, 356, 348-59. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.A.; Durocher, F.; Beauchemin, S.; Ziegler, D.; Chakra, C.N.A.; Dufresne, S.F. Breakthrough Invasive Fungal Infections in Patients With High-Risk Hematological Disorders Receiving Voriconazole and Posaconazole Prophylaxis: A Systematic Review. Clin. Infect. Dis. 2024, 79, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.R.; DiPippo, A.J.; Jiang, Y.; DiNardo, C.D.; Kadia, T.; Maiti, A.; Montalban-Bravo, G.; Ravandi, F. Kontoyiannis, D.P. Comparison of Mold Active Triazoles as Primary Antifungal Prophylaxis in Patients with Newly Diagnosed Acute Myeloid Leukemia in the Era of Molecularly Targeted Therapies. Clin. Infect. Dis. 2022, 75, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Calle-Miguel, L.; Garrido-Colino, C.; Santiago-García, B.; Moreno Santos, M.P.; Gonzalo Pascual, H.; Ponce Salas, B.; Beléndez Bieler, C.; Navarro Gómez, M.; Guinea Ortega, J.; Rincón-López, E.M. Changes in the epidemiology of invasive fungal disease in a Pediatric Hematology and Oncology Unit: The relevance of breakthrough infections. BMC Infect. Dis. 2023, 23, 348. [Google Scholar] [CrossRef]
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.A.; Groll, A.H.; Kurzai, O.; Lass-Florl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathia, G.; et al. Global guideline for the diagnosis and management of candidiasis: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293. [Google Scholar] [CrossRef]
- Aslam, S.; Rotstein, C. Candida infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transpl. 2019, 33, e13623. [Google Scholar] [CrossRef]
- Carugati, M.; Arif, S.; Yarrington, M.E.; King, L.Y.; Harris, M.; Evans, K.; Barbas, A.S.; Sudan, D.L.; Perfect, J.R.; Miller, R.A.; et al. Limitations of antifungal prophylaxis in preventing invasive Candida surgical site infections after liver transplant surgery. Antimicrob. Agents Chemother. 2024, 68, e0127923. [Google Scholar] [CrossRef]
- López-Medrano, F.; de la Espada, M.M.; Asín, M.A.P.; Fernández-Ruiz, M.; Herrero-Martínez, J.M.; Alonso-Carrillo, J.; San Juan, R.; Rodríguez-Goncer, I.; Andrés, A.; González, E.; et al. Fluconazole versus micafungin for initial antifungal prophylaxis against Candida in pancreas transplant recipients: A comparative study of two consecutive periods. Mycoses 2022, 65, 517–525. [Google Scholar] [CrossRef]
- Bloos, F.; Held, J.; Kluge, S.; Simon, P.; Kogelmann, K.; de Heer, G.; Kuhn, S.-O.; Jarczak, D.; Motsch, J.; Hempel, G.; et al. (1 → 3)-β-d-Glucan-guided antifungal therapy in adults with sepsis: The CandiSep randomized clinical trial. Intensive Care Med. 2022, 48, 865–875. [Google Scholar] [CrossRef]
- Erb, T.; Mihai, S.; Strauß, R.; Herbst, L.; Castellanos, I.; Diesch, K.; Cipa, F.; Bihlmaier, K.; Lang, A.-K.; Ganslmayer, M.; et al. β-(1→3)-D-glucan- and mannan-guided early termination of antifungal therapy in ICU patients: A randomized controlled study. Antimicrob. Agents Chemother. 2023, 67, e0072523. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Florl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Keighley, C.; Cooley, L.; Morris, A.J.; Ritchie, D.; Clark, J.E.; Boan, P.; Worth, L.J.; Australasian Antifungal Guidelines Steering Committee. Consensus guidelines for the diagnosis and management of invasive candidiasis in haematology, oncology and intensive care settings, 2021. Intern. Med. J. 2021, 51, 89–117. [Google Scholar] [CrossRef]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J.; Mycoses Study Group. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef]
- Wolfgruber, S.; Sedik, S.; Klingspor, L.; Tortorano, A.; Gow, N.A.R.; Lagrou, K.; Gangneux, J.-P.; Maertens, J.; Meis, J.F.; Lass-Florl, C.; et al. Insights from Three Pan-European Multicentre Studies on Invasive Candida Infections and Outlook to ECMM Candida IV. Mycopathologia 2024, 189, 70. [Google Scholar] [CrossRef]
- Maseda, E.; Martín-Loeches, I.; Zaragoza, R.; Pemán, J.; Fortún, J.; Grau, S.; Aguilar, G.; Varela, M.; Borges, M.; Giménez, M.-J.; et al. Critical appraisal beyond clinical guidelines for intraabdominal candidiasis. Crit. Care 2023, 27, 382. [Google Scholar] [CrossRef] [PubMed]
- Pais, M.M.; Zaragoza, R.; Martín-Loeches, I.; Gómez-Bertomeu, F.F.; Rodríguez, A. Management of Intra-Abdominal Candidiasis in Intensive Care Setting: A Narrative Review. J. Fungi 2025, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Baldelli, S.; Märtson, A.G.; Stocker, S.; Alffenaar, J.W.; Cattaneo, D.; Marriott, D.J. Therapeutic Drug Monitoring of the Echinocandin Antifungal Agents: Is There a Role in Clinical Practice? A Position Statement of the Anti-Infective Drugs Committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther. Drug Monit. 2022, 44, 198–214. [Google Scholar] [CrossRef]
- Beyda, N.D.; John, J.; Kilic, A.; Alam, M.J.; Lasco, T.M.; Garey, K.W. FKS mutant Candida glabrata: Risk factors and outcomes in patients with candidemia. Clin. Infect. Dis. 2014, 59, 819–825. [Google Scholar] [CrossRef]
- Hoenigl, M.; Arastehfar, A.; Arendrup, M.C.; Brüggemann, R.; Carvalho, A.; Chiller, T.; Chen, S.; Egger, M.; Feys, S.; Ganneux, J.-P.; et al. Novel antifungals and treatment approaches to tackle resistance and improve outcomes of invasive fungal disease. Clin. Microbiol. Rev. 2024, 37, e0007423. [Google Scholar] [CrossRef] [PubMed]
- Nagamizu, M.; Hotta, Y.; Noda, M.; Nakamura, D.; Hori, M.; Otsuka, Y.; Takemoto, R.; Horita, Y.; Wakita, E.; Morishita, N.; et al. Association of doses based on body constitutional parameters with the efficacy of micafungin in candidemia. J. Infect. Chemother. 2025, 31, 102654. [Google Scholar] [CrossRef]
- Alsowaida, Y.S.; Sulaiman, K.A.; Mahrous, A.J.; Alharbi, A.; Bifari, N.; Alshahrani, W.A.; Almangour, T.A.; Damfu, N.; Banamah, A.; Abu Raya, R.R.; et al. Evaluation of clinical outcomes of anidulafungin for the treatment of candidemia in hospitalized critically ill patients with obesity: A multicenter, retrospective cohort study. Int. J. Infect. Dis. 2024, 148, 107234. [Google Scholar] [CrossRef]
- Thompson, G.R.; Soriano, A.; Skoutelis, A.; Vazquez, J.A.; Honore, P.M.; Horcajada, J.P.; Spapen, H.; Bassetti, M.; Ostrosky-Zeichner, L.; Das, A.F.; et al. Rezafungin Versus Caspofungin in a Phase 2, Randomized, Double-blind Study for the Treatment of Candidemia and Invasive Candidiasis: The STRIVE Trial. Clin. Infect. Dis. 2021, 73, E3647–E3655. [Google Scholar] [CrossRef]
- Thompson, G.R.; Soriano, A.; Cornely, O.A.; Kullberg, B.J.; Kollef, M.; Vazquez, J.; Honore, P.M.; Bassetti, M.; Pullman, J.; Chayakulkeeree, M.; et al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): A multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet 2023, 401, 49–59. [Google Scholar] [CrossRef]
- Soriano, A.; Locke, J.B.; Cornely, O.A.; Roilides, E.; Ramos-Martinez, A.; Honoré, P.M.; Castanheira, M.; Carvalhaes, C.G.; Nseir, S.; Bassetti, M.; et al. Clinical and mycological outcomes of candidaemia and/or invasive candidiasis by Candida spp. and antifungal susceptibility: Pooled analyses of two randomized trials of rezafungin versus caspofungin. Clin. Microbiol. Infect. 2025, 31, 250–257. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E.; Betts, R.F.; Dupont, B.F.; Wu, C.; Buell, D.N.; Kovanda, L.; Lortholary, O. Early removal of central venous catheter in patients with candidemia does not improve outcome: Analysis of 842 patients from 2 randomized clinical trials. Clin. Infect. Dis. 2010, 51, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Braga, P.R.; Nouér, S.A.; Anaissie, E. Time of catheter removal in candidemia and mortality. Braz. J. Infect. Dis. 2018, 22, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Ryu, B.H.; Hong, S.I.; Cho, O.H.; Hong, K.W.; Bae, I.G.; Kwack, W.G.; Kim, Y.J.; Chung, E.K.; Kim, D.Y.; et al. Clinical impact of early reinsertion of a central venous catheter after catheter removal in patients with catheter-related bloodstream infections. Infect. Control. Hosp. Epidemiol. 2021, 42, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.S.; Liu, Y.; Cai, X.; Zhan, L.; Hu, K. Association of different Candida species with catheter-related candidemia, and the potential antifungal treatments against their adhesion properties and biofilm-forming capabilities. J. Clin. Lab. Anal. 2021, 35, e23738. [Google Scholar] [CrossRef] [PubMed]
- Breazzano, M.P.; Bond, J.B.; Bearelly, S.; Kim, D.H.; Donahue, S.P.; Lum, F.; Olsen, T.W.; American Academy of Ophthalmology. American Academy of Ophthalmology Recommendations on Screening for Endogenous Candida Endophthalmitis. Ophthalmology 2022, 129, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Breazzano, M.P.; Day, H.R.; Bloch, K.C.; Tanaka, S.; Cherney, E.F.; Sternberg, P.; Donahue, S.P.; Bond 3rd, J.B. Utility of Ophthalmologic Screening for Patients with Candida Bloodstream Infections: A Systematic Review. JAMA Ophthalmol. 2019, 137, 698–710. [Google Scholar] [CrossRef]
- Phongkhun, K.; Pothikamjorn, T.; Srisurapanont, K.; Manothummetha, K.; Sanguankeo, A.; Thongkam, A.; Chuleerarux, N.; Leksuwankun, S.; Meejun, T.; Thanakitcharu, J.; et al. Prevalence of Ocular Candidiasis and Candida Endophthalmitis in Patients with Candidemia: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2023, 76, 1738–1749. [Google Scholar] [CrossRef]
- Lehman, A.; Tessier, K.M.; Sattarova, V.; Montezuma, S.R.; Kline, S.; Erayil, S.E. Do Patients With Candidemia Need an Ophthalmologic Examination? Open Forum Infect. Dis. 2024, 11, ofae663. [Google Scholar] [CrossRef]
- Shin, S.U.; Yu, Y.H.; Kim, S.S.; Oh, T.H.; Kim, S.E.; Kim, U.J.; Kang, S.-J.; Jang, H.-C.; Park, K.-H.; Jung, S.I. Clinical characteristics and risk factors for complications of candidaemia in adults: Focus on endophthalmitis, endocarditis, and osteoarticular infections. Int. J. Infect. Dis. 2020, 93, 126–132. [Google Scholar] [CrossRef]
- Soriano, A.; Honore, P.M.; Puerta-Alcalde, P.; Garcia-Vidal, C.; Pagotto, A.; Gonçalves-Bradley, D.C.; Verweij, P.E. Invasive candidiasis: Current clinical challenges and unmet needs in adult populations. J. Antimicrob. Chemother. 2023, 78, 1569–1585. [Google Scholar] [CrossRef]
- Permpalung, N.; Sedik, S.; Pappas, P.G.; Hoenigl, M.; Ostrosky-Zeichner, L. Diagnosis and Management of Candida Endophthalmitis and Chorioretinitis. Clin. Microbiol. Infect. 2025; in press. [Google Scholar] [CrossRef]
- Turner, R.B.; Valcarlos, E.; Won, R.; Chang, E.; Schwartz, J. Impact of infectious diseases consultation on clinical outcomes of patients with Staphylococcus aureus bacteremia in a community health system. Antimicrob. Agents Chemother. 2016, 60, 5682–5687. [Google Scholar] [CrossRef]
- Kobayashi, T.; Marra, A.R.; Schweizer, M.L.; Eyck, P.T.; Wu, C.; Alzunitan, M.; Salinas, J.L.; Siegel, M.; Farmakiotis, D.; Auwaerter, P.G.; et al. Impact of infectious disease consultation in patients with candidemia: A retrospective study, systematic literature review, and meta-analysis. Open Forum Infect. Dis. 2020, 7, ofaa270. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.A.; Vo, D.T.; Zurko, J.C.; Griffin, R.L.; Rodriguez, J.M.; Camins, B.C. Infectious diseases consultation is associated with decreased mortality in enterococcal bloodstream infections. Open Forum Infect. Dis. 2020, 7, ofaa064. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; McQuillen, D.P.; Nahass, R.; Martinelli, L.; Rubin, M.; Schwebke, K.; Petrak, R.; Ritter, J.T.; Chansolme, D.; Slama, T.; et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin. Infect. Dis. 2014, 58, 22–28. [Google Scholar] [CrossRef]
- Alataby, H.; Atemnkeng, F.; Bains, S.S.; Kenne, F.M.; Diaz, K.; Nfonoyim, J. A COVID-19 case complicated by Candida dubliniensis and Klebsiella pneumoniae-carbapenem-resistant enterobacteriaceae. J. Med. Cases 2020, 11, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.L.; Fiuza, J.G.; Ferreira, G.; Almeida, M.D.; Moreira, D.; Neto, V.D. Embolic stroke and misidentification candida species endocarditis: Case presentation and literature review. Diagn. Microbiol. Infect. Dis. 2024, 108, 116133. [Google Scholar] [CrossRef]
- Vena, A.; Tiseo, G.; Falcone, M.; Bartalucci, C.; Marelli, C.; Cesaretti, M.; Di Pilato, V.; Escribano, P.; Forniti, A.; Giacobbe, D.R.; et al. Impact of fluconazole resistance on the outcomes of patients with Candida parapsilosis bloodstream infections: A retrospective multicenter study. Clin. Infect. Dis. 2025, 80, 540–550. [Google Scholar] [CrossRef]
- Javorova Rihova, Z.; Slobodova, L.; Hrabovska, A. Micafungin is an efficient treatment of multi drug-resistant Candida glabrata urosepsis: A case report. J. Fungi 2021, 7, 800. [Google Scholar] [CrossRef] [PubMed]
- Overgaauw, A.J.C.; de Leeuw, D.C.; Stoof, S.P.; van Dijk, K.; Bot, J.C.J.; Hendriks, E.J. Case report: Candida krusei spondylitis in an immunocompromised patient. BMC Infect. Dis. 2020, 20, 739. [Google Scholar] [CrossRef]
- Vrijders, L.; Ho, E.; Van der Beek, D.; Vrelust, I.; Nailis, H. More than meets the eye: Nocardia farcinica, Candida dubliniensis and Aspergillus spp. co-infection in a patient with multiple myeloma treated with multiple treatment regimens. BMC Infect. Dis. 2025, 25, 156. [Google Scholar] [CrossRef]
- Xie, L.; Long, Q.; Zhou, G.; Liu, S.; Wen, F.Q. Successful outcome of disseminated Candida tropicalis osteomyelitis on remission induction for childhood Philadelphia chromosome-positive acute lymphoblastic leukaemia—Case report. Ital. J. Pediatr. 2021, 47, 27. [Google Scholar] [CrossRef]
- Görkem, A.; Sav, H.; Kaan, Ö.; Eren, E. Coronavirus disease and candidemia infection: A case report. J. Mycol. Med. 2021, 31, 101155. [Google Scholar] [CrossRef]
- Rafie, R.A.; Soflaee, M.; Mortezaeian, H.; Jalali, A.; Feyzi, A.; Anafje, M.; Soheili, A. Comprehensive surgical and medical management of massive fungal endocarditis in a preterm neonate: A rare case of survival and full recovery. Diagn. Microbiol. Infect. Dis. 2025, 112, 116787. [Google Scholar] [CrossRef] [PubMed]
- Ünal, N.; Spruijtenburg, B.; Arastehfar, A.; Gümral, R.; de Groot, T.; Meijer, E.F.J.; Turk-Dag, H.; Birinci, A.; Hilmioglu-Polat, S.; Meis, J.F.; et al. Multicentre study of Candida parapsilosis blood isolates in Türkiye highlights an increasing rate of fluconazole resistance and emergence of echinocandin and multidrug resistance. Mycoses 2024, 67, e70000. [Google Scholar] [CrossRef] [PubMed]
| Resistance Mechanism | Type of Antifungal | Functional Basis of Resistance | Example | References |
|---|---|---|---|---|
| Over-expression of membrane transporters: ABC (Adenosine Triphosphate (ATP)-Binding Cassette) transporters are encoded by the CDR (Candida Drug Resistance) genes, and MF (Major Facilitator) transporters by the MDR (Multidrug Resistance) genes. Over-expression of ABC transporters is the most common cause of resistance to all azoles, while over-expression of MF only confers resistance to fluconazole. | Azoles | Alterations in azole transport, overexpression of ABC and MF transporters increase the cell’s ability to eliminate azoles, which normally enter by passive diffusion, reducing their intracellular concentration and, therefore, decreasing their effectiveness. | Overexpression of Cdr1p and Cdr2p in C. albicans; CgCdr1p in C. glabrata; and Cdr1p in C. auris. | [66,67] |
| Mutations and/or overexpression of genes involved in ergosterol biosynthesis: ERG11 is a gene that encodes lanosterol 14α-demethylase, which is crucial for the synthesis of ergosterol from the fungal cell membrane. Several point mutations have been identified that are associated with azole resistance. ERG3 is another gene that also participates in the ergosterol pathway, converting episterol to ergosta-5,7,24(28)-trienol. | Azoles | Mutations in the ERG11 sequence can affect the structure and function of lanosterol 14α-demethylase, reducing its affinity for azoles and decreasing the drug’s efficacy. Mutations in ERG3 inhibit ergosterol biosynthesis from episterol to ergosta-5,7,24(28)-trienol. | The A61V, A114S, Y132F, Y132H, and I471T mutations in ERG11 are associated with azole resistance in C. albicans; C108G, C423T, and A1581G in C. glabrata; A497C and G1570A in C. krusei. The Q139A mutations in ERG3 cause azole resistance in C. glabrata. | [66] |
| Sterol import: It has recently been reported that sterol import may be a resistance mechanism. | Azoles | The reduction in ergosterol levels can be compensated by the exogenous import of sterols through specific importers and under anaerobic and microaerophilic conditions. | C. glabrata imports sterols using the importers Aus1p and Pdr11p, leading to increased resistance to azoles. | [66] |
| Genomic plasticity: loss of heterozygosity and aneuploidy are genomic variations that can lead to azole resistance. | Azoles | If one allele of a gene is mutated, loss of heterozygosity can copy the mutation to the second allele, causing loss of gene function. | Loss of heterozygosity in CaTAC1, CaERG11 and CaMRR1 of C. albicans has been correlated with increased azole resistance. | [66] |
| Alterations in the ERG11 and ERG3 genes (lanosterol 14α-demethylase and sterol C-5 desaturase, respectively). | Polyenes | The loss of function of the ERG11 and ERG3 genes due to mutations alters the sterol composition of the cell membrane by the exchange of ergosterol for alternative sterols. | Loss of function of the ERG11 and ERG3 genes leads to the exchange of ergosterol for lanosterol, eburicol, and 4,14-dimethyl-zymosterol in the C. albicans membrane. | [68] |
| Alterations in the cell wall. | Polyenes | The increase in the 1,3-α-glucan fraction can physically inhibit the penetration of AmB through the cell wall. | An enlarged cell wall with increased levels of 1,3-β-glucan causes resistance to amphotericin B in C. tropicalis. | [69] |
| Point mutations in FKS1 and FKS2, which encode β-(1-3) D glucan synthase, an important enzyme for cell wall biosynthesis. | Echinocandins | Mutations in FKS1 and FKS2 modify the target site in β-(1-3) D glucan synthase, preventing the action of echinocandins. | In C. albicans, C. glabrata, and C. krusei, it has been observed that the mutation in FKS1 causes the substitution of serine 645 by proline, phenylalanine, and tyrosine, which modifies the target site and inhibits the binding of echinocandins to β-(1-3) D glucan synthase. | [67] |
| Mutation in the FCY1 and FCY2 genes, which encode the enzymes cytosine permease or cytosine deaminase which facilitate the absorption of fluorocytosine and its deamination, respectively. | 5-FC | Mutations in FCY1 and FCY2 lead to inactivation of the enzymes they encode and cause decreased uptake or metabolic transformation of 5-FC and, therefore, resistance to the drug. | In C. albicans, inactivation of cytosine permease and cytosine deaminase has been shown to be associated with resistance to 5-FC. | [70] |
| Diagnostic Method | Sample/Target/Test | Advantages and Limitations | References |
|---|---|---|---|
| Blood culture | Peripheral blood |
| [99] |
| Blood culture—Staining with calcofluor white | Peripheral blood |
| [102] |
| 1,3 β-D-Glucan | Peripheral blood |
| [120] |
| PCR | AurisID®, Fungiplex® Candida auris |
| [106] |
| CandID®, Fungiplex® Candida, Fungiplex® Universal, MycoReal Candida and MagicplexTM Sepsis |
| [106] | |
| RAPD-PCR | CDC3 and HIS3 |
| [105] |
| Microarrays | Hybcell Pathogens DNA xB, SepsiTestTM-UMD and MycoReal Fungi, 28 S, 18S e ITS |
| [106] |
| MALDI-TOF | Culture and peripheral blood |
| [16,99,115,116] |
| Next-generation sequencing | STI Region Dominino D1-D2 |
| [99] |
| T2 Candida | STI Region 5.8S and 28S rRNA |
| [110] |
| Biosensors | Electromechanical impedance, piezoelectric immunosensors, optical biosensor, loop-mediated isothermal amplification (LAMP), lateral flow strip, particle-mediated immunosensors, genosensors, fluorescence and flow cytometry, electromechanics, and microfluidic hydrodynamic cell trapping and epifluorescence |
| [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Guerrero, J.P.; García-Salazar, E.; Hernandez Silva, G.; Chinney Herrera, A.; Martínez-Herrera, E.; Pinto-Almazán, R.; Frías-De-León, M.G.; Castro-Fuentes, C.A. Candidemia: An Update on Epidemiology, Risk Factors, Diagnosis, Susceptibility, and Treatment. Pathogens 2025, 14, 806. https://doi.org/10.3390/pathogens14080806
Cabrera-Guerrero JP, García-Salazar E, Hernandez Silva G, Chinney Herrera A, Martínez-Herrera E, Pinto-Almazán R, Frías-De-León MG, Castro-Fuentes CA. Candidemia: An Update on Epidemiology, Risk Factors, Diagnosis, Susceptibility, and Treatment. Pathogens. 2025; 14(8):806. https://doi.org/10.3390/pathogens14080806
Chicago/Turabian StyleCabrera-Guerrero, Juan Pablo, Eduardo García-Salazar, Graciela Hernandez Silva, Alberto Chinney Herrera, Erick Martínez-Herrera, Rodolfo Pinto-Almazán, María Guadalupe Frías-De-León, and Carlos Alberto Castro-Fuentes. 2025. "Candidemia: An Update on Epidemiology, Risk Factors, Diagnosis, Susceptibility, and Treatment" Pathogens 14, no. 8: 806. https://doi.org/10.3390/pathogens14080806
APA StyleCabrera-Guerrero, J. P., García-Salazar, E., Hernandez Silva, G., Chinney Herrera, A., Martínez-Herrera, E., Pinto-Almazán, R., Frías-De-León, M. G., & Castro-Fuentes, C. A. (2025). Candidemia: An Update on Epidemiology, Risk Factors, Diagnosis, Susceptibility, and Treatment. Pathogens, 14(8), 806. https://doi.org/10.3390/pathogens14080806









