Re-Evaluation of a Hyperendemic Focus of Metastrongyloid Lungworm Infections in Gastropod Intermediate Hosts in Southern Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Gastropod Procession
2.3. Microscopical Analysis
2.4. DNA Extraction from Larvae
2.5. Molecular Identification of Lungworm Species
2.6. Information for Veterinary Practices and Pet Owners
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valente, R.; Robles, M.D.R.; Diaz, J.I. Gastropods as Intermediate Hosts of Angiostrongylus Spp. in the Americas: Bioecological Characteristics and Geographical Distribution. Mem. Inst. Oswaldo Cruz 2020, 115, e200236. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.A.A.; Daly, E.; Allen, S.; Rowson, B.; Greig, C.; Forman, D.; Morgan, E.R. Distribution of Angiostrongylus vasorum and Its Gastropod Intermediate Hosts along the Rural–Urban Gradient in Two Cities in the United Kingdom, Using Real Time PCR. Parasites Vectors 2016, 9, 56. [Google Scholar] [CrossRef]
- Colombo, M.; Traversa, D.; Grillotti, E.; Pezzuto, C.; De Tommaso, C.; Pampurini, F.; Schaper, R.; Drake, J.; Crisi, P.E.; Russi, I.; et al. Highly Variable Clinical Pictures in Dogs Naturally Infected with Angiostrongylus vasorum. Pathogens 2021, 10, 1372. [Google Scholar] [CrossRef]
- Bolt, G.; Monrad, J.; Koch, J.; Jensen, A.L. Canine Angiostrongylosis: A Review. Vet. Rec. 1994, 135, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.W.; Hertling, R.; Kennedy, M.J. Angiostrongylosis with Disseminated Larval Infection Associated with Signs of Ocular and Nervous Disease in an Imported Dog. Can. Vet. J. 1991, 32, 430–431. [Google Scholar]
- Bolt, G.; Monrad, J.; Henriksen, P.; Dietz, H.H.; Koch, J.; Bindseil, E.; Jensen, A.L. The Fox (Vulpes vulpes) as a Reservoir for Canine Angiostrongylosis in Denmark. Acta Vet. Scand. 1992, 33, 357–362. [Google Scholar] [CrossRef]
- Taylor, C.S.; Gato, R.G.; Learmount, J.; Aziz, N.A.; Montgomery, C.; Rose, H.; Coulthwaite, C.L.; Mcgarry, J.W.; Forman, D.W.; Allen, S.; et al. Increased Prevalence and Geographic Spread of the Cardiopulmonary Nematode Angiostrongylus vasorum in Fox Populations in Great Britain. Parasitology 2015, 142, 1190–1195. [Google Scholar] [CrossRef]
- De Liberato, C.; Grifoni, G.; Lorenzetti, R.; Meoli, R.; Cocumelli, C.; Mastromattei, A.; Scholl, F.; Rombolà, P.; Calderini, P.; Bruni, G.; et al. Angiostrongylus vasorum in Wolves in Italy: Prevalence and Pathological Findings. Parasites Vectors 2017, 10, 386. [Google Scholar] [CrossRef]
- Hermosilla, C.; Kleinertz, S.; Silva, L.M.R.; Hirzmann, J.; Huber, D.; Kusak, J.; Taubert, A. Protozoan and Helminth Parasite Fauna of Free-Living Croatian Wild Wolves (Canis lupus) Analyzed by Scat Collection. Vet. Parasitol. 2017, 233, 14–19. [Google Scholar] [CrossRef]
- Gavrilović, P.; Marinković, D.; Vidanović, D.; Dobrosavljević, I.; Gavrilović, A. Are Golden Jackals (Canis Aureus) Definitive Hosts for Angiostrongylus vasorum? Transbound. Emerg. Dis. 2019, 66, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Juhász, L.; Takács, A.; Lanszki, J.; Takács, P.; Heltai, M. Data on the Parasitological Status of Golden Jackal (Canis Aureus L., 1758) in Hungary. Acta. Vet. Hung. 2013, 62, 33–41. [Google Scholar] [CrossRef]
- Bružinskaitė-Schmidhalter, R.; Šarkūnas, M.; Malakauskas, A.; Mathis, A.; Torgerson, P.R.; Deplazes, P. Helminths of Red Foxes (Vulpes vulpes) and Raccoon Dogs (Nyctereutes procyonoides) in Lithuania. Parasitology 2012, 139, 120–127. [Google Scholar] [CrossRef]
- Magi, M.; Guardone, L.; Dell’Omodarme, M.; Prati, M.C.; Mignone, W.; Torracca, B.; Monni, G.; Macchioni, F. Angiostrongylus vasorum in Red Foxes (Vulpes vulpes) and Badgers (Meles meles) from Central and Northern Italy. Hystrix It. J. Mamm. 2009, 20, 121–126. [Google Scholar]
- Patterson-Kane, J.C.; Gibbons, L.M.; Jefferies, R.; Morgan, E.R.; Wenzlow, N.; Redrobe, S.P. Pneumonia from Angiostrongylus Vasorum Infection in a Red Panda (Ailurus Fulgens Fulgens). J. Vet. Diagn. Investig. 2009, 21, 270–273. [Google Scholar] [CrossRef]
- Segeritz, L.; Westhoff, K.M.; Schaper, R.; Hermosilla, C.; Taubert, A. Angiostrongylus vasorum, Aelurostrongylus abstrusus, Crenosoma vulpis and Troglostrongylus brevior Infections in Native Slug Populations of Bavaria and Baden-Wuerttemberg in Germany. Pathogens 2022, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Caron, Y.; Merveille, A.-C.; Losson, B.; Billen, F. Crenosoma vulpis Infection in Two Young Dogs in Belgium. Vet. Rec. Case Rep. 2014, 2, e000098. [Google Scholar] [CrossRef]
- Choi, S.; Sim, C.; Kim, H.-C.; Choi, H.-J.; Park, B.-K. Natural Infection of Crenosoma Vulpis (Nematoda: Crenosomatidae) in an Urban Korean Dog. Korean J. Vet. Res. 2014, 54, 127–129. [Google Scholar] [CrossRef][Green Version]
- Taubert, A.; Pantchev, N.; Vrhovec, M.G.; Bauer, C.; Hermosilla, C. Lungworm Infections (Angiostrongylus vasorum, Crenosoma vulpis, Aelurostrongylus abstrusus) in Dogs and Cats in Germany and Denmark in 2003–2007. Vet. Parasitol. 2009, 159, 175–180. [Google Scholar] [CrossRef]
- Schug, K.; Krämer, F.; Schaper, R.; Hirzmann, J.; Failing, K.; Hermosilla, C.; Taubert, A. Prevalence Survey on Lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) Infections of Wild Red Foxes (Vulpes Vulpes) in Central Germany. Parasites Vectors 2018, 11, 85. [Google Scholar] [CrossRef]
- Majeed, S.K.; Morris, P.A.; Cooper, J.E. Occurrence of the Lungworms Capillaria and Crenosoma Spp. in British Hedgehogs (Erinaceus europaeus). J. Comp. Pathol. 1989, 100, 27–36. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Youssefi, M.R.; Mousapour, A.; Dozouri, R.; Eshkevari, S.R.; Nikzad, M.; Nikzad, R.; Omidzahir, S. Histopathologic study of eosinophilic bronchointestinal pneumonia caused by Crenosoma striatum in the hedgehog. J. Zoo Wildl. Med. 2014, 45, 335–338. [Google Scholar] [CrossRef]
- Lehmann, S.; Dervas, E.; Ruiz Subira, A.; Eulenberger, U.; Gimmel, A.; Grimm, F.; Hetzel, U.; Kipar, A. Verminous Pneumonia in European Hedgehogs (Erinaceus europaeus). Vet. Pathol. 2024, 61, 256–268. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Wright, I.; Wang, B.; Schaper, R. Prevalence of Feline Lungworm Aelurostrongylus abstrusus in England. Vet. Parasitol. Reg. Stud. Rep. 2019, 16, 100271. [Google Scholar] [CrossRef]
- Traversa, D.; Lia, R.P.; Boari, A.; di Cesare, A.; Capelli, G.; Milillo, P.; Otranto, D. Feline Aelurostrongylosis: Epidemiological Survey in Central and Southern Italy. Parassitologia 2009, 23, 41–45. [Google Scholar]
- Traversa, D.; Lepri, E.; Veronesi, F.; Paoletti, B.; Simonato, G.; Diaferia, M.; Di Cesare, A. Metastrongyloid Infection by Aelurostrongylus abstrusus, Troglostrongylus brevior and Angiostrongylus chabaudi in a Domestic Cat. Int. J. Parasitol. 2015, 45, 685–690. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A.; Milillo, P.; Iorio, R.; Otranto, D. Aelurostrongylus abstrusus in a Feline Colony from Central Italy: Clinical Features, Diagnostic Procedures and Molecular Characterization. Parasitol. Res. 2008, 103, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Cavalera, M.A.; Iatta, R.; Colella, V.; Dantas-Torres, F.; Corsaro, A.; Brianti, E.; Otranto, D. Troglostrongylus brevior: A Feline Lungworm of Paediatric Concern. Vet. Parasitol. 2018, 253, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Crisi, P.E.; Di Cesare, A.; Boari, A. Feline Troglostrongylosis: Current Epizootiology, Clinical Features, and Therapeutic Options. Front. Vet. Sci. 2018, 5, 126. [Google Scholar] [CrossRef]
- Alić, A.; Traversa, D.; Duscher, G.G.; Kadrić, M.; Di Cesare, A.; Hodžić, A. Troglostrongylus Brevior in an Eurasian Lynx (Lynx lynx) from Bosnia and Herzegovina. Parasites Vectors 2015, 8, 653. [Google Scholar] [CrossRef]
- Haas, M.; Segeritz, L.; Anders, O.; Middelhoff, T.L.; Myat Tun, A.; Hasheminasab, S.S.; Cocchiararo, B.; Dusch, A.; Taubert, A.; Hermosilla, C. Patent Troglostrongylus brevior-, Aelurostrongylus abstrusus-, Angiostrongylus sp.-, and Crenosoma sp. Infections in Wild Eurasian Lynxes (Lynx Lynx) and Their Habitat-Sharing Gastropod Intermediate Hosts. Front. Vet. Sci. 2025, 12, 1515507. [Google Scholar] [CrossRef]
- Falsone, L.; Brianti, E.; Gaglio, G.; Napoli, E.; Anile, S.; Mallia, E.; Giannelli, A.; Poglayen, G.; Giannetto, S.; Otranto, D. The European Wildcats (Felis Silvestris Silvestris) as Reservoir Hosts of Troglostrongylus brevior (Strongylida: Crenosomatidae) Lungworms. Vet. Parasitol. 2014, 205, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Bourgoin, G.; Callait-Cardinal, M.-P.; Bouhsira, E.; Polack, B.; Bourdeau, P.; Roussel Ariza, C.; Carassou, L.; Lienard, E.; Drake, J. Prevalence of Major Digestive and Respiratory Helminths in Dogs and Cats in France: Results of a Multicenter Study. Parasites Vectors 2022, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; Shaw, S.E.; Brennan, S.F.; Waal, T.D.D.; Jones, B.R.; Mulcahy, G. Angiostrongylus vasorum: A Real Heartbreaker. Trends Parasitol. 2005, 21, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Barutzki, D.; Dyachenko, V.; Schaper, R. Lungworms in Germany 2002—2016: Is There an Increase in Occurrence and Geographical Spread? Parasitol. Res. 2017, 116, 11–30. [Google Scholar] [CrossRef][Green Version]
- Uribe, M.; Segeritz, L.; Schnyder, M.; Taubert, A.; Hermosilla, C.; López-Osorio, S.; Góngora-Orjuela, A.; Chaparro-Gutiérrez, J.J. Nationwide Seroprevalence Survey of Angiostrongylus vasorum-Derived Antigens and Specific Antibodies in Dogs from Colombia. Microorganisms 2022, 10, 1565. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Vélez, J.; Gutiérrez, J.; Hirzmann, J.; Chaparro-Gutiérrez, J.J.; Piedrahita, D.; Taubert, A.; Hermosilla, C. Regional Report on Angiostrongylus vasorum in Colombia: Genetic Similarity to European Lineage. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 21–23. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Lange, M.K.; Vélez, J.; Hirzmann, J.; Gutierrez-Arboleda, J.; Taubert, A.; Hermosilla, C.; Chaparro Gutierrez, J.J. The Invasive Giant African Snail Lissachatina fulica as Natural Intermediate Host of Aelurostrongylus abstrusus, Angiostrongylus vasorum, Troglostrongylus brevior, and Crenosoma vulpis in Colombia. PLoS Neglected Trop. Dis. 2019, 13, e0007277. [Google Scholar] [CrossRef]
- van Doorn, D.C.K.; van de Sande, A.H.; Nijsse, E.R.; Eysker, M.; Ploeger, H.W. Autochthonous Angiostrongylus Vasorum Infection in Dogs in The Netherlands. Vet. Parasitol. 2009, 162, 163–166. [Google Scholar] [CrossRef]
- Maksimov, P.; Hermosilla, C.; Taubert, A.; Staubach, C.; Sauter-Louis, C.; Conraths, F.J.; Vrhovec, M.G.; Pantchev, N. GIS-Supported Epidemiological Analysis on Canine Angiostrongylus vasorum and Crenosoma vulpis Infections in Germany. Parasites Vectors 2017, 10, 108. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Hirzmann, J.; Failing, K.; Schaper, R.; Van Bourgonie, Y.R.; Backeljau, T.; Hermosilla, C.; Taubert, A. Prevalence of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Crenosoma vulpis Larvae in Native Slug Populations in Germany. Vet. Parasitol. 2018, 254, 120–130. [Google Scholar] [CrossRef]
- Ash, L.R. Diagnostic Morphology of the Third-Stage Larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea). J. Parasitol. 1970, 56, 249–253. [Google Scholar] [CrossRef]
- Gasser, R.B.; Chilton, N.B.; Hoste, H.; Beveridge, I. Rapid Sequencing of rDNA from Single Worms and Eggs of Parasitic Helminths. Nucleic Acids Res. 1993, 21, 2525–2526. [Google Scholar] [CrossRef]
- Annoscia, G.; Latrofa, M.S.; Campbell, B.E.; Giannelli, A.; Ramos, R.A.N.; Dantas-Torres, F.; Brianti, E.; Otranto, D. Simultaneous Detection of the Feline Lungworms Troglostrongylus brevior and Aelurostrongylus abstrusus by a Newly Developed Duplex-PCR. Vet. Parasitol. 2014, 199, 172–178. [Google Scholar] [CrossRef]
- Ferdushy, T.; Kapel, C.M.O.; Webster, P.; Al-Sabi, M.N.S.; Grønvold, J.R. The Effect of Temperature and Host Age on the Infectivity and Development of Angiostrongylus vasorum in the Slug Arion Lusitanicus. Parasitol. Res. 2010, 107, 147–151. [Google Scholar] [CrossRef]
- Davies, S.M.; Davies, S.M. Arion flagellus Collinge and A. Lusitanicus Mabille in the British Isles: A Morphological, Biological and Taxonomic Investigation. J. Conchol. 1987, 32, 339–354. [Google Scholar] [CrossRef]
- Slotsbo, S. Ecophysiology and Life History of the Slug Arion lusitanicus. PhD Thesis, Aarhus Universitet, Aarhus, Denmark, 2012. [Google Scholar]
- Yousif, F.; Lämmler, G. The Effect of Some Biological and Physical Factors on Infection of Biomphalaria glabrata with Angiostrongylus cantonensis. Z. Parasitenkd. 1975, 47, 191–201. [Google Scholar] [CrossRef]
- Wallace, G.D.; Rosen, L. Studies on Eosinophilic Meningitis. V. Molluscan Hosts of Angiostrongylus cantonensis on Pacific Islands. Am. J. Trop. Med. Hyg. 1969, 18, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Bech, C.; Christiansen, M.T.; Kvernland, P.; Nygård, R.M.; Rypdal, E.; Sneltorp, S.K.; Trondrud, L.M.; Tvedten, Ø.G. The Standard Metabolic Rate of a Land Snail (Cepaea hortensis) Is a Repeatable Trait and Influences Winter Survival. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2020, 249, 110773. [Google Scholar] [CrossRef] [PubMed]
- Kafle, P.; Peacock, S.J.; Grond, S.; Orsel, K.; Kutz, S. Temperature-Dependent Development and Freezing Survival of Protostrongylid Nematodes of Arctic Ungulates: Implications for Transmission. Parasites Vectors 2018, 11, 400. [Google Scholar] [CrossRef]
- Anettová, L.; Šipková, A.; Izquierdo-Rodriguez, E.; Velič, V.; Modrý, D. Rat Lungworm Survives Winter: Experimental Overwintering of Angiostrongylus cantonensis Larvae in European Slugs. Parasitology 2023, 150, 950–955. [Google Scholar] [CrossRef]
- Rousi, E.; Fink, A.H.; Andersen, L.S.; Becker, F.N.; Beobide-Arsuaga, G.; Breil, M.; Cozzi, G.; Heinke, J.; Jach, L.; Niermann, D.; et al. The Extremely Hot and Dry 2018 Summer in Central and Northern Europe from a Multi-Faceted Weather and Climate Perspective. Nat. Hazards Earth Syst. Sci. 2023, 23, 1699–1718. [Google Scholar] [CrossRef]
- FAQ on the Spanish slug Arion vulgaris. Available online: https://www.molluskenforschung.at/en/2024/faq-on-the-spanish-slug-arion-vulgaris/ (accessed on 10 June 2025).
- Kozłowski, J.; Kozłowska, M. Food preferences of Deroceras reticulatum, Arion lusitanicus and Arion rufus for various medicinal herbs and oilseed rape. J. Plant Prot. Res. 2004, 44, 239–249. [Google Scholar]
- Beeston, H.; Beeston, H. Field Notes on Helicodonta Obvoluta Muller (Continued from Page 36). J. Conchol. 1919, 16, 44–50. [Google Scholar] [CrossRef]
- Karlin, E.J.; Bacon, C. Courtship, Mating, and Egg-Laying Behavior in the Limacidae (Mollusca). Trans. Am. Microsc. Soc. 1961, 80, 399–406. [Google Scholar] [CrossRef]
- Lankester, M.W.; Anderson, R.C. Small Mammals as Paratenic Hosts of Lungworms. Can. J. Zool. 1966, 44, 341–342. [Google Scholar] [CrossRef]
- Niebuhr, C.N.; Jarvi, S.I.; Kaluna, L.; Torres Fischer, B.L.; Deane, A.R.; Leinbach, I.L.; Siers, S.R. Occurrence of Rat Lungworm (Angiostrongylus cantonensis) in Invasive Coqui Frogs (Eleutherodactylus coqui) and Other Hosts in Hawaii, USA. J. Wildl. Dis. 2019, 56, 203–207. [Google Scholar] [CrossRef]
- Morgan, J.W.M.; Eric, R. Detection and Diagnosis of Dog Lungworm Larvae and Eggs. Available online: https://www.theveterinarynurse.com/content/clinical/detection-and-diagnosis-of-dog-lungworm-larvae-and-eggs/ (accessed on 11 February 2025).
- Helm, J.; Morgan, E. Canine and Feline Lungworm Infections in the UK. Practice 2017, 39, 298–315. [Google Scholar] [CrossRef]
- Nolan, T.J.; Lok, J.B. Macrocyclic Lactones in the Treatment and Control of Parasitism in Small Companion Animals. Curr. Pharm. Biotechnol. 2012, 13, 1078–1094. [Google Scholar] [CrossRef]
- Rivory, P.; Lee, R.; Šlapeta, J. Rat Lungworm (Angiostrongylus cantonensis) Active Larval Emergence from Deceased Bubble Pond Snails (Bullastra lessoni) into Water. Parasitology 2023, 150, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Adema, C.M. Sticky Problems: Extraction of Nucleic Acids from Molluscs. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200162. [Google Scholar] [CrossRef]
- Wilson, I.G. Inhibition and Facilitation of Nucleic Acid Amplification. Appl. Environ. Microbiol. 1997, 63, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
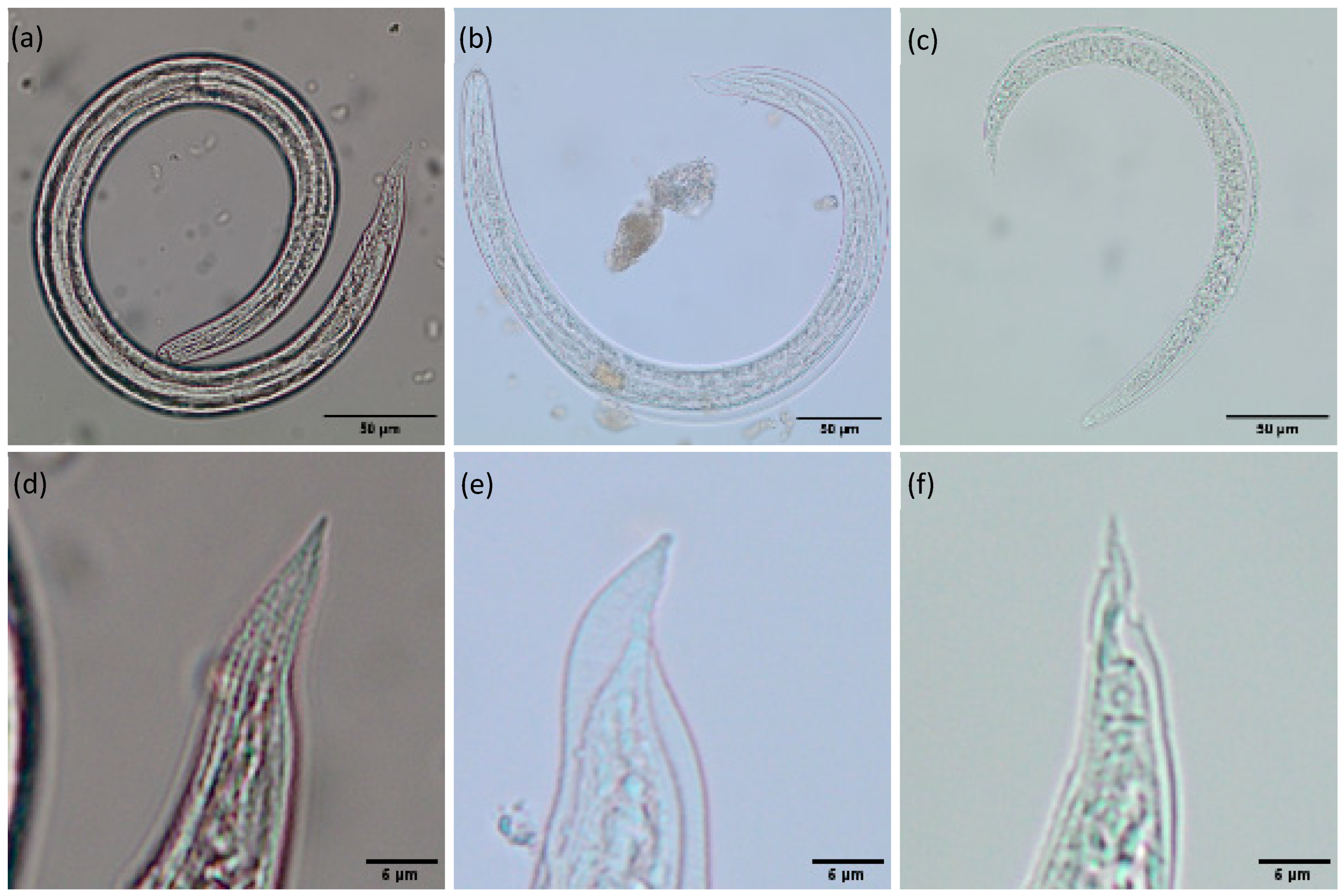
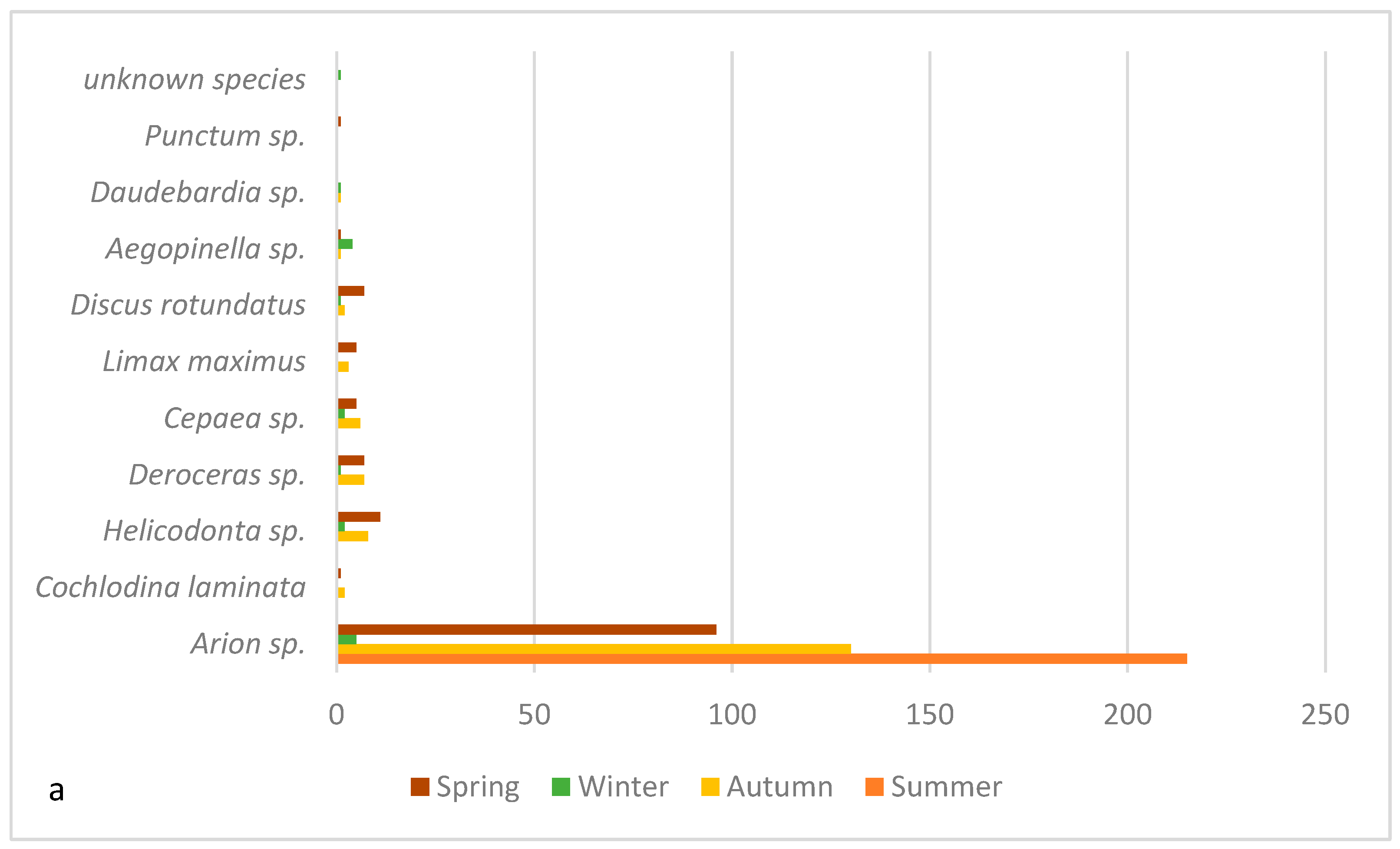
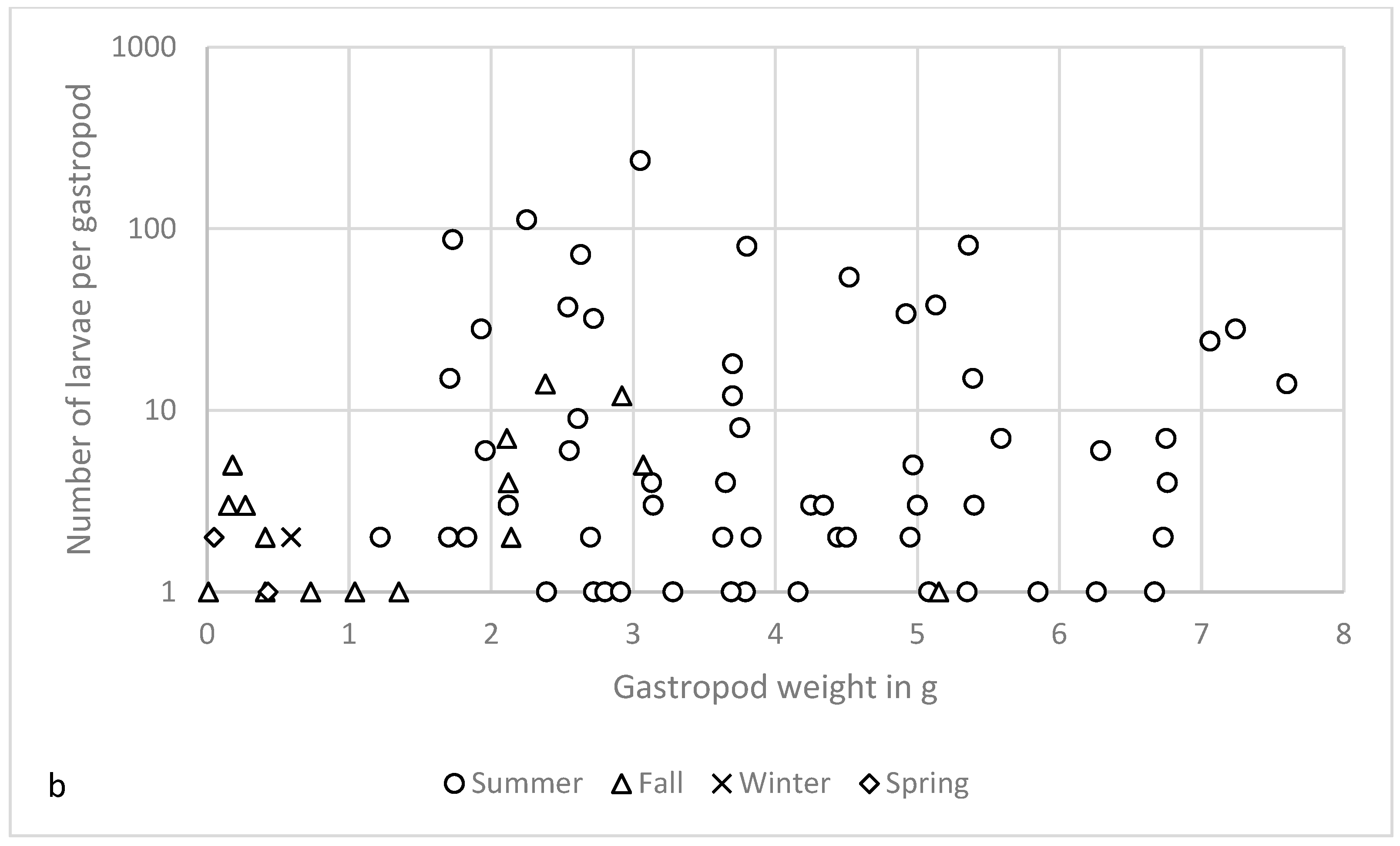
| Spring | Summer | Fall | Winter | |
|---|---|---|---|---|
| A. vasorum | 0.74% | 22.79% | 8.7% | 0% |
| Crenosoma sp. | 0% | 1.40% | 1.2% | 0% |
| Ae. abstrusus | 0% | 0.47% | 0.6% | 0% |
| Metastrongyloidea | 0.74% | 2.80% | 0% | 5.56% |
| Total | 1.48% | 27.46% | 10% | 5.56% |
| Species | Accession number | Season |
|---|---|---|
| A. vasorum | PV917150 | Summer |
| Crenosoma sp. | PV917152 | Summer |
| A. vasorum | PV917158 | Summer |
| Ae. abstrusus | PV917161 | Summer |
| A. vasorum | PV917159 | Summer |
| C. striatum | PV917154 | Summer |
| Crenosoma sp. | PV917156 | Summer |
| Ae. abstrusus | PV917171 | Fall |
| Crenosoma sp. | PV917167 | Fall |
| Crenosoma sp. | PV917170 | Fall |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dusch, A.; Segeritz, L.; Schmiedel, J.; Taubert, A.; Hermosilla, C. Re-Evaluation of a Hyperendemic Focus of Metastrongyloid Lungworm Infections in Gastropod Intermediate Hosts in Southern Germany. Pathogens 2025, 14, 800. https://doi.org/10.3390/pathogens14080800
Dusch A, Segeritz L, Schmiedel J, Taubert A, Hermosilla C. Re-Evaluation of a Hyperendemic Focus of Metastrongyloid Lungworm Infections in Gastropod Intermediate Hosts in Southern Germany. Pathogens. 2025; 14(8):800. https://doi.org/10.3390/pathogens14080800
Chicago/Turabian StyleDusch, Alena, Lisa Segeritz, Judith Schmiedel, Anja Taubert, and Carlos Hermosilla. 2025. "Re-Evaluation of a Hyperendemic Focus of Metastrongyloid Lungworm Infections in Gastropod Intermediate Hosts in Southern Germany" Pathogens 14, no. 8: 800. https://doi.org/10.3390/pathogens14080800
APA StyleDusch, A., Segeritz, L., Schmiedel, J., Taubert, A., & Hermosilla, C. (2025). Re-Evaluation of a Hyperendemic Focus of Metastrongyloid Lungworm Infections in Gastropod Intermediate Hosts in Southern Germany. Pathogens, 14(8), 800. https://doi.org/10.3390/pathogens14080800







