Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assay
2.3. Treatments
2.4. Viruses
2.5. Time Course and MOI for VSV-SARS-CoV-2-S∆21
2.6. Viral Titration Protocol for SARS-CoV-2
2.7. Models of Infection
- Pre-treatment model: Vero E6 cells were pre-treated with the specific hormone, seeded in 96-wells plate, and the day after the treatment was removed, the virus was added in presence of the compounds; after 3 h of viral adsorption, the viral inoculum was removed, cells were washed, and then medium without treatments was added.
- Pre–post-treatment model: Infection was performed as in pre-treatment model, but after the 3 h of viral adsorption, medium with the different treatments was added.
2.8. Real Time qPCR
2.9. Bio-Plex Multiplex System
2.10. Statistical Analysis
3. Results
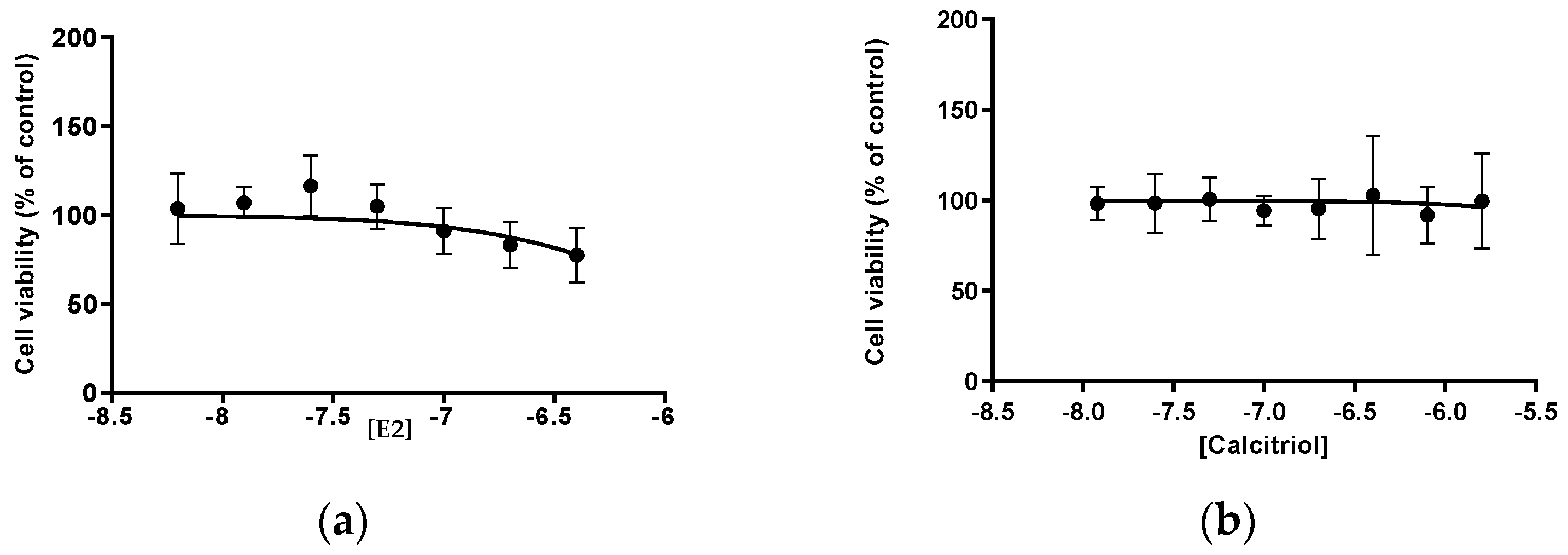
3.1. MTT E2 and Calcitriol
3.2. VSV-Based Pseudovirus SARS-CoV-2 Infection: MOI and Time Course
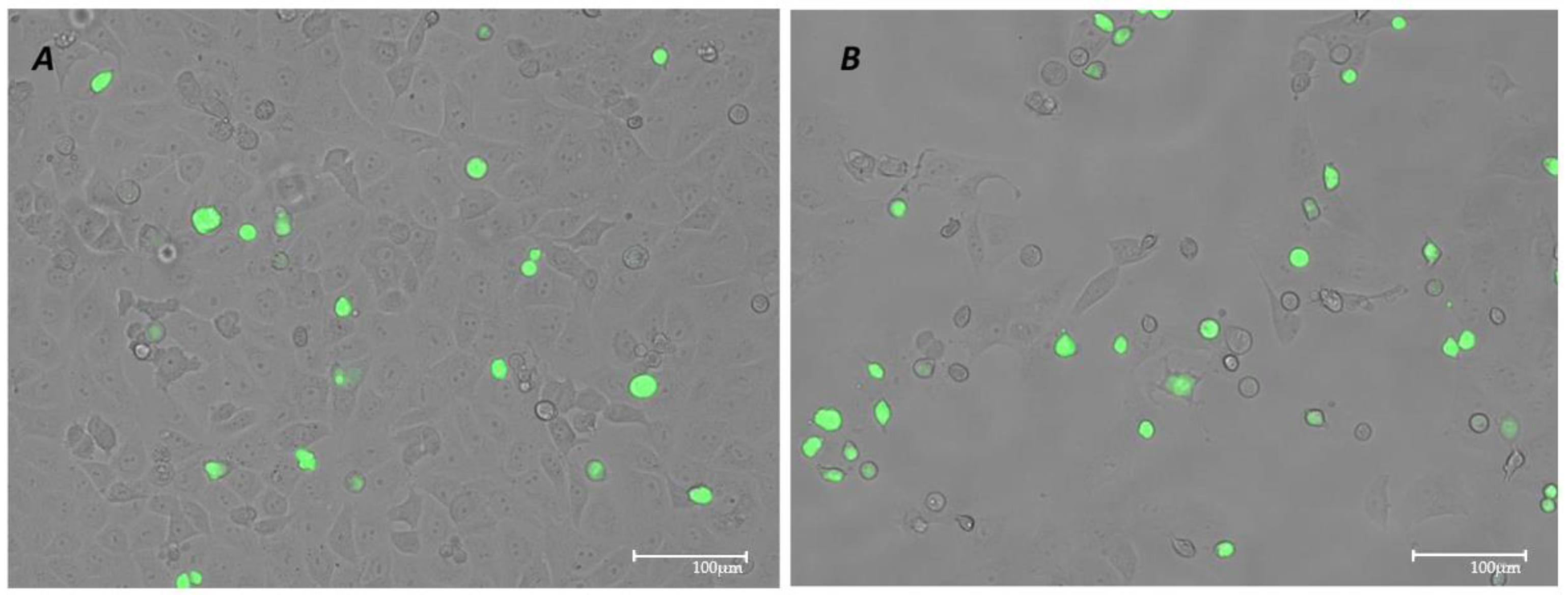
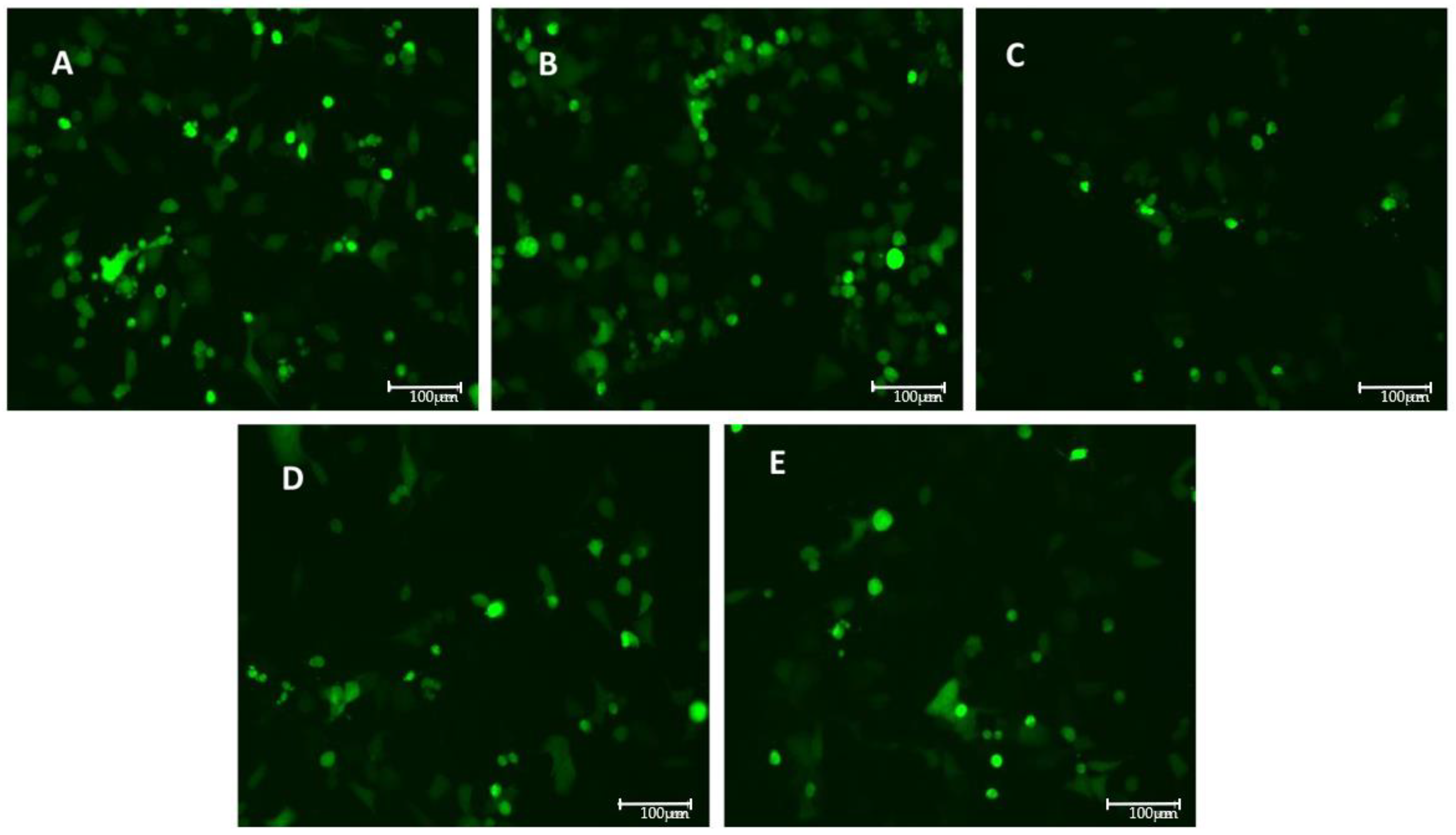
3.3. Models of Infection and Plaque Assay: VSV-Based Pseudovirus SARS-CoV-2
3.4. Models of Infection and Viral Titre (TCID50/mL) SARS-CoV-2
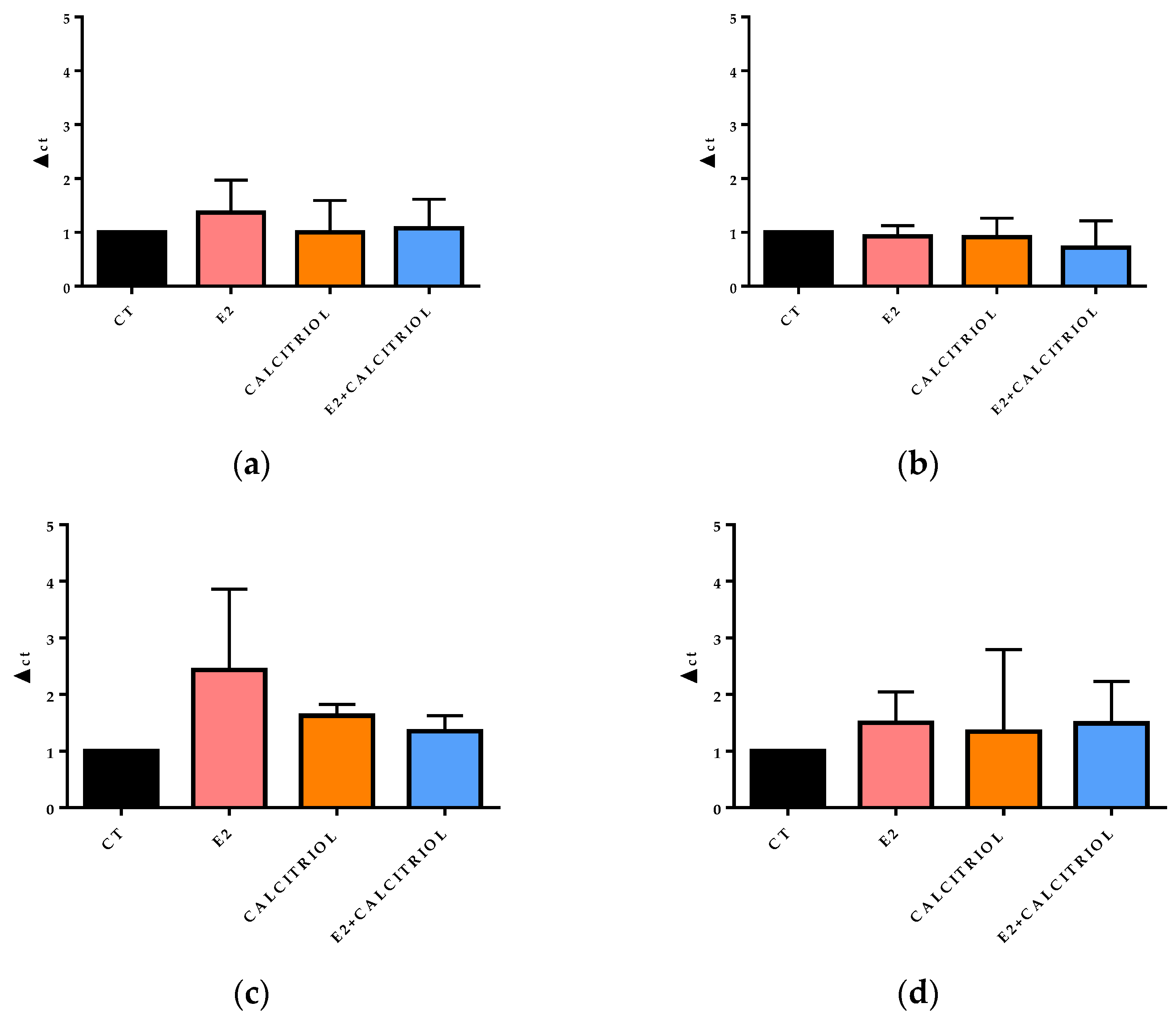
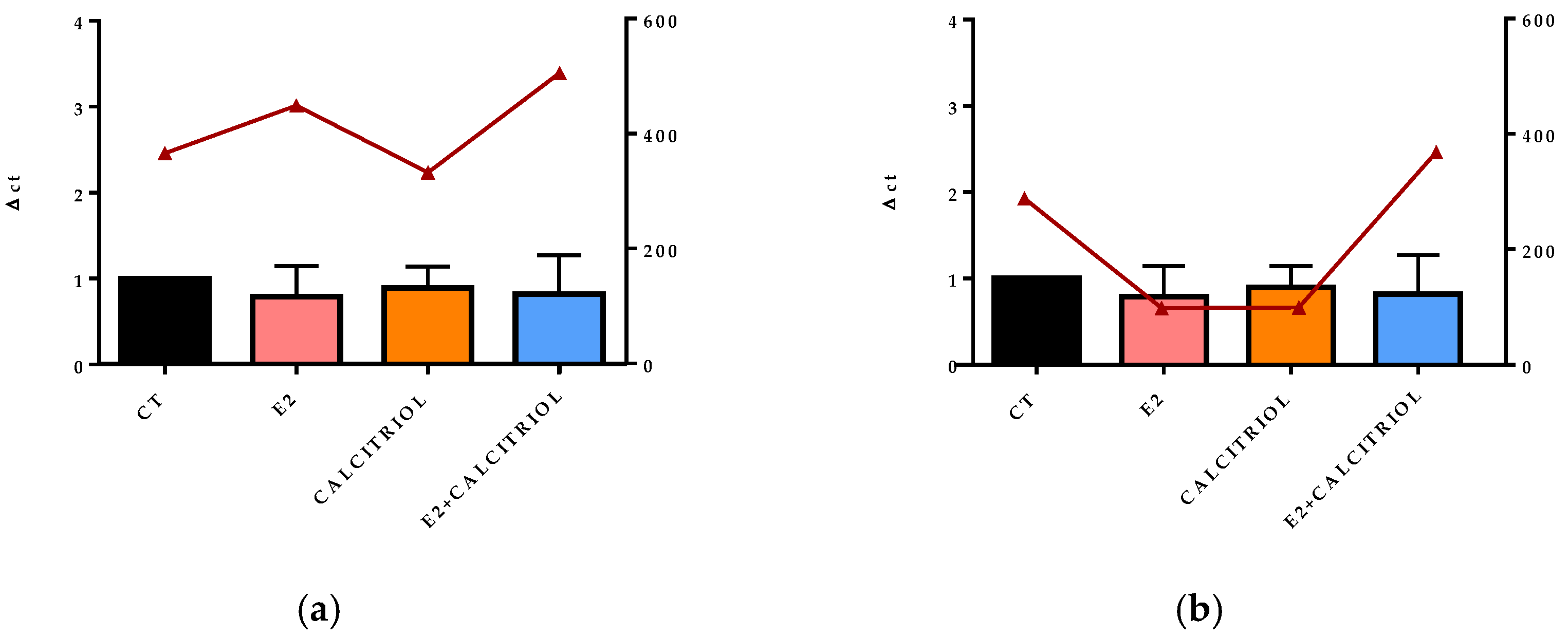
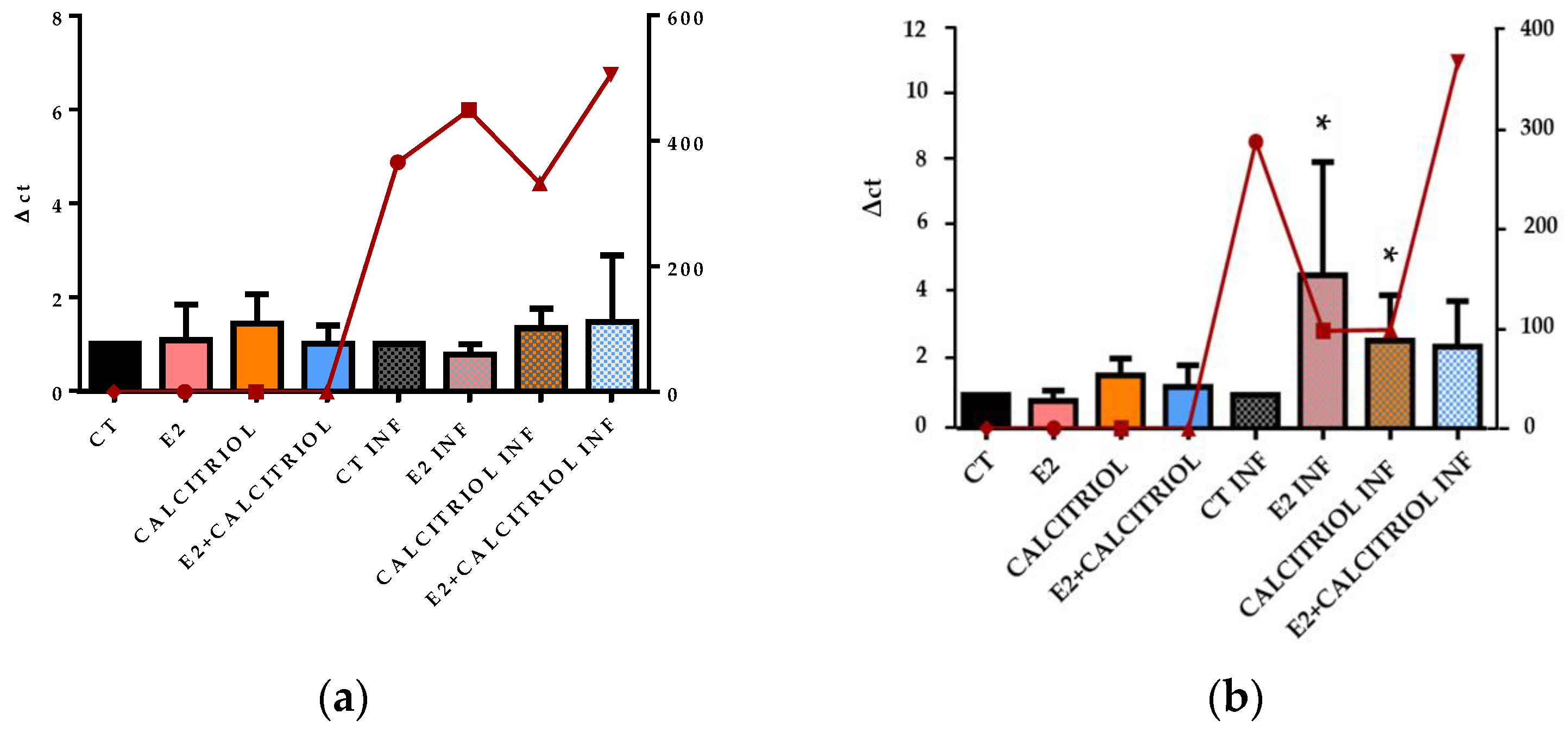
3.5. VSV-Based Pseudovirus SARS-CoV-2 and SARS-CoV-2 Entry: Gene Expression of ACE2 and VDR
3.6. Cytokines
3.7. SARS-CoV-2 Infection and PD-L1 Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus-2 |
| COVID-19 | Coronavirus Disease 19 |
| E2 | 17,β-Estradiol |
| Calcitriol | 1,25 α Dihydroxyvitamin D3 |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| VDR | Vitamin D Receptor |
| PD-L1 | Programmed Death-Ligand 1 |
| WHO | World Health Organization |
| SARS-CoV | Severe Acute Respiratory Syndrome Coronavirus-1 |
| MERS-CoV | Middle East Respiratory Syndrome |
| +ssRNA | Positive Single-Stranded RNA |
| TMPRSS2 | Transmembrane Serine Protease 2 |
| CSS | Cytokine Storm Syndrome |
| sPD-L1 | Soluble Programmed Death-Ligand 1 |
| PD-1 | Programmed Death-1 |
| TGFβ | Transforming Growth Factor-β |
| NO | Nitric Oxide |
| CVDs | Cardiovascular Disease |
| E1 | Estrone |
| E3 | Estriol |
| E4 | Estetrol |
| ERs | Estrogen Receptors |
| EREs | Estrogen Response Elements |
| Ap1 | Activator Protein-1 |
| Sp1 | Specificity Protein-1 |
| PI3K | Phosphatidylinositol 3-Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| RXRs | Retinoid X Receptors |
| VDRE | Vitamin D Receptor Element |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal Bovin Serum |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| VSV | Vesicular Stomatitis Virus |
| GFP | Green Fluorescent Protein |
| FFU | Foci Forming Unit |
| CPE | Cytopathic Effect |
| TCID 50 | 50% Tissue Culture Infectious Dose |
| TEM | Transmission Electronic Microscope |
| ON | Overnight |
| LL-37 | Antimicrobial Peptide Cathelicidin |
| VEGF | Vascular Endothelial Growth Factor |
| ARDS | Acute Respiratory Distress Syndrome |
Appendix A

| Treatment | ||||
|---|---|---|---|---|
| Pre-Treatment | ||||
| CT INF | E2 INF | CALCITRIOL INF | E2 + CALCITRIOL INF | |
| 130 | 105 | 117 | 108 | |
| 158 | 95 | 107 | 145 | |
| 313 | 101 | 110 | 122 | |
| 137 | 118 | 125 | 97 | |
| 130 | 70 | 103 | 65 | |
| 114 | 70 | 98 | 54 | |
| 138 | 87 | 81 | 112 | |
| 130 | 77 | 83 | 75 | |
| 151 | 67 | 80 | 70 | |
| Pre–Post-Treatment | ||||
| 146 | 84 | 133 | 128 | |
| 194 | 97 | 121 | 126 | |
| 131 | 90 | 115 | 127 | |
| 114 | 94 | 94 | 115 | |
| 118 | 108 | 128 | 104 | |
| 115 | 90 | 142 | 130 | |
| 180 | 84 | 119 | 102 | |
| 119 | 94 | 80 | 110 | |
| 153 | 90 | 104 | 77 | |
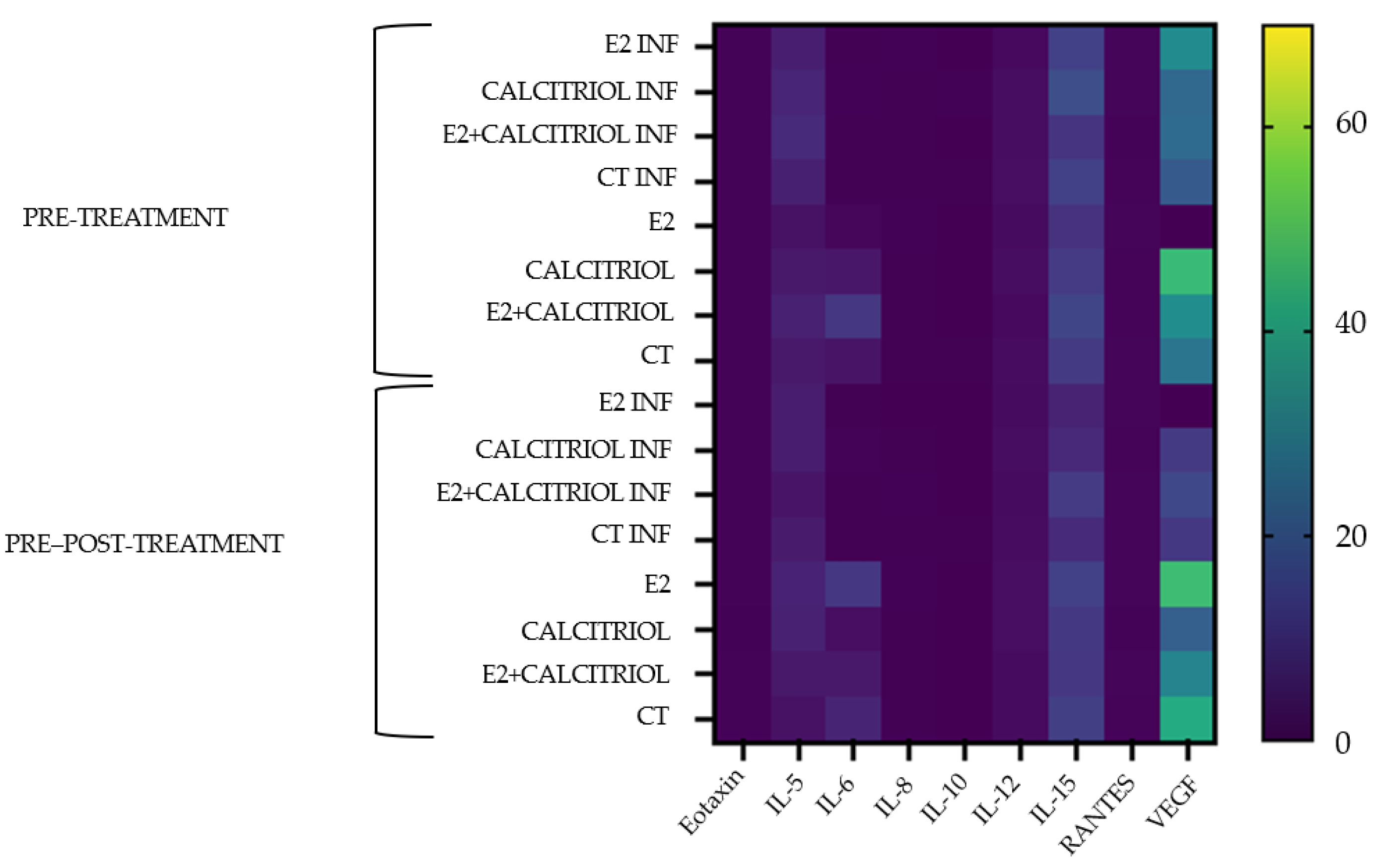
| Cytokines/Chemokines and Growth Factors Average Concentrations (pg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eotoxin | IL-5 | IL-6 | IL-8 | IL-10 | IL-12 | IL-15 | Rantes | VEGF | |
| Pre-Treatment | |||||||||
| E2 INF | 0.34 | 5.6 | 0.07 | 0.23 | 0.00 | 1.43 | 13.04 | 0.57 | 33.36 |
| CALCITRIOL INF | 0.40 | 7.01 | 0.17 | 0.12 | 0.26 | 2.09 | 16.38 | 0.68 | 23.08 |
| E2 + CALCITRIOL INF | 0.38 | 8.16 | 0.08 | 0.05 | 0.00 | 2.09 | 10.40 | 0.32 | 24.05 |
| CT INF | 0.36 | 5.84 | 0.07 | 0.10 | 0.05 | 2.26 | 12.98 | 0.5 | 19.31 |
| E2 | 0.34 | 3.00 | 0.96 | 0.26 | 0.00 | 1.76 | 9.87 | 0.57 | 0.00 |
| CALCITRIOL | 0.38 | 4.45 | 4.11 | 0.13 | 0.00 | 2.09 | 12.07 | 0.45 | 47.25 |
| E2 + CALCITRIOL | 0.38 | 6.16 | 10.95 | 0.05 | 0.00 | 1.43 | 14.02 | 0.44 | 33.87 |
| CT | 0.37 | 4.44 | 3.32 | 0.12 | 0.02 | 1.93 | 11.72 | 0.62 | 27.00 |
| Pre–Post-Treatment | |||||||||
| E2 INF | 0.34 | 5.3 | 0.13 | 0.00 | 0.00 | 1.76 | 6.56 | 0.57 | 0.00 |
| CALCITRIOL INF | 0.38 | 5.31 | 0.39 | 0.05 | 0.00 | 2.09 | 7.75 | 0.45 | 11.65 |
| E2 + CALCITRIOL INF | 0.38 | 3.54 | 0.08 | 0.17 | 0.00 | 1.76 | 12.07 | 0.62 | 14.93 |
| CT INF | 0.37 | 4.72 | 0.05 | 0.08 | 0.02 | 2.01 | 8.14 | 0.59 | 11.05 |
| E2 | 0.41 | 6.44 | 10.98 | 0.18 | 0.00 | 2.42 | 13.11 | 0.57 | 48.09 |
| CALCITRIOL | 0.27 | 6.17 | 2.22 | 0.12 | 0.00 | 2.42 | 11.03 | 0.38 | 20.66 |
| E2 + CALCITRIOL | 0.38 | 4.16 | 4.44 | 0.05 | 0.00 | 1.76 | 10.91 | 0.57 | 31.32 |
| CT | 0.39 | 2.97 | 6.62 | 0.11 | 0.00 | 1.84 | 12.74 | 0.50 | 42.78 |
References
- Adil, M.T.; Rahman, R.; Whitelaw, D.; Jain, V.; Al-Taan, O.; Rashid, F.; Munasinghe, A.; Jambulingam, P. SARS-CoV-2 and the Pandemic of COVID-19. Postgrad. Med. J. 2021, 97, 110–116. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. Sars-Cov-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef] [PubMed]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The Molecular Virology of Coronaviruses. J. Biol. Chem. 2020, 295, 1910–1934. [Google Scholar] [CrossRef] [PubMed]
- Samavati, L.; Uhal, B.D. ACE2, Much More Than Just a Receptor for SARS-COV-2. Front. Cell Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular Mechanism of Interaction between SARS-CoV-2 and Host Cells and Interventional Therapy. Signal Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 Infection: An Overview on Cytokine Storm and Related Interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- Manik, M.; Singh, R.K. Role of Toll-like Receptors in Modulation of Cytokine Storm Signaling in SARS-CoV-2-Induced COVID-19. J. Med. Virol. 2022, 94, 869–877. [Google Scholar] [CrossRef]
- Beserra, D.R.; Alberca, R.W.; Branco, A.C.C.C.; De Mendonça Oliveira, L.; De Souza Andrade, M.M.; Gozzi-Silva, S.C.; Teixeira, F.M.E.; Yendo, T.M.; Da Silva Duarte, A.J.; Sato, M.N. Upregulation of PD-1 Expression and High SPD-L1 Levels Associated with COVID-19 Severity. J. Immunol. Res. 2022, 2022, 9764002. [Google Scholar] [CrossRef]
- Sabbatino, F.; Conti, V.; Franci, G.; Sellitto, C.; Manzo, V.; Pagliano, P.; De Bellis, E.; Masullo, A.; Salzano, F.A.; Caputo, A.; et al. PD-L1 Dysregulation in COVID-19 Patients. Front. Immunol. 2021, 12, 695242. [Google Scholar] [CrossRef]
- R Bonam, S.; Hu, H.; Bayry, J. Role of the PD-1 and PD-L1 Axis in COVID-19. Future Microbiol. 2022, 17, 985–988. [Google Scholar] [CrossRef]
- Madeleine, M.B.; Altfeld, M. Implications of Sex Differences in Immunity for SARS-CoV-2 Pathogenesis and Design of Therapeutic Interventions. Immunity 2020, 53, 19–21. [Google Scholar]
- Peruzzu, D.; Pagano, M.T.; Pierdominici, M.; Ruggieri, A.; Antinori, A.; D’Offizi, G.; Petrosillo, N.; Palmieri, F.; Piselli, P.; Boumis, E.; et al. Synergy Between Vitamin D and Sex Hormones in Respiratory Functionality of Patients Affected by COVID-19. Front. Pharmacol. 2021, 12, 683529. [Google Scholar] [CrossRef] [PubMed]
- Lemes, R.M.R.; Costa, A.J.; Bartolomeo, C.S.; Bassani, T.B.; Nishino, M.S.; Pereira, G.J.; Smaili, S.S.; Maciel, R.M.; Braconi, C.T.; da Cruz, E.F.; et al. 17β-Estradiol Reduces SARS-CoV-2 Infection in Vitro. Physiol. Rep. 2021, 9, e14707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Hua, M.; Jiu, T.; Ge, R.L.; Bai, Z. Biofunctional Roles of Estrogen in Coronavirus Disease 2019: Beyond a Steroid Hormone. Front. Pharmacol. 2022, 13, 1003469. [Google Scholar] [CrossRef] [PubMed]
- Tingskov, S.J.; Mutsaers, H.A.M.; Nørregaard, R. Estrogen Regulates Aquaporin-2 Expression in the Kidney. In Vitamins and Hormones; Academic Press Inc.: New York, NY, USA, 2020; Volume 112, pp. 243–264. ISBN 9780128208090. [Google Scholar]
- Borborema, M.E.A.; Lucena, T.M.C.; Silva, J.A. Vitamin D and Estrogen Steroid Hormones and Their Immunogenetic Roles in Infectious Respiratory (TB and COVID-19) Diseases. Genet. Mol. Biol. 2023, 1, e20220158. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of Estrogen Receptors in Health and Disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Briceno Noriega, D.; Savelkoul, H.F.J. Vitamin D: A Potential Mitigation Tool for the Endemic Stage of the COVID-19 Pandemic? Front. Public Health 2022, 10, 888168. [Google Scholar] [CrossRef]
- Apaydin, T.; Polat, H.; Dincer Yazan, C.; Ilgin, C.; Elbasan, O.; Dashdamirova, S.; Bayram, F.; Tukenmez Tigen, E.; Unlu, O.; Tekin, A.F.; et al. Effects of Vitamin D Receptor Gene Polymorphisms on the Prognosis of COVID-19. Clin. Endocrinol. 2022, 96, 819–830. [Google Scholar] [CrossRef]
- Mohan, M.; Cherian, J.J.; Sharma, A. Exploring Links between Vitamin D Deficiency and Covid-19. PLoS Pathog. 2020, 16, e1008874. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The Role of Vitamin D in Autoimmune Diseases: Could Sex Make the Difference? Biol. Sex Differ. 2021, 12, 12. [Google Scholar] [CrossRef]
- Condor Capcha, J.M.; Lambert, G.; Dykxhoorn, D.M.; Salerno, A.G.; Hare, J.M.; Whitt, M.A.; Pahwa, S.; Jayaweera, D.T.; Shehadeh, L.A. Generation of SARS-CoV-2 Spike Pseudotyped Virus for Viral Entry and Neutralization Assays: A 1-Week Protocol. Front. Cardiovasc. Med. 2021, 7, 618651. [Google Scholar] [CrossRef]
- Smither, S.J.; Lear-Rooney, C.; Biggins, J.; Pettitt, J.; Lever, M.S.; Olinger, G.G. Comparison of the Plaque Assay and 50% Tissue Culture Infectious Dose Assay as Methods for Measuring Filovirus Infectivity. J. Virol. Methods 2013, 193, 565–571. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. AJE 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Qaderi, K.; Hosseinirad, H.; Kalhor, M.; Zangeneh, S.; Pournaghi, M.; Khodavirdilou, R.; Keshavarz, M.; Eghdampour, F.; Mirmolaei, S.T.; Jesmani, Y.; et al. The Relationship between Sex Steroids (E2, Progesterone, and AMH) Levels and Severity and Fatality of COVID-19: A Systematic Review. Heliyon 2023, 9, e14218. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Agrawal, P.; Gautam, A.; Pursnani, N.; Agarwal, M.; Agarwal, A.; Parihar, A.; Pandey, A. Covid-19 Outcomes in Postmenopausal and Perimenopausal Females: Is Estrogen Hormone Attributing to Gender Differences? J. Midlife Health 2020, 11, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.-K.; Ng, Y.L.; Ahidjo, B.A.; Aw, Z.Q.; Chen, H.; Wong, Y.H.; Lee, R.C.H.; Loe, M.W.C.; Liu, J.; Tan, K.S.; et al. Evaluation of In Vitro and In Vivo Antiviral Activities of Vitamin D for SARS-CoV-2 and Variants. Pharmaceutics 2023, 15, 925. [Google Scholar] [CrossRef]
- Pagano, M.T.; Peruzzu, D.; Ruggieri, A.; Ortona, E.; Gagliardi, M.C. Vitamin D and Sex Differences in COVID-19. Front. Endocrinol. 2020, 11, 567824. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Hafezi, S.; Saheb Sharif-Askari, F.; Saheb Sharif-Askari, N.; Ali Hussain Alsayed, H.; Alsafar, H.; Al Anouti, F.; Hamid, Q.; Halwani, R. Vitamin D Enhances Type I IFN Signaling in COVID-19 Patients. Sci. Rep. 2022, 12, 17778. [Google Scholar] [CrossRef]
- Schwartz, N.; Verma, A.; Bivens, C.B.; Schwartz, Z.; Boyan, B.D. Rapid Steroid Hormone Actions via Membrane Receptors. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2289–2298. [Google Scholar] [CrossRef]
- Geerling, E.; Pinski, A.N.; Stone, T.E.; DiPaolo, R.J.; Zulu, M.Z.; Maroney, K.J.; Brien, J.D.; Messaoudi, I.; Pinto, A.K. Roles of Antiviral Sensing and Type I Interferon Signaling in the Restriction of SARS-CoV-2 Replication. iScience 2022, 25, 103553. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Liu, J.; Xia, H.; Zou, J.; Muruato, A.E.; Periasamy, S.; Kurhade, C.; Plante, J.A.; Bopp, N.E.; et al. A Live-Attenuated SARS-CoV-2 Vaccine Candidate with Accessory Protein Deletions. Nat. Commun. 2022, 13, 4337. [Google Scholar] [CrossRef]
- Gupta, A.; Jayakumar, M.N.; Saleh, M.A.; Kannan, M.; Halwani, R.; Qaisar, R.; Ahmad, F. SARS-CoV-2 Infection- Induced Growth Factors Play Differential Roles in COVID-19 Pathogenesis. Life Sci. 2022, 304, 120703. [Google Scholar] [CrossRef]
- Lampropoulou, D.I.; Bala, V.M.; Zerva, E.; Pliakou, E.; Filippou, D.; Gazouli, M.; Aravantinos, G. The Potential Role of the Combined PARP-1 and VEGF Inhibition in Severe SARS-CoV-2 (COVID-19) Infection. J. Infect. Dev. Ctries. 2022, 16, 101–111. [Google Scholar] [CrossRef]
- Mura, M.; Dos Santos, C.C.; Stewart, D.; Liu, M. Vascular Endothelial Growth Factor and Related Molecules in Acute Lung Injury. J. Appl. Physiol. 2004, 97, 1605–1617. [Google Scholar] [CrossRef]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.W.A.L.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Barcena, M.; et al. SARS-Coronavirus-2 Replication in Vero E6 Cells: Replication Kinetics, Rapid Adaptation and Cytopathology. J. Gen. Virol. 2020, 101, 925–940. [Google Scholar] [CrossRef]

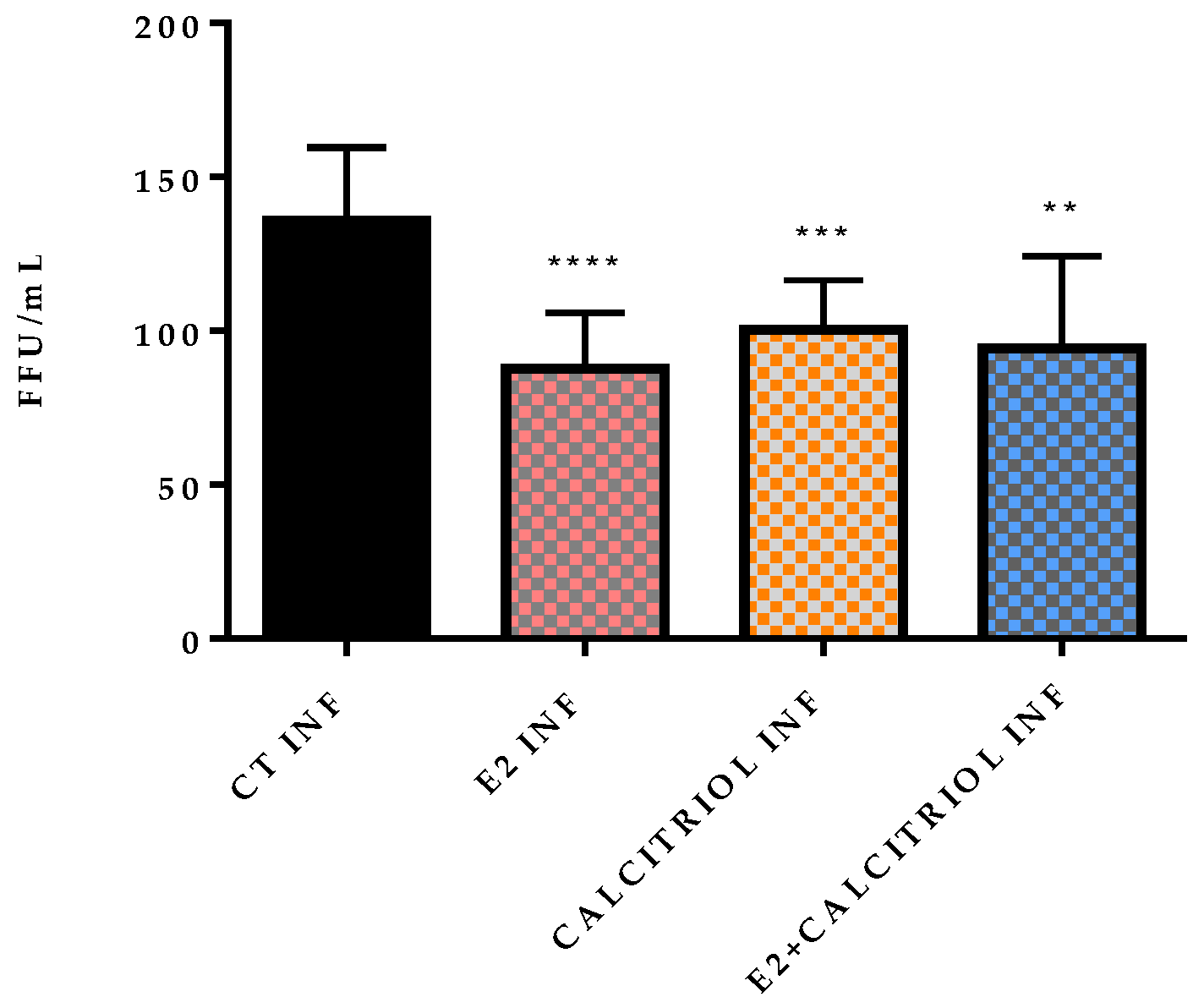
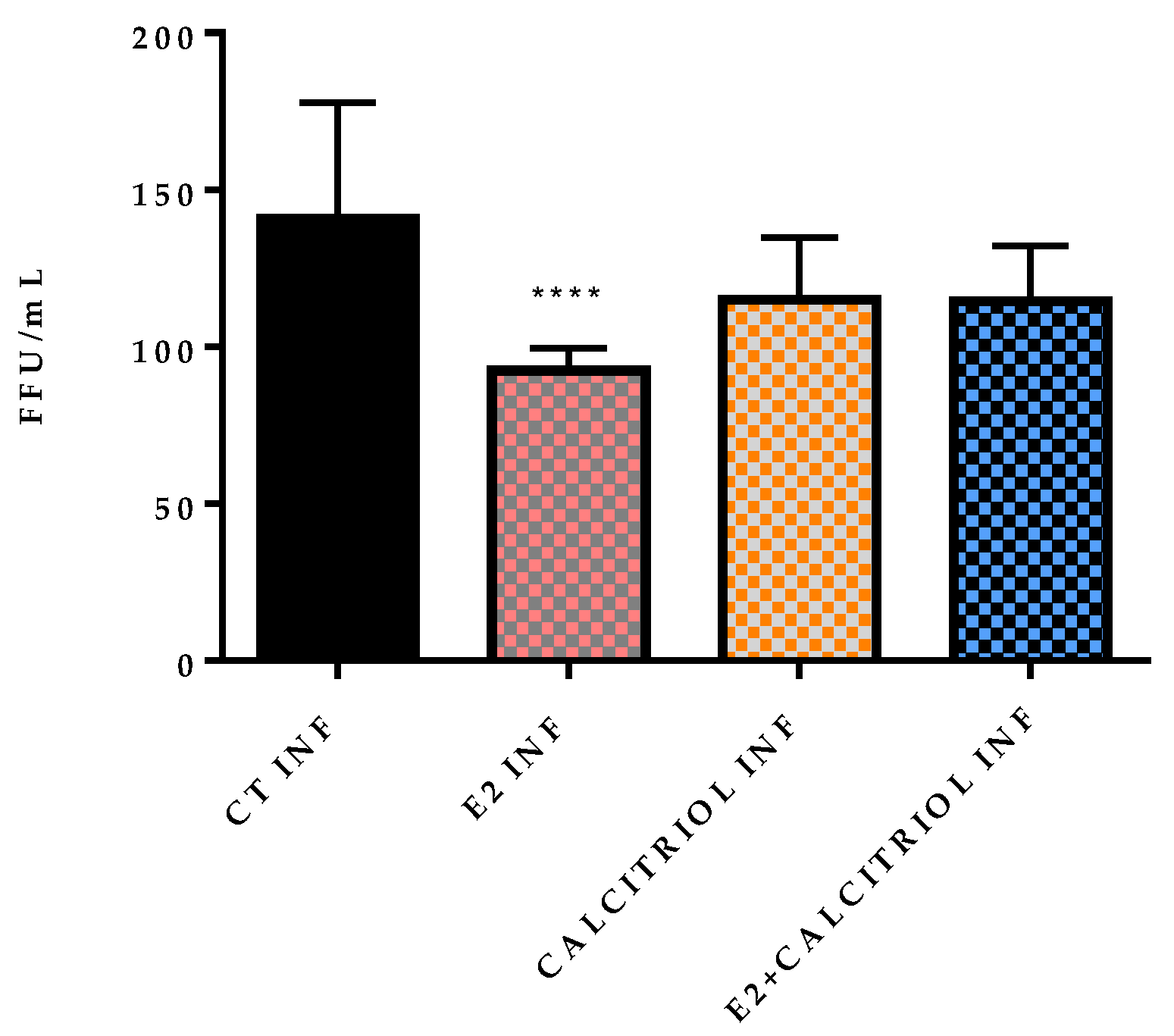

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vercellino, N.; Ferrari, A.; Sammartino, J.C.; Bellan, M.; Iskandar, E.; Lilleri, D.; Minisini, R. Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry. Pathogens 2025, 14, 765. https://doi.org/10.3390/pathogens14080765
Vercellino N, Ferrari A, Sammartino JC, Bellan M, Iskandar E, Lilleri D, Minisini R. Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry. Pathogens. 2025; 14(8):765. https://doi.org/10.3390/pathogens14080765
Chicago/Turabian StyleVercellino, Nicole, Alessandro Ferrari, José Camilla Sammartino, Mattia Bellan, Elizabeth Iskandar, Daniele Lilleri, and Rosalba Minisini. 2025. "Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry" Pathogens 14, no. 8: 765. https://doi.org/10.3390/pathogens14080765
APA StyleVercellino, N., Ferrari, A., Sammartino, J. C., Bellan, M., Iskandar, E., Lilleri, D., & Minisini, R. (2025). Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry. Pathogens, 14(8), 765. https://doi.org/10.3390/pathogens14080765








