A Major Facilitator Superfamily Transporter Is Critical for the Metabolism and Biogenesis of the Apicoplast
Abstract
1. Introduction
2. Materials and Methods
3. Results
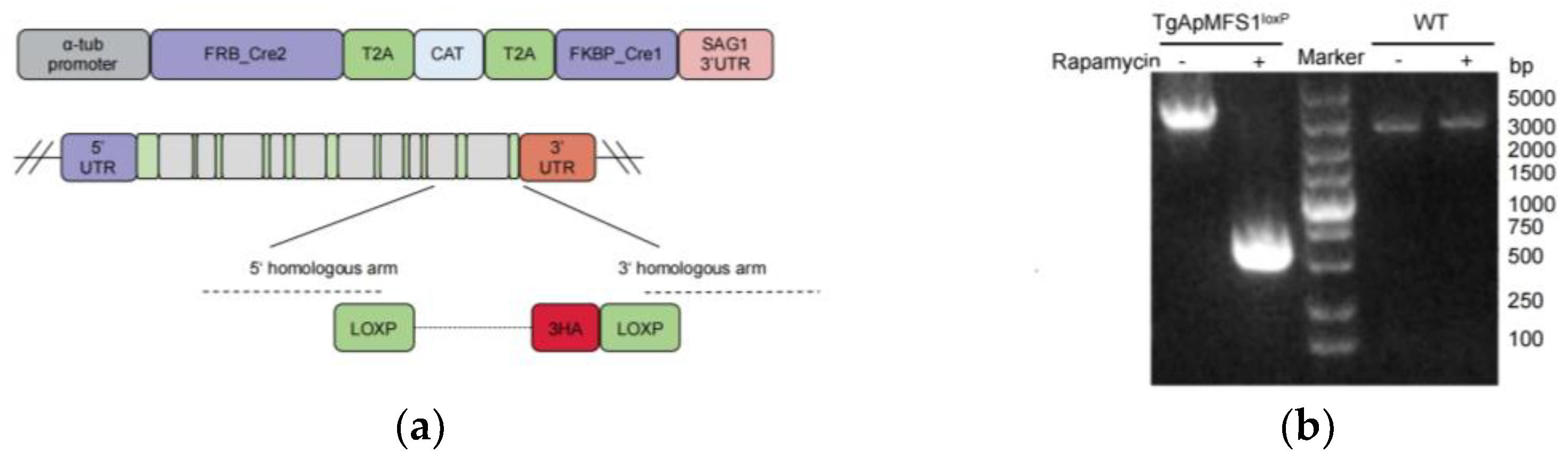
3.1. Establishment of a Conditional Knockout Strain for TgApMFS1
3.2. TgApMFS1 Is Required for the Survival of T. gondii
3.3. TgApMFS1 Is Required for the Inheritance of the Apicoplast
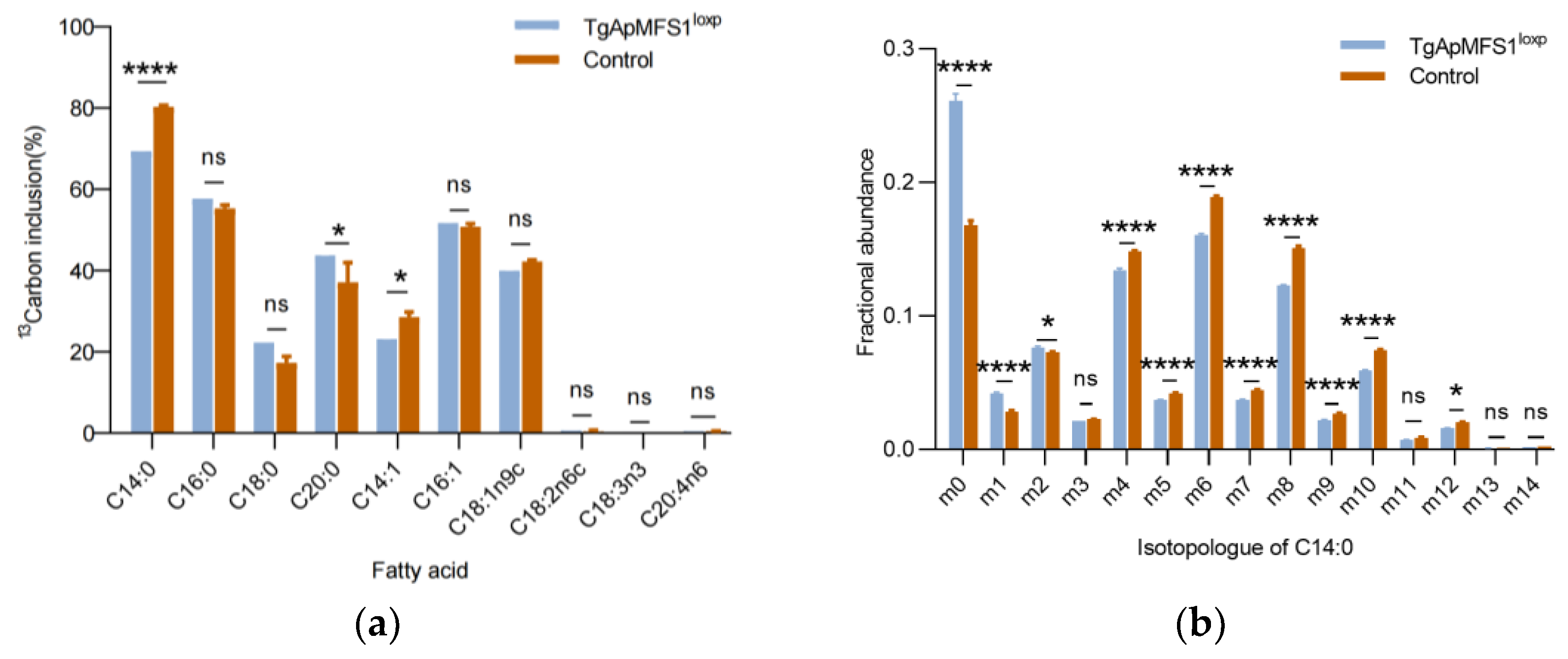
3.4. Depletion of TgApMFS1 Disturb the Fatty Acid Synthesis of the Parasites
3.5. Depletion of TgApMFS1 Hinder the Sugar Metabolism
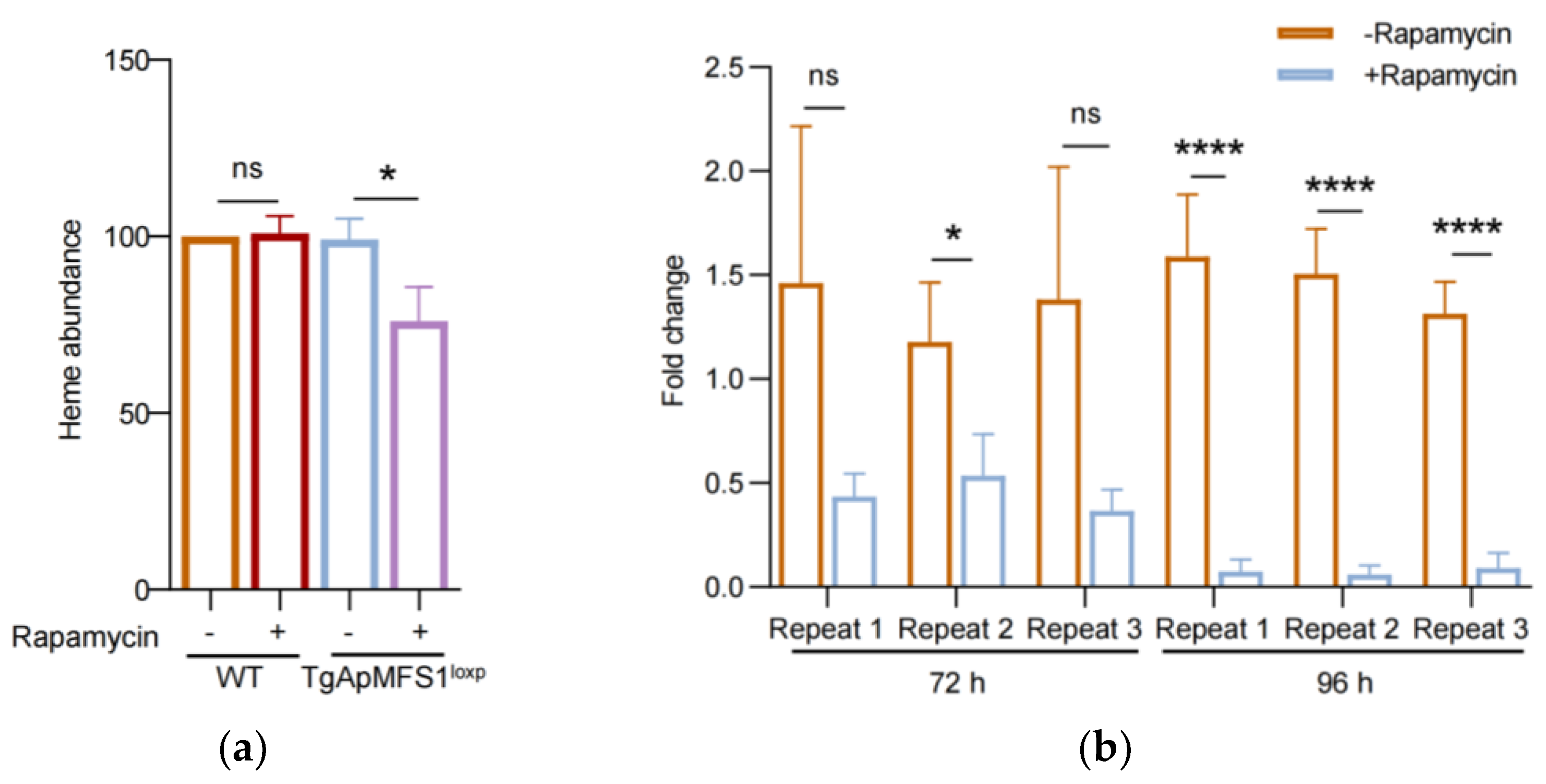
3.6. Depletion of TgApMFS1 Disturbs the Heme Synthesis and Obstructs the Replication of the Apicoplast Genome in the Parasites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TgApMFS1 | T. gondii apicoplast MFS transporter 1 |
| GC-MS | gas chromatography-mass spectrometry |
| IFA | immunofluorescence assay |
| TgCPN60 | T. gondii Chaperonin 60 |
| DMEM | dulbecco’s modified eagle medium |
| FBS | fetal bovine serum |
| PfiTPT | P. falciparum innermost triosephosphate/phosphate transporter |
| PfoTPT | P. falciparum outermost triosephosphate/phosphate transporter |
| TgAPT1 | T. gondii apicoplast phosphate transporter 1 |
| ER | endoplasmic reticulum |
| IPP | isopentenyl pyrophosphate |
| MEP | methylerythritol phosphate |
| LPA | lysophosphatidic acid |
| FA | fatty acids |
| gRNA | guide RNA |
| PCR | polymerase chain reaction |
| TBS | Tris-buffered saline |
| TgSAG2 | T. gondii surface antigen 2 |
| hyperLOPIT | hyperplexed localization analysis of organelle proteins |
| MID | mass isotopologue distributions |
| FAME | fatty ccid methyl ester |
| TCA | tricarboxylic acid cycle |
References
- Dubey, J.P. The history of Toxoplasma gondii—The first 100 years. J. Eukaryot. Microbiol. 2008, 55, 467–475. [Google Scholar] [CrossRef]
- El Bissati, K.; Levigne, P.; Lykins, J.; Adlaoui, E.B.; Barkat, A.; Berraho, A.; Laboudi, M.; El Mansouri, B.; Ibrahimi, A.; Rhajaoui, M.; et al. Global initiative for congenital toxoplasmosis: An observational and international comparative clinical analysis. Emerg. Microbes Infect. 2018, 7, 165. [Google Scholar] [CrossRef]
- Kloehn, J.; Lacour, C.E.; Soldati-Favre, D. The metabolic pathways and transporters of the plastid organelle in Apicomplexa. Curr. Opin. Microbiol. 2021, 63, 250–258. [Google Scholar] [CrossRef]
- Chen, X.; Suo, X.; Zhu, G.; Shen, B. The apicoplast biogenesis and metabolism: Current progress and questions. Trends Parasitol. 2024, 40, 1144–1158. [Google Scholar] [CrossRef]
- Brooks, C.F.; Johnsen, H.; van Dooren, G.G.; Muthalagi, M.; Lin, S.S.; Bohne, W.; Fischer, K.; Striepen, B. The toxoplasma apicoplast phosphate translocator links cytosolic and apicoplast metabolism and is essential for parasite survival. Cell Host Microbe 2010, 7, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; King, T.P.; Ayong, L.; Asady, B.; Cai, X.; Rahman, T.; Vella, S.A.; Coppens, I.; Patel, S.; Moreno, S.N.J. A plastid two-pore channel essential for inter-organelle communication and growth of Toxoplasma gondii. Nat. Commun. 2021, 12, 5802. [Google Scholar] [CrossRef] [PubMed]
- Fleige, T.; Fischer, K.; Ferguson, D.J.; Gross, U.; Bohne, W. Carbohydrate metabolism in the Toxoplasma gondii apicoplast: Localization of three glycolytic isoenzymes, the single pyruvate dehydrogenase complex, and a plastid phosphate translocator. Eukaryot. Cell 2007, 6, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Karnataki, A.; Derocher, A.; Coppens, I.; Nash, C.; Feagin, J.E.; Parsons, M. Cell cycle-regulated vesicular trafficking of Toxoplasma APT1, a protein localized to multiple apicoplast membranes. Mol. Microbiol. 2007, 63, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Linka, M.; Mullin, K.A.; Weber, A.P.; McFadden, G.I. The carbon and energy sources of the non-photosynthetic plastid in the malaria parasite. FEBS. Lett. 2010, 584, 549–554. [Google Scholar] [CrossRef]
- Mullin, K.A.; Lim, L.; Ralph, S.A.; Spurck, T.P.; Handman, E.; McFadden, G.I. Membrane transporters in the relict plastid of malaria parasites. Proc. Natl. Acad. Sci. USA 2006, 103, 9572–9577. [Google Scholar] [CrossRef]
- Dong, H.; Yang, J.; He, K.; Zheng, W.B.; Lai, D.H.; Liu, J.; Ding, H.Y.; Wu, R.B.; Brown, K.M.; Hide, G.; et al. The Toxoplasma monocarboxylate transporters are involved in the metabolism within the apicoplast and are linked to parasite survival. Elife 2024, 12, RP88866. [Google Scholar] [CrossRef]
- Chen, P.; Chen, Y.; Xia, N.; Fan, B.; Niu, Z.; He, Z.; Wang, X.; Yuan, J.; Gupta, N.; Shen, B. A pyruvate transporter in the apicoplast of apicomplexan parasites. Proc. Natl. Acad. Sci. USA 2024, 121, e2314314121. [Google Scholar] [CrossRef]
- Chen, X.; Tang, T.; Ding, H.; Dong, H.; Long, S.; Suo, X. The Major Facilitator Superfamily Transporter HAP12 Is Critical in Toxoplasma gondii Survival and Virulence. Int. J. Mol. Sci. 2025, 26, 3910. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Chen, H.; Liang, X.; Fu, J.; Wang, S.; Zheng, J.; Zhang, Z.; Pang, Y.; Wang, J.; Shen, B.; et al. The Sec1/Munc18-like proteins TgSec1 and TgVps45 play pivotal roles in assembly of the pellicle and sub-pellicle network in Toxoplasma gondii. Mol. Microbiol. 2020, 113, 208–221. [Google Scholar] [CrossRef]
- Cao, S.; Yang, J.; Fu, J.; Chen, H.; Jia, H. The Dissection of SNAREs Reveals Key Factors for Vesicular Trafficking to the Endosome-like Compartment and Apicoplast via the Secretory System in Toxoplasma gondii. mBio 2021, 12, e0138021. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, Z.; Jia, H.; Zheng, Y. Characterization of aspartyl aminopeptidase from Toxoplasma gondii. Sci. Rep. 2016, 6, 34448. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhao, L.; Pang, Y.; Chen, H.; Yamamoto, H.; Chen, Y.; Li, Z.; Mizushima, N.; Jia, H. Apicoplast biogenesis mediated by ATG8 requires the ATG12-ATG5-ATG16L and SNAP29 complexes in Toxoplasma gondii. Autophagy 2023, 19, 1258–1276. [Google Scholar] [CrossRef]
- Barylyuk, K.; Koreny, L.; Ke, H.; Butterworth, S.; Crook, O.M.; Lassadi, I.; Gupta, V.; Tromer, E.; Mourier, T.; Stevens, T.J.; et al. A Comprehensive Subcellular Atlas of the Toxoplasma Proteome via hyperLOPIT Provides Spatial Context for Protein Functions. Cell Host Microbe 2020, 28, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.M.; Huet, D.; Ganesan, S.M.; Huynh, M.H.; Wang, T.; Nasamu, A.S.; Thiru, P.; Saeij, J.P.J.; Carruthers, V.B.; Niles, J.C.; et al. A Genome-wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell 2016, 166, 1423–1435 e12. [Google Scholar] [CrossRef]
- Roos, D.S.; Crawford, M.J.; Donald, R.G.K.; Kissinger, J.C.; Klimczak, L.J.; Striepen, B. Origin, targeting, and function of the apicomplexan plastid. Curr. Opin. Microbiol. 1999, 2, 426–432. [Google Scholar] [CrossRef]
- DeRocher, A.E.; Karnataki, A.; Vaney, P.; Parsons, M. Apicoplast targeting of a Toxoplasma gondii transmembrane protein requires a cytosolic tyrosine-based motif. Traffic 2012, 13, 694–704. [Google Scholar] [CrossRef]
- Prasad, A.; Mastud, P.; Patankar, S. Dually localised proteins found in both the apicoplast and mitochondrion utilize the Golgi-dependent pathway for apicoplast targeting in Toxoplasma gondii. Biol. Cell 2021, 113, 58–78. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Cao, S.; Yang, J.; Fu, J. Reply to Prasad and Patankar, “Recognition of Two Distinct Pathways for Trafficking of Proteins to the Apicoplast”. mBio 2021, 12, e0310721. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.; North, R.A.; Nagarathinam, K.; Tanabe, M. Structures and General Transport Mechanisms by the Major Facilitator Superfamily (MFS). Chem. Rev. 2021, 121, 5289–5335. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Qi, W.; Fu, J.; Jia, H. A Major Facilitator Superfamily Transporter Is Critical for the Metabolism and Biogenesis of the Apicoplast. Pathogens 2025, 14, 763. https://doi.org/10.3390/pathogens14080763
Liang Y, Qi W, Fu J, Jia H. A Major Facilitator Superfamily Transporter Is Critical for the Metabolism and Biogenesis of the Apicoplast. Pathogens. 2025; 14(8):763. https://doi.org/10.3390/pathogens14080763
Chicago/Turabian StyleLiang, Yumeng, Wei Qi, Jiawen Fu, and Honglin Jia. 2025. "A Major Facilitator Superfamily Transporter Is Critical for the Metabolism and Biogenesis of the Apicoplast" Pathogens 14, no. 8: 763. https://doi.org/10.3390/pathogens14080763
APA StyleLiang, Y., Qi, W., Fu, J., & Jia, H. (2025). A Major Facilitator Superfamily Transporter Is Critical for the Metabolism and Biogenesis of the Apicoplast. Pathogens, 14(8), 763. https://doi.org/10.3390/pathogens14080763





