Urban Mangroves Under Threat: Metagenomic Analysis Reveals a Surge in Human and Plant Pathogenic Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection
2.2. Sample Collection
2.3. Sample Storage and Processing
2.4. DNA Extraction
2.5. Amplification and Sequencing
2.6. Sequencing Platform and Read Type
2.7. Raw Data Processing
2.8. TGS Data Processing and Quality Control
2.9. Bioinformatics Analysis
2.10. Microbial Diversity Analysis
2.11. Statistical Analyses
3. Results
3.1. Diversity and Sequencing Metrics
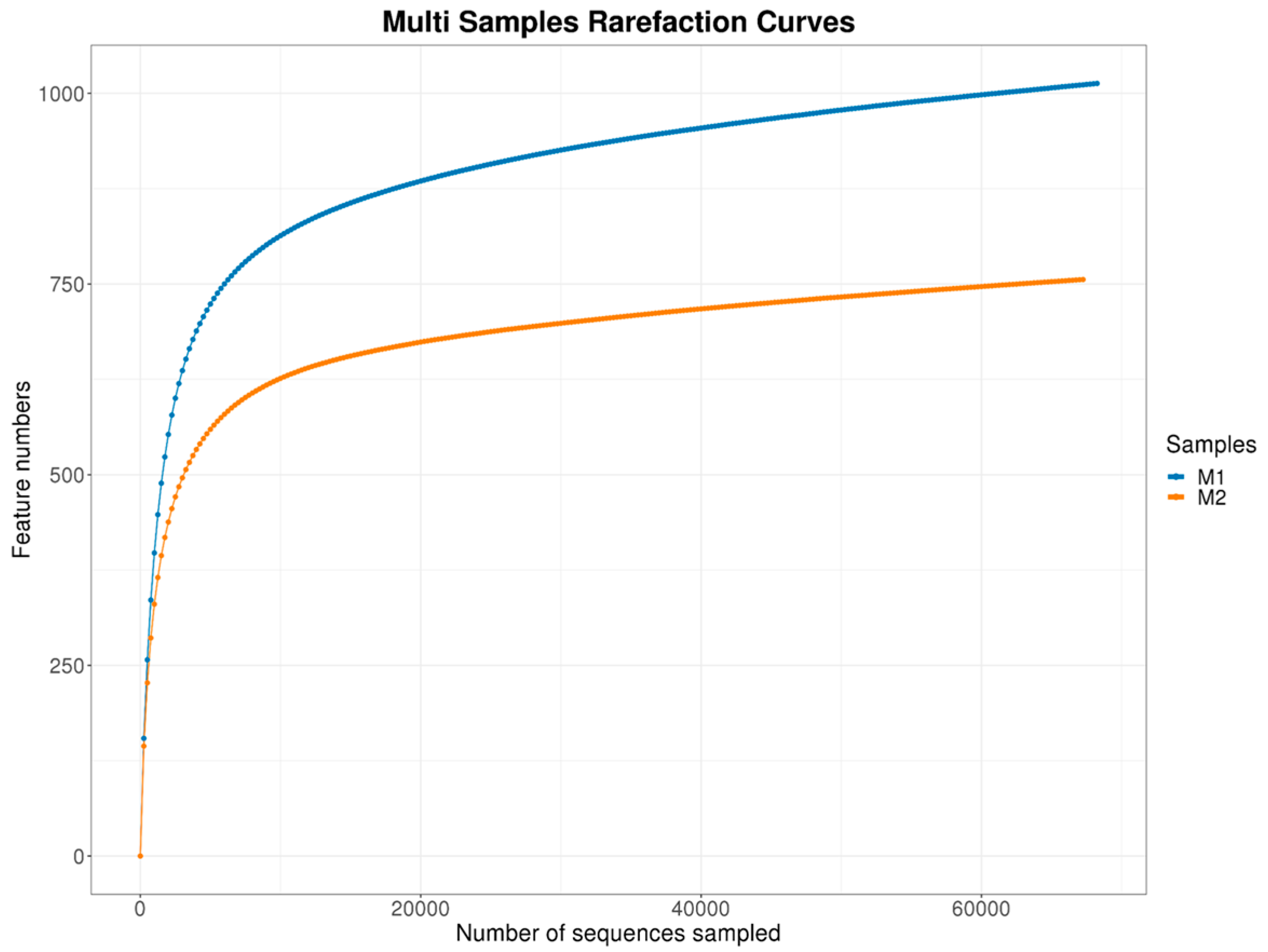
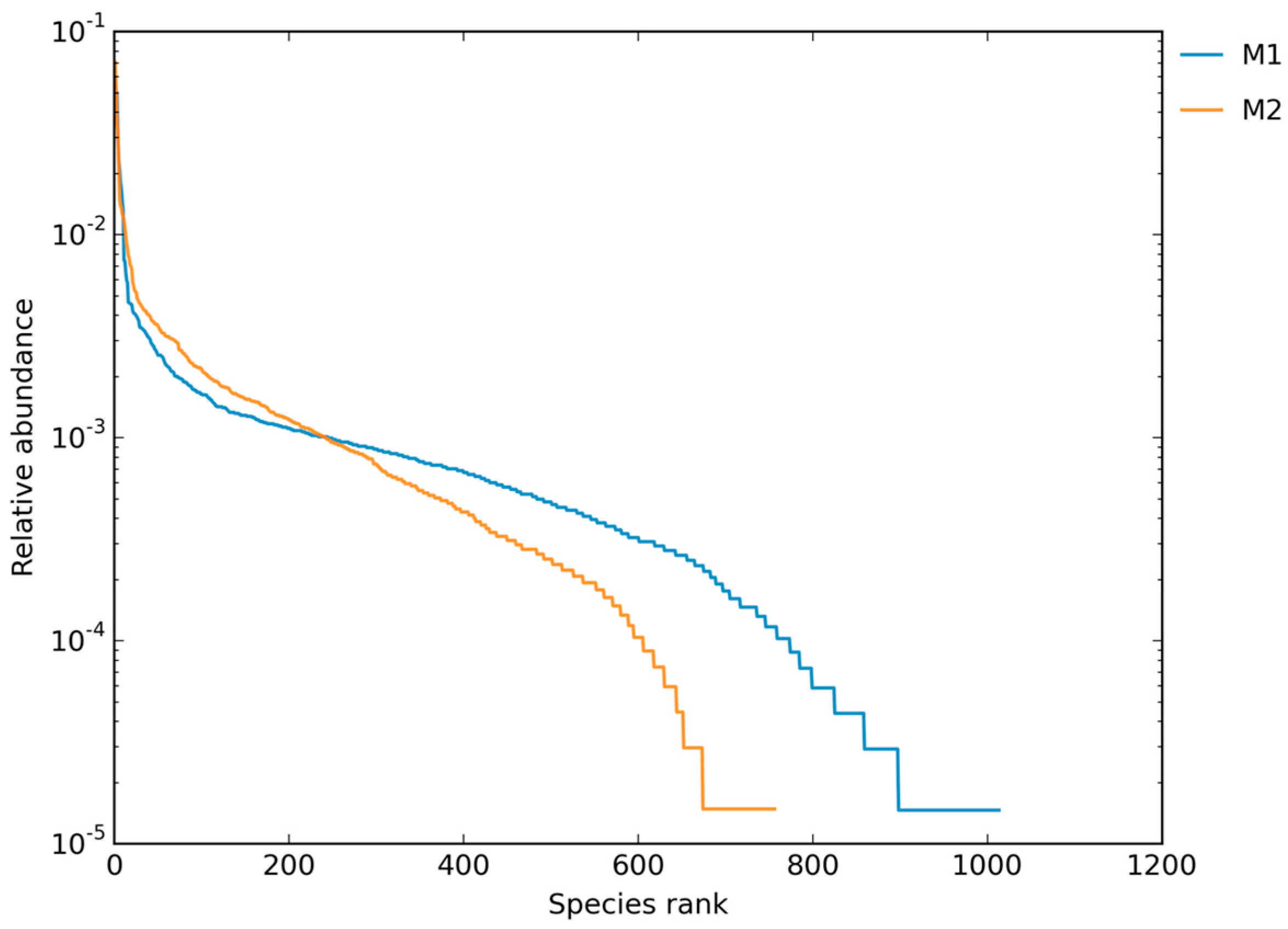
3.1.1. Rarefaction and Community Evenness
3.1.2. ASV/OTU Richness and Read Counts
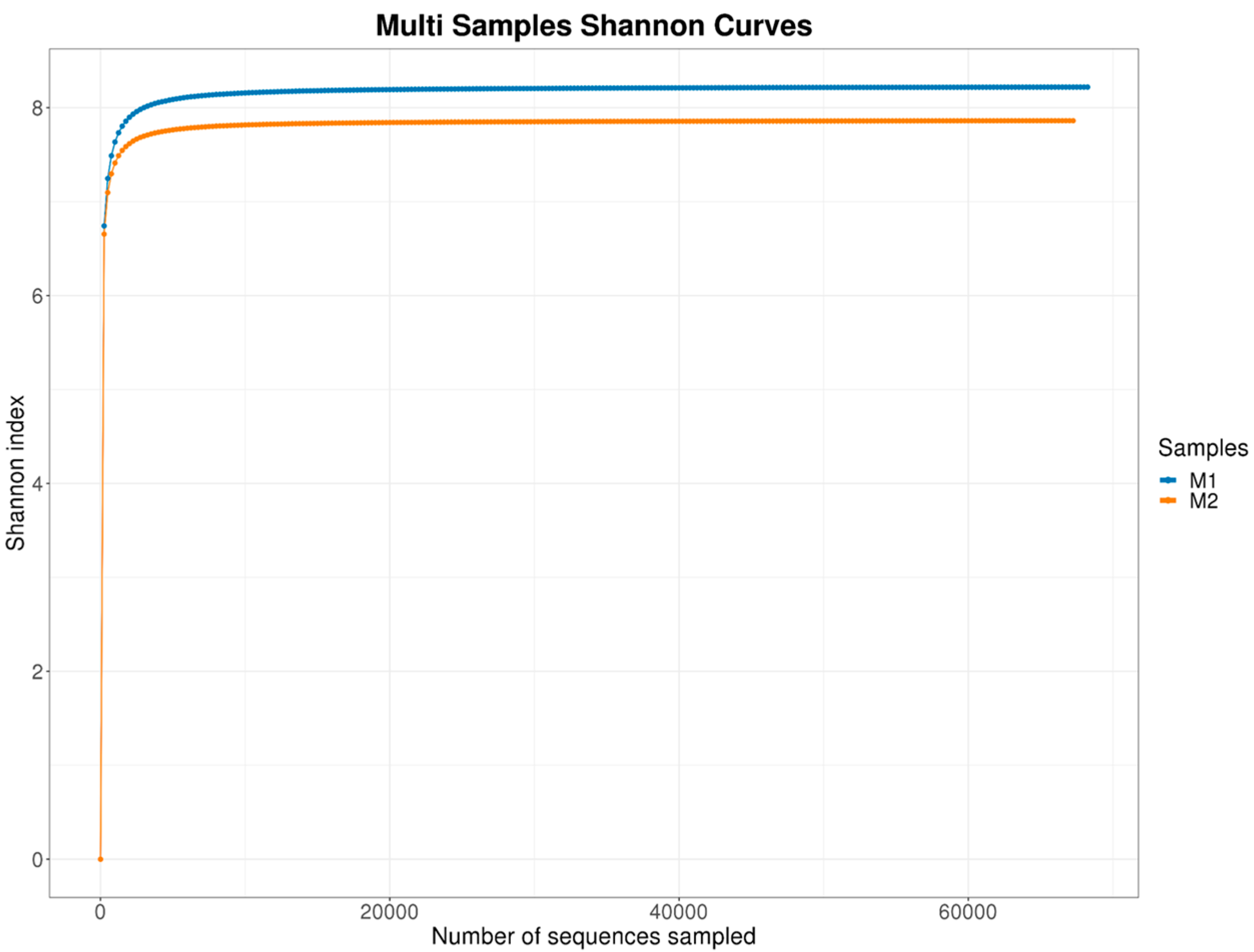
3.1.3. Alpha Diversity
3.2. Taxonomic and Functional Characterization
3.2.1. Phylum-Level Distribution
3.2.2. Class-Level Functional Profiles
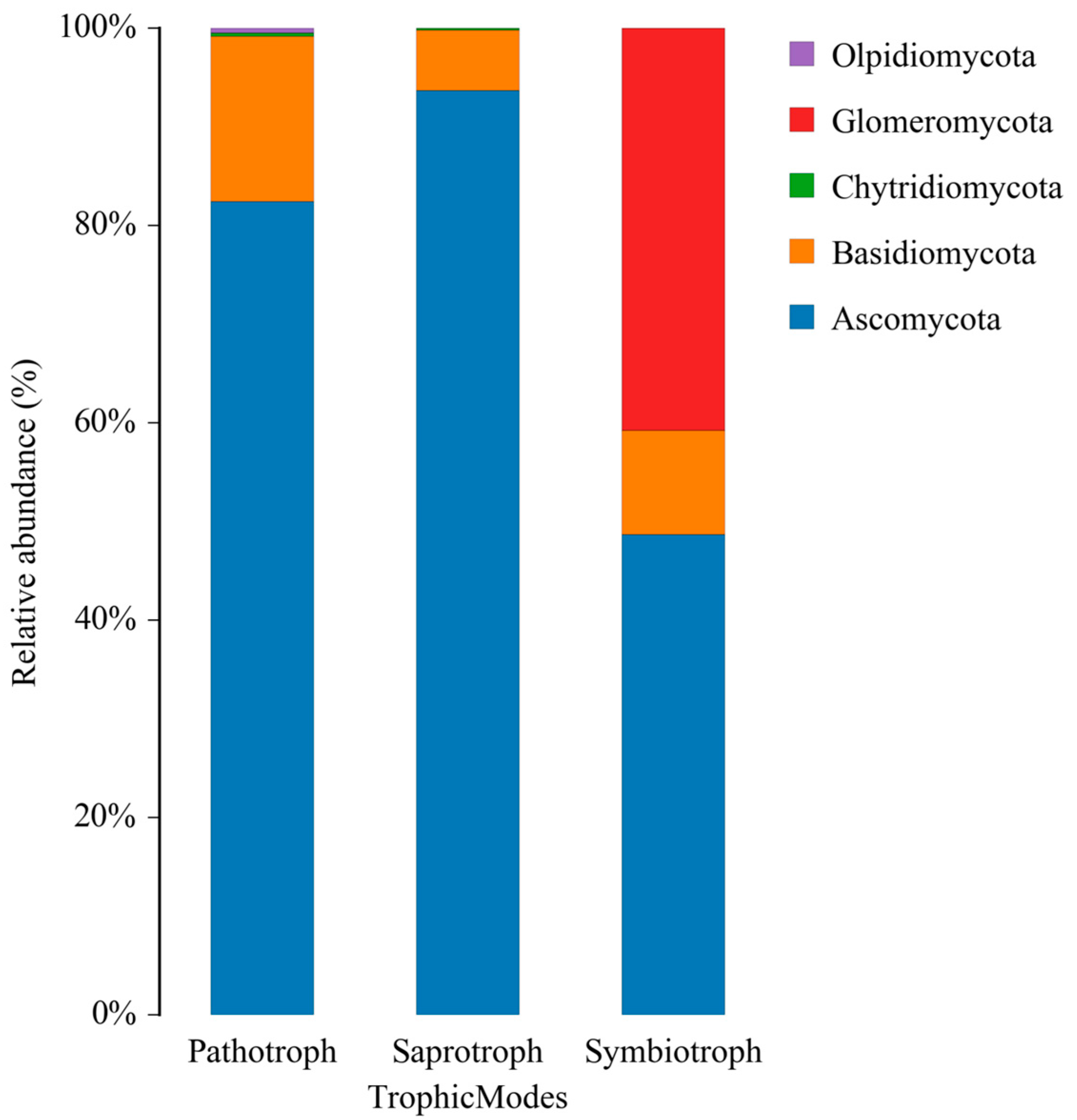
3.3. Pathogenic Potential and Ecological Implications
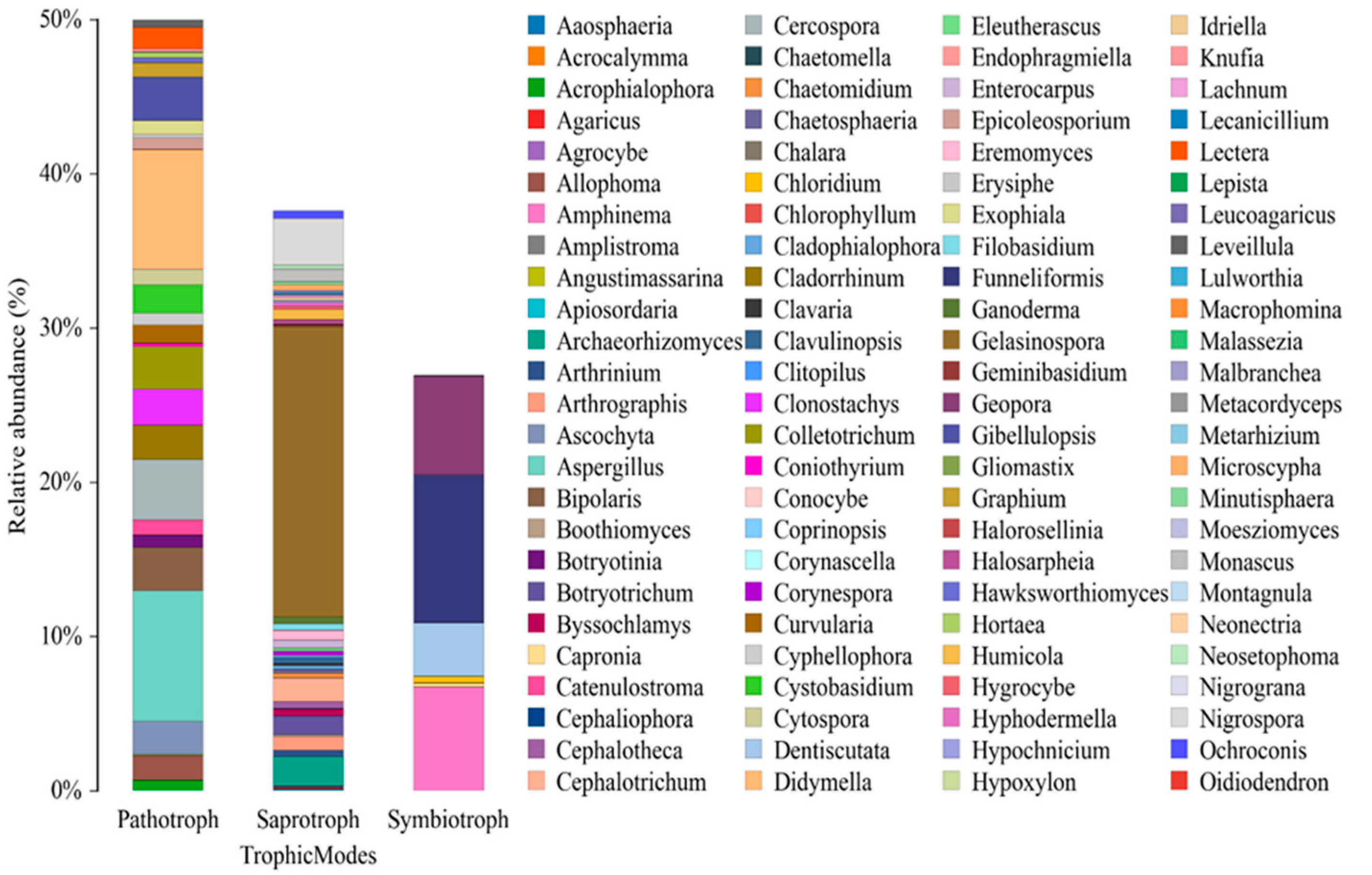
Fungal Genera and Trophic Modes
3.4. Functional Ecology of Fungal Communities
Guild Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Britto Martins De Oliveira, J.; Corrêa Junior, D.; Parente, C.E.T.; Frases, S. Fungi in Mangrove: Ecological Importance, Climate Change Impacts, and the Role in Environmental Remediation. Microorganisms 2025, 13, 878. [Google Scholar] [CrossRef]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Valiela, I.; Bowen, J.L.; York, J.K. Mangrove Forests: One of the World’s Threatened Major Tropical Environments. BioScience 2001, 51, 807. [Google Scholar] [CrossRef]
- Moitinho, M.A.; Chiaramonte, J.B.; Bononi, L.; Gumiere, T.; Melo, I.S.; Taketani, R.G. Fungal succession on the decomposition of three plant species from a Brazilian mangrove. Sci. Rep. 2022, 12, 14547. [Google Scholar] [CrossRef]
- Hyde, K.D.; Lee, S.Y. Ecology of mangrove fungi and their role in nutrient cycling: What gaps occur in our knowledge? Hydrobiologia 1995, 295, 107–118. [Google Scholar] [CrossRef]
- Marcial Gomes, N.C.; Borges, L.R.; Paranhos, R.; Pinto, F.N.; Mendonça-Hagler, L.C.S.; Smalla, K. Exploring the diversity of bacterial communities in sediments of urban mangrove forests: Sediment bacterial communities in urban mangrove forests. FEMS Microbiol. Ecol. 2008, 66, 96–109. [Google Scholar] [CrossRef]
- Silva, F.S.R.; Da Silva, Y.J.A.B.; Maia, A.J.; Biondi, C.M.; Araújo, P.R.M.; Barbosa, R.S.; Silva, C.M.C.A.C.; Luiz, T.C.S.; Araújo, A.F.V. Prediction of heavy metals in polluted mangrove soils in Brazil with the highest reported levels of mercury using near-infrared spectroscopy. Environ. Geochem. Health 2023, 45, 8337–8352. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, Y.; Cao, W.; Xing, M.; Xu, X.; Wang, Z.; Sun, J. Metagenomic analysis reveals antibiotic resistance genes and virulence factors in the saline-alkali soils from the Yellow River Delta, China. Environ. Res. 2022, 214, 113823. [Google Scholar] [CrossRef]
- Hau, P.-T.; Shiu, A.; Tam, E.W.-T.; Chau, E.C.-T.; Murillo, M.; Humer, E.; Po, W.-W.; Yu, R.C.-W.; Fung, J.; Seto, S.-W.; et al. Diversity and Antifungal Susceptibilities of Yeasts from Mangroves in Hong Kong, China—A One Health Aspect. JoF 2024, 10, 728. [Google Scholar] [CrossRef]
- Xiao, Y.; He, M.; Xie, J.; Liu, L.; Zhang, X. Effects of heavy metals and organic matter fractions on the fungal communities in mangrove sediments from Techeng Isle, South China. Ecotoxicol. Environ. Saf. 2021, 222, 112545. [Google Scholar] [CrossRef]
- Yi, J.; Lo, L.S.H.; Liu, H.; Qian, P.-Y.; Cheng, J. Study of Heavy Metals and Microbial Communities in Contaminated Sediments Along an Urban Estuary. Front. Mar. Sci. 2021, 8, 741912. [Google Scholar] [CrossRef]
- Meng, S.; Peng, T.; Pratush, A.; Huang, T.; Hu, Z. Interactions between heavy metals and bacteria in mangroves. Mar. Pollut. Bull. 2021, 172, 112846. [Google Scholar] [CrossRef]
- Shuaib, M.; Azam, N.; Bahadur, S.; Romman, M.; Yu, Q.; Xuexiu, C. Variation and succession of microbial communities under the conditions of persistent heavy metal and their survival mechanism. Microb. Pathog. 2021, 150, 104713. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.M.; Hypolito, R.; Moura, C.L.; Nascimento, S.C. Caracterização da contaminação por metais pesados em área de mangüezal, Município de Santos (SP). Rev. Inst. Geol. 2012, 33, 57–69. [Google Scholar] [CrossRef]
- Fontana, L.F.; Belart, P.; Bonetti, C.; Junior, D.S.; Frontalini, F.; Martínez-Colón, M.; Bouchet, V.M.P.; Laut, L. Foraminifera and geomicrobiology as indicators of the environmental recovery in a mangrove affected by oil spills in the Guanabara Bay (Brazil). Sci. Total Environ. 2024, 957, 177650. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Worthington, T.A.; Riul, P. Brazilian Mangroves: Blue Carbon Hotspots of National and Global Relevance to Natural Climate Solutions. Front. For. Glob. Change 2022, 4, 787533. [Google Scholar] [CrossRef]
- Farias, C.O.; Hamacher, C.; Wagener, A.D.L.R.; Campos, R.C.D.; Godoy, J.M. Trace metal contamination in mangrove sediments, Guanabara Bay, Rio de Janeiro, Brazil. J. Braz. Chem. Soc. 2007, 18, 1194–1206. [Google Scholar] [CrossRef]
- Blankespoor, B.; Dasgupta, S.; Lange, G.-M. Mangroves as a protection from storm surges in a changing climate. Ambio 2017, 46, 478–491. [Google Scholar] [CrossRef]
- Krishna, S.; Lemmen, C.; Örey, S.; Rehren, J.; Pane, J.D.; Mathis, M.; Püts, M.; Hokamp, S.; Pradhan, H.K.; Hasenbein, M.; et al. Interactive effects of multiple stressors in coastal ecosystems. Front. Mar. Sci. 2025, 11, 1481734. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, S.; Xu, L.; Nethmini, R.T.; Zhang, Y.; Huang, L.; Dong, K.; Zhao, H.; Pan, L. Shifts in Soil Fungal Community and Trophic Modes During Mangrove Ecosystem Restoration. JoF 2025, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, S.; Akache, B.; Weber, S.; De Deken, X.; Raymond, M.; Turcotte, B. Candida albicans Zinc Cluster Protein Upc2p Confers Resistance to Antifungal Drugs and Is an Activator of Ergosterol Biosynthetic Genes. Antimicrob. Agents Chemother. 2005, 49, 1745–1752. [Google Scholar] [CrossRef]
- Seidel, D.; Wurster, S.; Jenks, J.D.; Sati, H.; Gangneux, J.-P.; Egger, M.; Alastruey-Izquierdo, A.; Ford, N.P.; Chowdhary, A.; Sprute, R.; et al. Impact of climate change and natural disasters on fungal infections. Lancet Microbe 2024, 5, e594–e605. [Google Scholar] [CrossRef]
- Merhaby, D.; Rabodonirina, S.; Net, S.; Ouddane, B.; Halwani, J. Overview of sediments pollution by PAHs and PCBs in mediterranean basin: Transport, fate, occurrence, and distribution. Mar. Pollut. Bull. 2019, 149, 110646. [Google Scholar] [CrossRef]
- Gajewska, J.; Floryszak-Wieczorek, J.; Sobieszczuk-Nowicka, E.; Mattoo, A.; Arasimowicz-Jelonek, M. Fungal and oomycete pathogens and heavy metals: An inglorious couple in the environment. IMA Fungus 2022, 13, 6. [Google Scholar] [CrossRef]
- Cabral, L.; Júnior, G.V.L.; Pereira de Sousa, S.T.; Dias, A.C.F.; Lira Cadete, L.; Andreote, F.D.; Hess, M.; de Oliveira, V.M. Anthropogenic impact on mangrove sediments triggers differential responses in the heavy metals and antibiotic resistomes of microbial communities. Environ. Pollut. 2016, 216, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.; Akinbobola, A.; Woodford, L.; Quilliam, R.S. Thermotolerance, virulence, and drug resistance of human pathogenic Candida species colonising plastic pollution in aquatic ecosystems. Environ. Sci. Pollut. Res. 2025, 32, 14993–15005. [Google Scholar] [CrossRef]
- Erazo, N.G.; Bowman, J.S. Sensitivity of the mangrove-estuarine microbial community to aquaculture effluent. iScience 2021, 24, 102204. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, Y.; Su, L.; Yan, L.; Lin, X.; Long, Y.; Zhang, A.; Zhao, Q. Significant Enrichment of Potential Pathogenic Fungi in Soil Mediated by Flavonoids, Phenolic Acids, and Organic Acids. JoF 2025, 11, 154. [Google Scholar] [CrossRef]
- Barroso, G.C.; Abril, G.; Machado, W.; Abuchacra, R.C.; Peixoto, R.B.; Bernardes, M.; Marques, G.S.; Sanders, C.J.; Oliveira, G.B.; Oliveira Filho, S.R.; et al. Linking eutrophication to carbon dioxide and methane emissions from exposed mangrove soils along an urban gradient. Sci. Total Environ. 2022, 850, 157988. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Wei, D.; Deng, X.; Weng, S.; He, Z.; He, J. Environmental Factors Shape Water Microbial Community Structure and Function in Shrimp Cultural Enclosure Ecosystems. Front. Microbiol. 2017, 8, 2359. [Google Scholar] [CrossRef] [PubMed]
- Laux, M.; Ciapina, L.P.; De Carvalho, F.M.; Gerber, A.L.; Guimarães, A.P.C.; Apolinário, M.; Paes, J.E.S.; Jonck, C.R.; De Vasconcelos, A.T.R. Living in mangroves: A syntrophic scenario unveiling a resourceful microbiome. BMC Microbiol. 2024, 24, 228. [Google Scholar] [CrossRef]
- Tavares, T.C.L.; Bezerra, W.M.; Normando, L.R.O.; Rosado, A.S.; Melo, V.M.M. Brazilian Semi-Arid Mangroves-Associated Microbiome as Pools of Richness and Complexity in a Changing World. Front. Microbiol. 2021, 12, 715991. [Google Scholar] [CrossRef]
- Ghizelini, A.M.; Mendonça-Hagler, L.C.S.; Macrae, A. Microbial diversity in Brazilian mangrove sediments: A mini review. Braz. J. Microbiol. 2012, 43, 1242–1254. [Google Scholar] [CrossRef]
- Fistarol, G.O.; Coutinho, F.H.; Moreira, A.P.B.; Venas, T.; Cánovas, A.; de Paula, S.E.M.; Coutinho, R.; de Moura, R.L.; Valentin, J.L.; Tenenbaum, D.R.; et al. Environmental and Sanitary Conditions of Guanabara Bay, Rio de Janeiro. Front. Microbiol. 2015, 6, 1232. [Google Scholar] [CrossRef]
- Vilela, C.G.; Figueira, B.O.; Macedo, M.C.; Baptista Neto, J.A. Late Holocene evolution and increasing pollution in Guanabara Bay, Rio de Janeiro, SE Brazil. Mar. Pollut. Bull. 2014, 79, 175–187. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Aguiar, V.M.; De Lima, M.N.; Abuchacra, R.C.; Abuchacra, P.F.F.; Neto, J.A.B.; Borges, H.V.; De Oliveira, V.C. Ecological risks of trace metals in Guanabara Bay, Rio de Janeiro, Brazil: An index analysis approach. Ecotoxicol. Environ. Saf. 2016, 133, 306–315. [Google Scholar] [CrossRef]
- Abreu, F.E.L.; Batista, R.M.; Zanardi-Lamardo, E.; Yogui, G.T.; Amado, L.L.; Ribeiro-Brasil, D.R.G.; Franco, T.C.R.D.S.; Viana, J.L.M.; Fernandez, M.A.; Castro, I.B.; et al. Antifouling paint residues in areas impacted by maritime activities along 6000 km of Brazilian coastline. Sci. Total Environ. 2025, 981, 179559. [Google Scholar] [CrossRef]
- Do Carmo Fernández, T.; Souto-Neto, J.A.; Villar Freret-Meurer, N.; De Assis Machado, L.; Do Carmo Vaccani, A.; Dos Santos Cabiró, G.; Bezerra, J.J.V.; De Almeida, R.F.; Saint’Pierre, T.D.; Hauser-Davis, R.A. First report on Technology-Critical Elements in seahorses from Rio de Janeiro, Southeastern Brazil. Mar. Pollut. Bull. 2025, 218, 118042. [Google Scholar] [CrossRef] [PubMed]
- World Reference Base|FAO SOILS PORTAL|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/soils-portal/data-hub/soil-classification/world-reference-base/en/ (accessed on 23 July 2025).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, J.; Miao, L.; Hou, J. Effects of sediment physicochemical factors and heavy metals on the diversity, structure, and functions of bacterial and fungal communities from a eutrophic river. Environ. Pollut. 2022, 303, 119129. [Google Scholar] [CrossRef]
- Jacob, J.K.S.; Witzel, K.; Dela Cruz, T.E.E. Comparative Diversity and Functional Traits of Fungal Endophytes in Response to Elevated Mineral Content in a Mangrove Ecosystem. JoF 2023, 9, 1186. [Google Scholar] [CrossRef]
- Ghizelini, A.M.; Martins, K.G.; Gießelmann, U.C.; Santoro, E.; Pasqualette, L.; Mendonça-Hagler, L.C.S.; Rosado, A.S.; Macrae, A. Fungal communities in oil contaminated mangrove sediments—Who is in the mud? Mar. Pollut. Bull. 2019, 139, 181–188. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Villarreal Ruiz, L.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Fungal biogeography. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Devadatha, B.; Jones, E.B.G.; Pang, K.L.; Abdel-Wahab, M.A.; Hyde, K.D.; Sakayaroj, J.; Bahkali, A.H.; Calabon, M.S.; Sarma, V.V.; Sutreong, S.; et al. Occurrence and geographical distribution of mangrove fungi. Fungal Divers. 2021, 106, 137–227. [Google Scholar] [CrossRef]
- Tedersoo, L.; Tooming-Klunderud, A.; Anslan, S. PacBio metabarcoding of Fungi and other eukaryotes: Errors, biases and perspectives. New Phytol. 2018, 217, 1370–1385. [Google Scholar] [CrossRef]
- Taghavi Ghasemkheili, F.; Pirdashti, H.; Ghanbary, M.A.T.; Emadi, M.; Ghadirnezhad Shiade, S.R. Quantifying the Fungal Population Dynamics in Contaminated Soil Using Spatial Analysis. SSRN Electron. J. 2021, 4, 787533. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, A.; Tomasek, A.; McMillan, S.; Yufra, S.; Yupanqui, M.; Rondon, R.; Hoagland, L. Composition and potential functional roles of soil fungal communities on arid farms in Arequipa (Southern Peru) characterized using SMRT sequencing. Appl. Soil. Ecol. 2022, 169, 104228. [Google Scholar] [CrossRef]
- de S Araújo, G.R.; de Souza, W.; Frases, S. The Hidden Pathogenic Potential of Environmental Fungi. Future Microbiol. 2017, 12, 1533–1540. [Google Scholar] [CrossRef]
- El-Sharkawy, M.; Alotaibi, M.O.; Li, J.; Du, D.; Mahmoud, E. Heavy Metal Pollution in Coastal Environments: Ecological Implications and Management Strategies: A Review. Sustainability 2025, 17, 701. [Google Scholar] [CrossRef]
- Cabral, L.; Siqueira, J.O.; Soares, C.R.F.S.; Pinto, J.E.B.P. Retenção de metais pesados em micélio de fungos micorrízicos arbusculares. Quím. Nova 2010, 33, 25–29. [Google Scholar] [CrossRef]
- Godoy, M.D.P.; Lacerda, L.D.D. Mangroves Response to Climate Change: A Review of Recent Findings on Mangrove Extension and Distribution. An. Acad. Bras. Ciênc. 2015, 87, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.R. Diversity of fungi in mangrove ecosystems. In Microbial Diversity: Current Perspectives and Potential Applications; I.K. International Publishing House and Dreamtech Press: New Delhi, India, 2005; pp. 129–147. [Google Scholar] [CrossRef]
- Chynel, M.; Rockomanovic, S.; Abril, G.; Barroso, G.; Marotta, H.; Machado, W.; Sanders, C.J.; Thiney, N.; Meziane, T. Contrasting organic matter composition in pristine and eutrophicated mangroves revealed by fatty acids and stable isotopes (Rio de Janeiro, Brazil). Estuar. Coast. Shelf Sci. 2022, 277, 108061. [Google Scholar] [CrossRef]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, 1. [Google Scholar] [CrossRef]
- Paulussen, C.; Hallsworth, J.E.; Álvarez-Pérez, S.; Nierman, W.C.; Hamill, P.G.; Blain, D.; Rediers, H.; Lievens, B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb. Biotechnol. 2017, 10, 296–322. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Short, B.; Brown, J.; Delaney, C.; Sherry, L.; Williams, C.; Ramage, G.; Kean, R. Candida auris exhibits resilient biofilm characteristics in vitro: Implications for environmental persistence. J. Hosp. Infect. 2019, 103, 92–96. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal Biofilm Resistance. Int. J. Microbiol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Baptista Neto, J.A.; Smith, B.J.; McAllister, J.J. Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, Southeast Brazil. Environ. Pollut. 2000, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, J.; Song, J.; Wang, Q.; Liu, H.; Tang, X. Habitat suitability index model of the sea cucumber Apostichopus japonicus (Selenka): A case study of Shandong Peninsula, China. Mar. Pollut. Bull. 2017, 122, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Lee, J.; Wang, W.; Lee, Y.; Oh, E.; Park, K.-H.; Park, C.; Woo, G.-E.; Son, Y.-J.; Kang, H. Austalide K from the Fungus Penicillium rudallense Prevents LPS-Induced Bone Loss in Mice by Inhibiting Osteoclast Differentiation and Promoting Osteoblast Differentiation. Int. J. Mol. Sci. 2021, 22, 5493. [Google Scholar] [CrossRef] [PubMed]

| Sample | ASVs | Sequences (Reads) |
|---|---|---|
| Sample 1 | 1013 | 68,281 |
| Sample 2 | 756 | 67,345 |
| Total | 1769 | 135,626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Britto Martins de Oliveira, J.; Barbieri, M.; Corrêa-Junior, D.; Schmitt, M.; Santos, L.L.R.; Bahia, A.C.; Parente, C.E.T.; Frases, S. Urban Mangroves Under Threat: Metagenomic Analysis Reveals a Surge in Human and Plant Pathogenic Fungi. Pathogens 2025, 14, 759. https://doi.org/10.3390/pathogens14080759
Britto Martins de Oliveira J, Barbieri M, Corrêa-Junior D, Schmitt M, Santos LLR, Bahia AC, Parente CET, Frases S. Urban Mangroves Under Threat: Metagenomic Analysis Reveals a Surge in Human and Plant Pathogenic Fungi. Pathogens. 2025; 14(8):759. https://doi.org/10.3390/pathogens14080759
Chicago/Turabian StyleBritto Martins de Oliveira, Juliana, Mariana Barbieri, Dario Corrêa-Junior, Matheus Schmitt, Luana Lessa R. Santos, Ana C. Bahia, Cláudio Ernesto Taveira Parente, and Susana Frases. 2025. "Urban Mangroves Under Threat: Metagenomic Analysis Reveals a Surge in Human and Plant Pathogenic Fungi" Pathogens 14, no. 8: 759. https://doi.org/10.3390/pathogens14080759
APA StyleBritto Martins de Oliveira, J., Barbieri, M., Corrêa-Junior, D., Schmitt, M., Santos, L. L. R., Bahia, A. C., Parente, C. E. T., & Frases, S. (2025). Urban Mangroves Under Threat: Metagenomic Analysis Reveals a Surge in Human and Plant Pathogenic Fungi. Pathogens, 14(8), 759. https://doi.org/10.3390/pathogens14080759







