Association Between ABO Blood Groups and SARS-CoV-2 RNAemia, Spike Protein Mutations, and Thrombotic Events in COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Sample Collection
2.3. Molecular Virology Analysis
2.3.1. SARS-CoV-2 RNA Extraction
2.3.2. Quantitative Reverse Transcription PCR (RT-qPCR) Assay
2.3.3. Detection of the SARS-CoV-2 RNAemia Variants by Whole-Genome Sequencing
2.3.4. Laboratory Analysis for Coagulation Markers
2.4. Statistical Analysis
3. Results
3.1. Laboratory Test
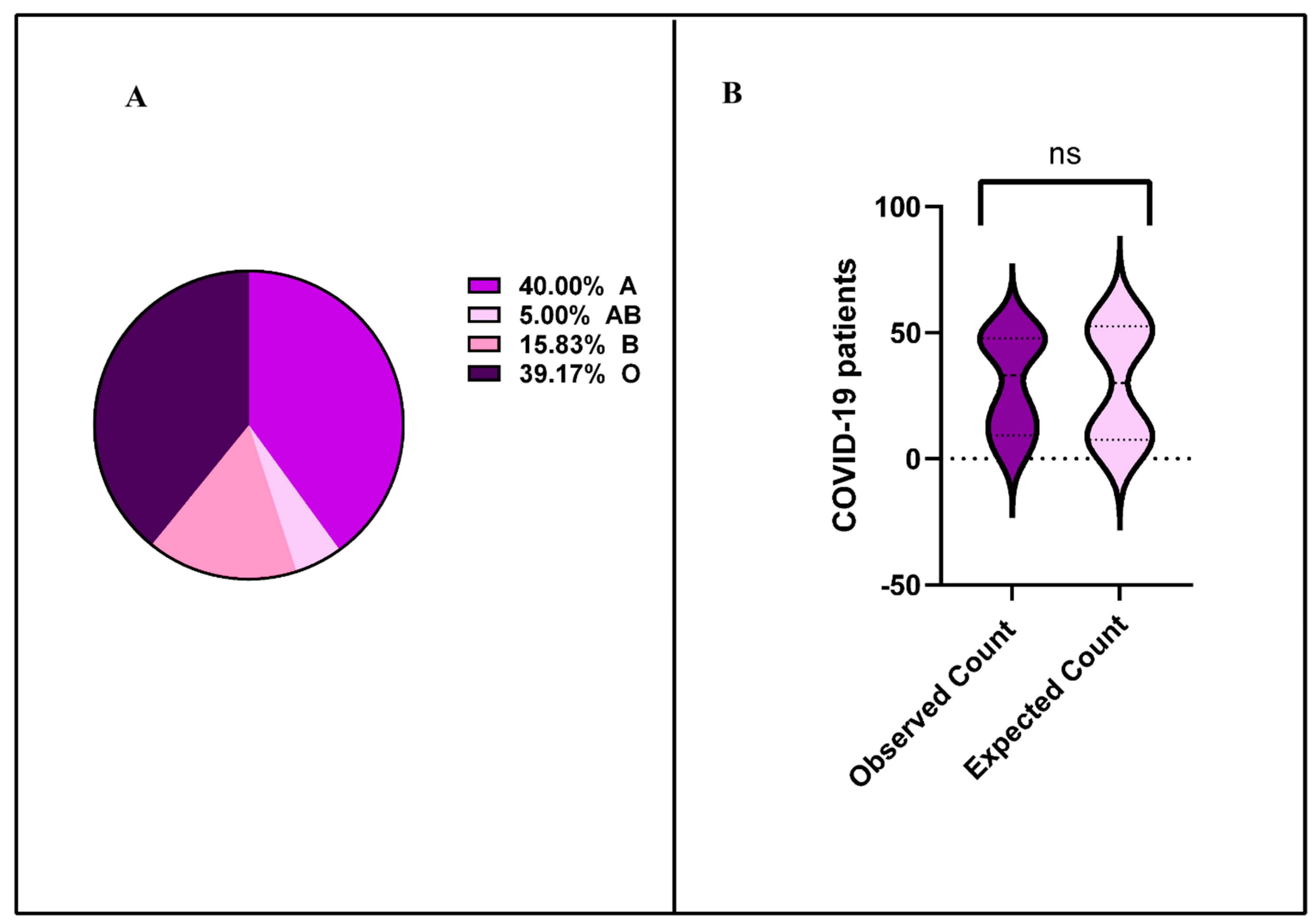
3.2. Distribution of SARS-CoV-2 RNAemia Across Blood Groups
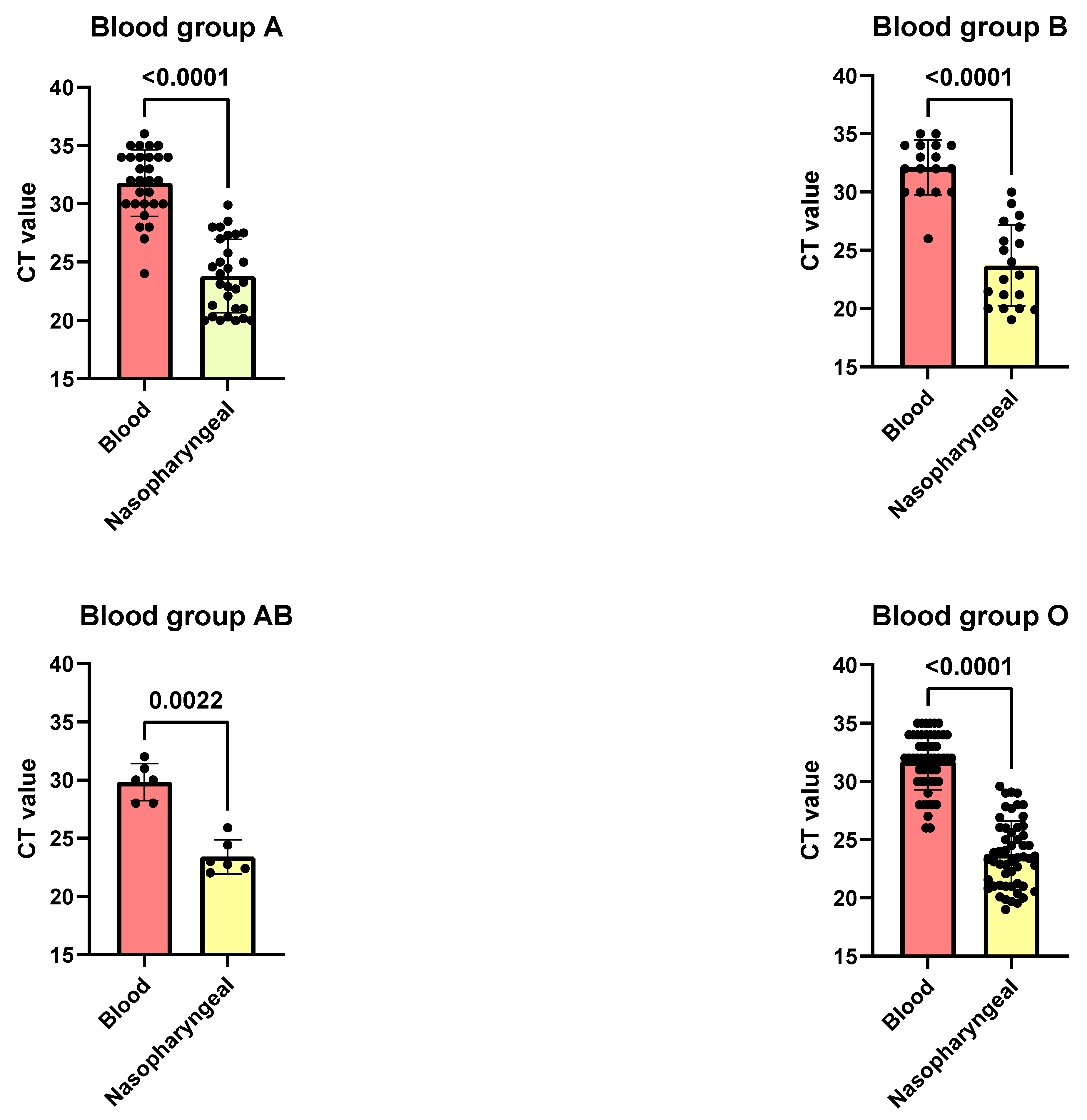
3.3. Correlation Between SARS-CoV-2 CT Values in Blood and Nasopharyngeal Swabs Across ABO Blood Groups
3.4. Distribution of SARS-CoV-2 Variants of Concern (VOCs) and Non-VOCs Across Blood Groups in COVID-19 Patients with RNAemia
3.5. Prevalence of SARS-CoV-2 Spike Gene Mutations Across ABO Blood Groups in COVID-19 Patients
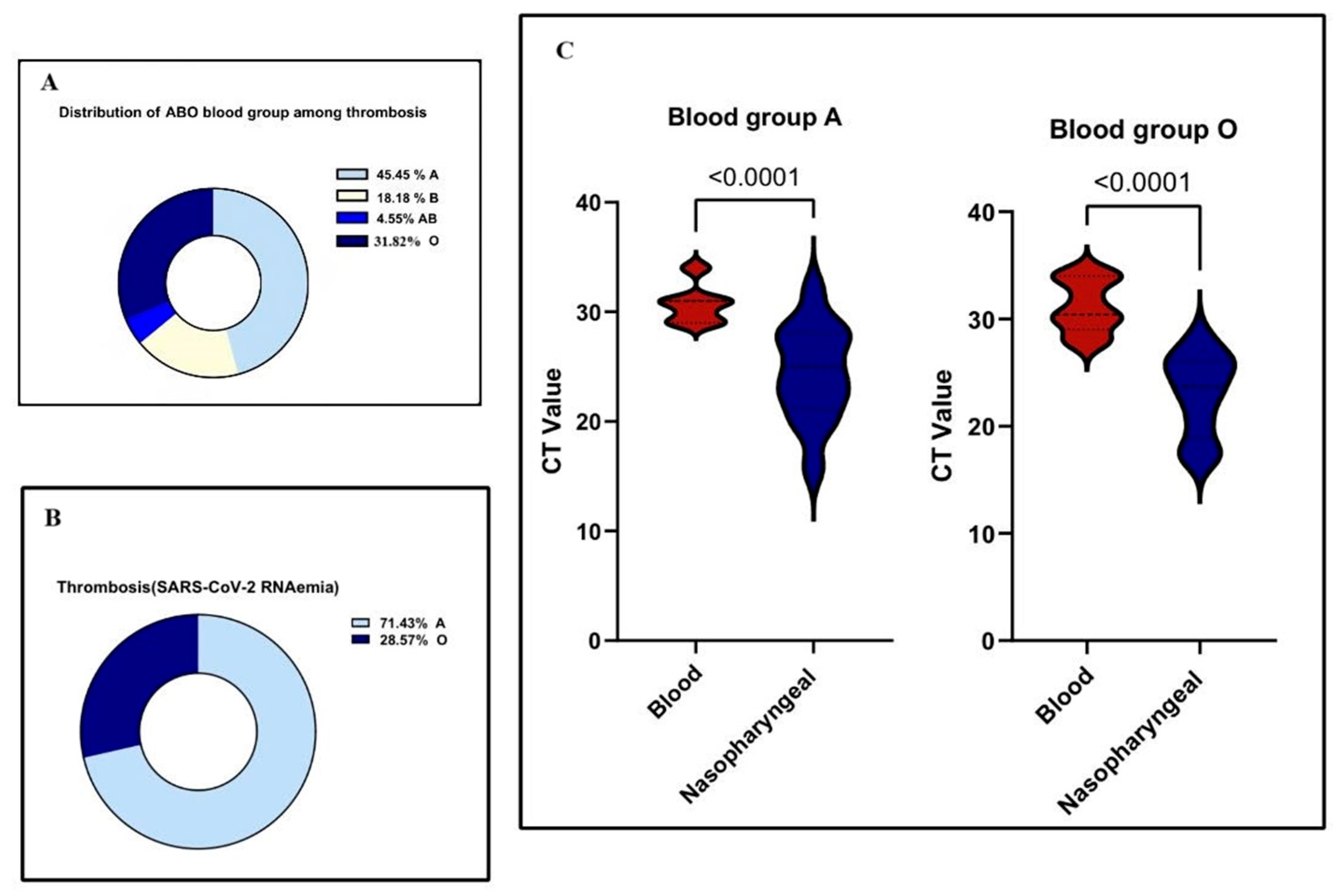
3.6. Association Between Blood Group Types and Thrombosis in COVID-19 Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Syapitri, H.; Sinaga, J.; Purba, I.E.; Aritonang, J.; Saragih, M. The potential of coronavirus (COVID-19) transmission in Medan city, Indonesia. J. Southwest Jiaotong Univ. 2021, 56, 724–732. [Google Scholar] [CrossRef]
- Hu, F.H.; Jia, Y.J.; Zhao, D.Y.; Fu, X.L.; Zhang, W.Q.; Tang, W.; Hu, S.Q.; Wu, H.; Ge, M.W.; Du, W.; et al. Clinical outcomes of the severe acute respiratory syndrome coronavirus 2 Omicron and Delta variant: Systematic review and meta-analysis of 33 studies covering 6,037,144 coronavirus disease 2019-positive patients. Clin. Microbiol. Infect. 2023, 29, 835–844. [Google Scholar] [CrossRef]
- Soares, D.M.; Araújo, D.A.; de Souza, J.L.; Maurício, R.B.; Soares, E.M.; Neto, F.D.; Pinheiro, M.S.; de Vasconcelos Gama, V.C.; Braga-Neto, P.; Nóbrega, P.R.; et al. Correlation between ABO blood type, susceptibility to SARS-CoV-2 infection and COVID-19 disease severity: A systematic review. Hematol. Transfus. Cell Ther. 2023, 45, 483–494. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, G.; Chui, C.H.; Lau, F.Y.; Chan, P.K.; Ng, M.H.; Sung, J.J.; Wong, R.S. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA 2005, 293, 1450–1451. [Google Scholar] [CrossRef]
- Cooling, L. Blood groups in infection and host susceptibility. Clin. Microbiol. Rev. 2015, 28, 801–870. [Google Scholar] [CrossRef]
- Rowe, J.A.; Handel, I.G.; Thera, M.A.; Deans, A.M.; Lyke, K.E.; Koné, A.; Diallo, D.A.; Raza, A.; Kai, O.; Marsh, K.; et al. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc. Natl. Acad. Sci. USA 2007, 104, 17471–17476. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xue, L.; Gao, J.; Wu, A.; Kou, X. ABO blood group-associated susceptibility to norovirus infection: A systematic review and meta-analysis. Infect. Genet. Evol. 2020, 81, 104245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Y.; Huang, H.; Li, D.; Gu, D.; Lu, X.; Zhang, Z.; Liu, L.; Liu, T.; Liu, Y.; et al. Relationship Between the ABO Blood Group and the Coronavirus Disease 2019 (COVID-19) Susceptibility. Clin. Infect. Dis. 2021, 73, 328–331. [Google Scholar] [CrossRef]
- Zietz, M.; Tatonetti, N.P. Testing the association between blood type and COVID-19 infection, intubation, and death. Nat. Commun. 2020, 11, 5761. [Google Scholar] [CrossRef]
- Asad, L.; Mirza, T.; Kumar, S.; Khatoon, A. Effect of ABO blood group on the severity and clinico-pathological parameters of COVID-19. Pak. J. Med. Sci. 2024, 40, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Arthur, C.M.; Jan, H.M.; Garcia-Beltran, W.F.; Patel, K.R.; Rathgeber, M.F.; Verkerke, H.P.; Cheedarla, N.; Jajosky, R.P.; Paul, A.; et al. Blood group A enhances SARS-CoV-2 infection. Blood 2023, 142, 742–747. [Google Scholar] [CrossRef]
- Sardu, C.; Marfella, R.; Maggi, P.; Messina, V.; Cirillo, P.; Codella, V.; Gambardella, J.; Sardu, A.; Gatta, G.; Santulli, G.; et al. Implications of AB0 blood group in hypertensive patients with COVID-19. BMC Cardiovasc. Disord. 2020, 20, 373. [Google Scholar] [CrossRef]
- Mielke, N.; Gorz, R.; Bahl, A.; Zhao, L.; Berger, D.A. Association between ABO blood type and coronavirus disease 2019 severe outcomes across dominant variant strains. J. Am. Coll. Emerg. Physicians Open 2024, 5, e13115. [Google Scholar] [CrossRef]
- Hogan, C.A.; Stevens, B.A.; Sahoo, M.K.; Huang, C.; Garamani, N.; Gombar, S.; Yamamoto, F.; Murugesan, K.; Kurzer, J.; Zehnder, J.; et al. High Frequency of SARS-CoV-2 RNAemia and Association with Severe Disease. Clin. Infect. Dis. 2021, 72, e291–e295. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Elsayid, M. A Study of Prevalence of Blood Group of Saudi Patients in King Abdulaziz Medical City-Riyadh. Sci. J. Public Health 2015, 3, 559. [Google Scholar] [CrossRef][Green Version]
- Alhamlan, F.S.; Bakheet, D.M.; Bohol, M.F.; Alsanea, M.S.; Alahideb, B.M.; Alhadeq, F.M.; Alsuwairi, F.A.; Al-Abdulkareem, M.A.; Asiri, M.S.; Almaghrabi, R.S.; et al. SARS-CoV-2 spike gene Sanger sequencing methodology to identify variants of concern. Biotechniques 2023, 74, 69–75. [Google Scholar] [CrossRef]
- Jawdat, D.; Hajeer, A.; Massadeh, S.; Aljawini, N.; Abedalthagafi, M.S.; Alaamery, M. Correlation between ABO Blood Group Phenotype and the Risk of COVID-19 Infection and Severity of Disease in a Saudi Arabian Cohort. J. Epidemiol. Glob. Health 2022, 12, 85–91. [Google Scholar] [CrossRef]
- Rezoagli, E.; Gatti, S.; Villa, S.; Villa, G.; Muttini, S.; Rossi, F.; Faraldi, L.; Fumagalli, R.; Grasselli, G.; Foti, G.; et al. ABO blood types and major outcomes in patients with acute hypoxaemic respiratory failure: A multicenter retrospective cohort study. PLoS ONE 2018, 13, e0206403. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.A.; Parikh, R.; Grandi, S.M.; Ray, J.G.; Cohen, E. ABO and Rh blood groups and risk of infection: Systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 797. [Google Scholar] [CrossRef]
- Kawasuji, H.; Morinaga, Y.; Tani, H.; Yoshida, Y.; Takegoshi, Y.; Kaneda, M.; Murai, Y.; Kimoto, K.; Ueno, A.; Miyajima, Y.; et al. SARS-CoV-2 RNAemia with a higher nasopharyngeal viral load is strongly associated with disease severity and mortality in patients with COVID-19. J. Med. Virol. 2021, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, C.; Zeng, J.; Luo, C.; Hu, S.; Peng, Y.; Li, W.; Xie, Z.; Ling, Y.; Zhang, X.; et al. Analysis of viral load in different specimen types and serum antibody levels of COVID-19 patients. J. Transl. Med. 2021, 19, 30. [Google Scholar] [CrossRef]
- Salto-Alejandre, S.; Berastegui-Cabrera, J.; Camacho-Martinez, P.; Infante-Dominguez, C.; Carretero-Ledesma, M.; Crespo-Rivas, J.C.; Marquez, E.; Lomas, J.M.; Bueno, C.; Amaya, R.; et al. SARS-CoV-2 viral load in nasopharyngeal swabs is not an independent predictor of unfavorable outcome. Sci. Rep. 2021, 11, 12931. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, Z.; Li, P.; Yu, Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin. Chim. Acta 2020, 509, 220–223. [Google Scholar] [CrossRef]
- Gérard, C.; Maggipinto, G.; Minon, J.-M. COVID-19 and ABO blood group: Another viewpoint. Br. J. Haematol. 2020, 190, e93–e94. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Z.; Xiao, J.; Wu, Y.; Xia, N.; Yuan, Q. Evolution of the SARS-CoV-2 Omicron Variants: Genetic Impact on Viral Fitness. Viruses 2024, 16, 184. [Google Scholar] [CrossRef]
- Halawani, A.J.; Alhamoud, A.H.; Kabrah, S.M.; Al Eissa, M.M.; Daowd, R.A.; Algarni, A.M.; Alqarni, A.H.; Alshahrani, M.M.; Khan, A.A.; Jalal, N.A.; et al. Lack of association of ABO and RhD blood groups with COVID-19 mortality: A 2-center cross-sectional study in Saudi Arabia. Medicine 2024, 103, e39673. [Google Scholar] [CrossRef]
- Wu, B.-B.; Gu, D.-Z.; Yu, J.-N.; Yang, J.; Shen, W.-Q. Association between ABO blood groups and COVID-19 infection, severity and demise: A systematic review and meta-analysis. Infect. Genet. Evol. 2020, 84, 104485. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, C.K.; Ayres, F.; Hermanus, T.; Madzivhandila, M.; Kgagudi, P.; Oosthuysen, B.; Lambson, B.E.; De Oliveira, T.; Vermeulen, M.; Van der Berg, K.; et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021, 27, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Krieger, J.M.; Banerjee, A.; Xiang, Y.; Kaynak, B.; Shi, Y.; Arditi, M.; Bahar, I. Impact of new variants on SARS-CoV-2 infectivity and neutralization: A molecular assessment of the alterations in the spike-host protein interactions. iScience 2022, 25, 103939. [Google Scholar] [CrossRef] [PubMed]
- Magazine, N.; Zhang, T.; Wu, Y.; McGee, M.C.; Veggiani, G.; Huang, W. Mutations and Evolution of the SARS-CoV-2 Spike Protein. Viruses 2022, 14, 640. [Google Scholar] [CrossRef]
- Seyran, M.; Takayama, K.; Uversky, V.N.; Lundstrom, K.; Palù, G.; Sherchan, S.P.; Attrish, D.; Rezaei, N.; Aljabali, A.A.; Ghosh, S.; et al. The structural basis of accelerated host cell entry by SARS-CoV-2†. FEBS J. 2021, 288, 5010–5020. [Google Scholar] [CrossRef]
- Kazybay, B.; Ahmad, A.; Mu, C.; Mengdesh, D.; Xie, Y. Omicron N501Y mutation among SARS-CoV-2 lineages: Insilico analysis of potent binding to tyrosine kinase and hypothetical repurposed medicine. Travel Med. Infect. Dis. 2022, 45, 102242. [Google Scholar] [CrossRef]
- Tian, F.; Tong, B.; Sun, L.; Shi, S.; Zheng, B.; Wang, Z.; Dong, X.; Zheng, P. N501Y mutation of spike protein in SARS-CoV-2 strengthens its binding to receptor ACE2. eLife 2021, 10, e69091. [Google Scholar] [CrossRef]
- Pasko, B.E.; Abbott, D.; Bocsi, G.T.; Draper, N.L. ABO Blood Groups Are Not Associated With COVID-19 Disease Incidence and Severity When Correcting for Ethnicity Differences in Blood Type. Am. J. Clin. Pathol. 2022, 158, 249–253. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chang, W.C.; Prakash, E.; Peng, Y.J.; Tu, Z.J.; Lin, C.H.; Hsu, P.H.; Chang, C.F. Carbohydrate Ligands for COVID-19 Spike Proteins. Viruses 2022, 14, 330. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. Cell Rep. 2022, 39, 110829. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.; Bouman, C.C.; Beenen, L.F.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Canale, M.P.; Menghini, R.; Martelli, E.; Federici, M. COVID-19-Associated Endothelial Dysfunction and Microvascular Injury: From Pathophysiology to Clinical Manifestations. Card. Electrophysiol. Clin. 2022, 14, 21–28. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med. 2015, 13, 7. [Google Scholar] [CrossRef]
| Blood Group | COVID-19 Cohort (n = 446) | Saudi Population [19] |
|---|---|---|
| A | 30.94% (138 patients) | 27% |
| B | 19.06% (85 patients) | 22% |
| AB | 3.59% (16 patients) | 3% |
| O | 46.41% (207 patients) | 48% |
| All (n = 446) | A (n = 138) | B (n = 85) | AB (n = 16) | O (n = 207) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| References Range | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | p Value | |
| WBC (109/L) | 4–11 | 5.94 | 0.38–192 | 5.86 | 1.37–27.09 | 6.06 | 1.18–191.6 | 5.66 | 1.88–16.6 | 5.98 | 0.38–43.75 | 0.1059 |
| Hb (g/L) | 118–148 | 116 | 8.22–179 | 117 | 64–168 | 112 | 57–162 | 115 | 8.22–149 | 118 | 51–179 | 0.3143 |
| Platelets count (109/L) | 150–450 | 207 | 9–878 | 191.5 | 18–641 | 214 | 16–593 | 230 | 96–431 | 209 | 9–878 | 0.5820 |
| INR | 0.8–1.10 | 1.1 | 0.2–21 | 1.1 | 0.8–10 | 1.1 | 0.9–5.60 | 1.1 | 0.9–21 | 1.1 | 0.2–4.3 | 0.0002 |
| PT (s) | 10–141 | 14.9 | 11.6–72.6 | 15 | 11–59.2 | 17.4 | 12.30–72.6 | 14.9 | 12.30–36.6 | 15.11 | 11.8–44.50 | 0.8558 |
| aPTT (s) | 26–40 | 38.75 | 26.3–150 | 38.10 | 26.30–150.0 | 37.80 | 27.30–150 | 36.85 | 26.30–76.50 | 45 | 27.00–150 | 0.7831 |
| D-dimer (µg/mL) | >0.5 | 1.16 | 0.27-20 | 1.19 | 0.27–20 | 1.140 | 0.27–20 | 1.34 | 0.34–20 | 1.15 | 0.27–75 | 0.5071 |
| Fibrinogen (g/L) | 1.4–4.40 | 4.8 | 0.65–9.71 | 4.73 | 0.65–8.73 | 4.59 | 0.80–43.20 | 1.05 | 0.48–7.98 | 4.920 | 0.87–35.40 | <0.0001 |
| CRP (mg/dL) | <0.9 | 49.1 | 0.3–559 | 44.30 | 0.6–300 | 54.20 | 0.50–300 | 142.3 | 0.40–300 | 52.50 | 0.30–559 | 0.0624 |
| Serum Creatinine (mg/dL) | M (0.7–1.3) | 1 | 0.84–35 | 73.50 | 26–598 | 88 | 1–1697 | 93.50 | 59–326 | 78.00 | 0.95–2335 | 0.0332 |
| F (0.6–1.1) | ||||||||||||
| Blood Group | Alpha (n, %) | Beta (n, %) | Delta (n, %) | Omicron (n, %) | Non-VOCs (n, %) |
|---|---|---|---|---|---|
| A (n = 48) | 3 (6.25%) | 4 (8.33%) | 1 (2.08%) | 30 (62.5%) | 10 (20.83%) |
| B (n = 19) | 1 (5.26%) | 6 (31.58%) | 1 (5.26%) | 3 (15.79%) | 8 (42.11%) |
| AB (n = 6) | 2 (33.33%) | 0 (0%) | 0 (0%) | 2 (33.33%) | 2 (33.33%) |
| O (n = 47) | 4 (8.51%) | 10 (21.28%) | 0 (0%) | 23 (48.94%) | 10 (21.28%) |
| Mutation | Blood Group Types | p-Value | |||||||||
| SPIKE GENE | Spike | A | B | AB | O | ||||||
| N501Y | Positive | 14 | 29.20% | 9 | 47.70% | 2 | 33.33% | 25 | 53.20% | 0.1 | |
| Negative | 34 | 70.80% | 10 | 52.30% | 4 | 66.66% | 22 | 46.80% | |||
| D614G | Positive | 11 | 23.00% | 6 | 31.60% | 2 | 33.33% | 20 | 42.55% | 0.24 | |
| Negative | 37 | 77.00% | 13 | 68.40% | 4 | 66.66% | 27 | 57.45% | |||
| K417N | Positive | 8 | 16.70% | 7 | 36.90% | 0 | 0.00% | 13 | 27.66% | 0.2 | |
| Negative | 40 | 83.30% | 12 | 63.10% | 0 | 0.00% | 34 | 72.34% | |||
| N440K | Positive | 7 | 14.60% | 3 | 15.80% | 0 | 0.00% | 14 | 29.80% | 0.16 | |
| Negative | 41 | 85.40% | 16 | 84.20% | 0 | 0.00% | 33 | 70.20% | |||
| E484K | Positive | 7 | 14.60% | 5 | 26.30% | 0 | 0.00% | 13 | 27.70% | 0.16 | |
| Negative | 41 | 85.40% | 14 | 73.70% | 0 | 0.00% | 34 | 72.30% | |||
| P681R | Positive | 13 | 27.70% | 4 | 21.00% | 1 | 16.70% | 0 | 0.00% | 0.36 | |
| Negative | 34 | 72.30% | 15 | 79.00% | 5 | 83.30% | 0 | 0.00% | |||
| T547K | Positive | 5 | 10.40% | 3 | 15.80% | 0 | 0.00% | 13 | 27.70% | 0.16 | |
| Negative | 43 | 89.60% | 16 | 84.20% | 0 | 0.00% | 34 | 72.30% | |||
| R346T–R346Y | Positive | 5 | 10.40% | 4 | 21.00% | 0 | 0.00% | 11 | 23.40% | 0.16 | |
| Negative | 43 | 89.60% | 15 | 79% | 0 | 0.00% | 36 | 76.60% | |||
| H69del | Positive | 5 | 10.40% | 4 | 21.00% | 2 | 33.33% | 13 | 27.70% | 0.16 | |
| Negative | 43 | 89.60% | 15 | 79% | 4 | 66.70% | 34 | 72.30% | |||
| V70del | Positive | 5 | 10.40% | 4 | 21.00% | 2 | 33.30% | 13 | 27.70% | 0.16 | |
| Negative | 43 | 89.60% | 15 | 79.00% | 4 | 66.70% | 34 | 72.30% | |||
| Variable | Adjusted OR | 95% CI | p-Value |
|---|---|---|---|
| Blood Group A vs Non-A | 2.08 | 1.28–3.42 | 0.002 |
| Age (per year increase) | 1.04 | 1.01–1.07 | 0.010 |
| Sex (Male vs. Female) | 1.12 | 0.68–1.85 | 0.640 |
| BMI (per 1 kg/m2 increase) | 1.03 | 0.98–1.08 | 0.210 |
| Diabetes | 1.26 | 0.72–2.21 | 0.420 |
| Hypertension | 1.18 | 0.69–2.02 | 0.540 |
| Vaccination Status (Unvaccinated vs. Vaccinated) | 1.34 | 0.78–2.30 | 0.290 |
| Corticosteroid Use | 0.91 | 0.52–1.60 | 0.750 |
| Anticoagulant Use | 0.65 | 0.43–0.98 | 0.030 |
| Dominant Variant Period (Omicron vs Pre-Omicron) | 1.21 | 0.72–2.03 | 0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudouleh, E.; Owaidah, T.; Alhamlan, F.; Al-Qahtani, A.A.; Al Sarar, D.; Alkathiri, A.; Alghannam, S.; Bagasi, A.; Alkhulaifi, M.M.; Al-Qahtani, A.A. Association Between ABO Blood Groups and SARS-CoV-2 RNAemia, Spike Protein Mutations, and Thrombotic Events in COVID-19 Patients. Pathogens 2025, 14, 758. https://doi.org/10.3390/pathogens14080758
Abudouleh E, Owaidah T, Alhamlan F, Al-Qahtani AA, Al Sarar D, Alkathiri A, Alghannam S, Bagasi A, Alkhulaifi MM, Al-Qahtani AA. Association Between ABO Blood Groups and SARS-CoV-2 RNAemia, Spike Protein Mutations, and Thrombotic Events in COVID-19 Patients. Pathogens. 2025; 14(8):758. https://doi.org/10.3390/pathogens14080758
Chicago/Turabian StyleAbudouleh, Esra’a, Tarek Owaidah, Fatimah Alhamlan, Arwa A. Al-Qahtani, Dalia Al Sarar, Abdulrahman Alkathiri, Shouq Alghannam, Arwa Bagasi, Manal M. Alkhulaifi, and Ahmed A. Al-Qahtani. 2025. "Association Between ABO Blood Groups and SARS-CoV-2 RNAemia, Spike Protein Mutations, and Thrombotic Events in COVID-19 Patients" Pathogens 14, no. 8: 758. https://doi.org/10.3390/pathogens14080758
APA StyleAbudouleh, E., Owaidah, T., Alhamlan, F., Al-Qahtani, A. A., Al Sarar, D., Alkathiri, A., Alghannam, S., Bagasi, A., Alkhulaifi, M. M., & Al-Qahtani, A. A. (2025). Association Between ABO Blood Groups and SARS-CoV-2 RNAemia, Spike Protein Mutations, and Thrombotic Events in COVID-19 Patients. Pathogens, 14(8), 758. https://doi.org/10.3390/pathogens14080758






