AI Methods Tailored to Influenza, RSV, HIV, and SARS-CoV-2: A Focused Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Emerging Artificial Intelligence Tools in Viral Pandemics: Opportunities, Challenges, and Future Directions
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keshavarzi Arshadi, A.; Webb, J.; Salem, M.; Cruz, E.; Calad-Thomson, S.; Ghadirian, N.; Collins, J.; Diez-Cecilia, E.; Kelly, B.; Goodarzi, H.; et al. Artificial Intelligence for COVID-19 Drug Discovery and Vaccine Development. Front. Artif. Intell. 2020, 3, 65. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Link, K.E.; Daneshjou, R.; Cortés-Penfield, N. Black Box Warning: Large Language Models and the Future of Infectious Diseases Consultation. Clin. Infect. Dis. 2024, 78, 860–866. [Google Scholar] [CrossRef]
- Wagner, M.W.; Ertl-Wagner, B.B. Accuracy of Information and References Using ChatGPT-3 for Retrieval of Clinical Radiological Information. Can. Assoc. Radiol. J. 2024, 75, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S. Explainability and artificial intelligence in medicine. Lancet Digit. Health 2022, 4, e214–e215. [Google Scholar] [CrossRef]
- Rizzo, A.; Mensa, E.; Giacomelli, A. The future of large language models in fighting emerging outbreaks: Lights and shadows. Lancet Microbe 2024, 5, 100954. [Google Scholar] [CrossRef]
- van Hoek, A.J.; Funk, S.; Flasche, S.; Quilty, B.J.; van Kleef, E.; Camacho, A.; Kucharski, A.J. Importance of investing time and money in integrating large language model-based agents into outbreak analytics pipelines. Lancet Microbe. 2024, 5, 100881. [Google Scholar] [CrossRef] [PubMed]
- Tomic, A.; Tomic, I.; Rosenberg-Hasson, Y.; Dekker, C.L.; Maecker, H.T.; Davis, M.M. SIMON, an Automated Machine Learning System, Reveals Immune Signatures of Influenza Vaccine Responses. J. Immunol. 2019, 203, 749–759. [Google Scholar] [CrossRef]
- Hu, H.; Wang, H.; Wang, F.; Langley, D.; Avram, A.; Liu, M. Prediction of influenza-like illness based on the improved artificial tree algorithm and artificial neural network. Sci. Rep. 2018, 8, 4895. [Google Scholar] [CrossRef]
- Reich, N.G.; McGowan, C.J.; Yamana, T.K.; Tushar, A.; Ray, E.L.; Osthus, D.; Kandula, S.; Brooks, L.C.; Crawford-Crudell, W.; Gibson, G.C.; et al. Accuracy of real-time multi-model ensemble forecasts for seasonal influenza in the U. S. PLoS Comput. Biol. 2019, 15, e1007486. [Google Scholar] [CrossRef] [PubMed]
- Borkenhagen, L.K.; Allen, M.W.; Runstadler, J.A. Influenza virus genotype to phenotype predictions through machine learning: A systematic review. Emerg. Microbes Infect. 2021, 10, 1896–1907. [Google Scholar] [CrossRef]
- Arık, S.; Shor, J.; Sinha, R.; Yoon, J.; Ledsam, J.R.; Le, L.T.; Dusenberry, M.W.; Yoder, N.C.; Popendorf, K.; Epshteyn, A.; et al. A prospective evaluation of AI-augmented epidemiology to forecast COVID-19 in the USA and Japan. NPJ Digit. Med. 2021, 4, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef]
- Wolk, D.M.; Lanyado, A.; Tice, A.M.; Shermohammed, M.; Kinar, Y.; Goren, A.; Chabris, C.F.; Meyer, M.N.; Shoshan, A.; Abedi, V.; et al. Prediction of Influenza Complications: Development and Validation of a Machine Learning Prediction Model to Improve and Expand the Identification of Vaccine-Hesitant Patients at Risk of Severe Influenza Complications. J. Clin. Med. 2022, 11, 4342. [Google Scholar] [CrossRef]
- Marquez, E.; Barrón-Palma, E.V.; Rodríguez, K.; Savage, J.; Sanchez-Sandoval, A.L. Supervised Machine Learning Methods for Seasonal Influenza Diagnosis. Diagnostics 2023, 13, 3352. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, H.; Long, J.; Jian, Y.; Feng, L.; Hu, L.; Zhou, H.; Zhu, W.; Yuan, Z.; Chen, Y.; et al. Developing Machine Learning Models Based on Clinical Manifestations to Predict Influenza—Chongqing Municipality, China, 2022–2023. China CDC Wkly. 2025, 7, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.K.; Wu, C.C.; Singh, A.; Li, J.H.; Lee, C.; Chou, E.H.; Pekosz, A.; Rothman, R.; Chen, K.F. Developing and validating clinical features-based machine learning algorithms to predict influenza infection in influenza-like illness patients. Biomed. J. 2023, 46, 100561. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, W.; Duan, L.; McDonald, W.; Osgood, N. Comparison of pretrained transformer-based models for influenza and COVID-19 detection using social media text data in Saskatchewan, Canada. Front. Digit. Health 2023, 5, 1203874. [Google Scholar] [CrossRef] [PubMed]
- Baccega, D.; Castagno, P.; Fernández Anta, A.; Sereno, M. Enhancing COVID-19 forecasting precision through the integration of compartmental models, machine learning and variants. Sci. Rep. 2024, 14, 19220. [Google Scholar] [CrossRef]
- Baik, S.M.; Hong, K.S.; Park, D.J. Deep learning approach for early prediction of COVID-19 mortality using chest X-ray and electronic health records. BMC Bioinformatics. 2023, 24, 190. [Google Scholar] [CrossRef]
- Dipaola, F.; Gatti, M.; Giaj Levra, A.; Menè, R.; Shiffer, D.; Faccincani, R.; Raouf, Z.; Secchi, A.; Rovere Querini, P.; Voza, A.; et al. Multimodal deep learning for COVID-19 prognosis prediction in the emergency department: A bi-centric study. Sci. Rep. 2023, 13, 10868. [Google Scholar] [CrossRef]
- Akter, T.; Hossain, M.F.; Ullah, M.S.; Akter, R.; Kumar, R. Mortality prediction in COVID-19 using time series and machine learning techniques. Comput. Math. Methods Med. 2024, 2024, 5891177. [Google Scholar] [CrossRef]
- Zakariaee, S.S.; Naderi, N.; Ebrahimi, M.; Kazemi-Arpanahi, H. Comparing machine learning algorithms to predict COVID-19 mortality using a dataset including chest computed tomography severity score data. Sci. Rep. 2023, 13, 11343. [Google Scholar] [CrossRef]
- Kawamoto, S.; Morikawa, Y.; Yahagi, N. Novel Approach for Detecting Respiratory Syncytial Virus in Pediatric Patients Using Machine Learning Models Based on Patient-Reported Symptoms: Model Development and Validation Study. JMIR Form. Res. 2024, 8, e52412. [Google Scholar] [CrossRef]
- Soriano-Arandes, A.; Andrés, C.; Perramon-Malavez, A.; Creus-Costa, A.; Gatell, A.; Martín-Martín, R.; Solà-Segura, E.; Riera-Bosch, M.T.; Fernández, E.; Biosca, M.; et al. Implementing Symptom-Based Predictive Models for Early Diagnosis of Pediatric Respiratory Viral Infections. Viruses 2025, 17, 546. [Google Scholar] [CrossRef]
- Tso, C.F.; Lam, C.; Calvert, J.; Mao, Q. Machine learning early prediction of respiratory syncytial virus in pediatric hospitalized patients. Front. Pediatr. 2022, 10, 886212. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhao, J. Prediction of HIV-1 and HIV-2 proteins by using Chou’s pseudo amino acid compositions and different classifiers. Sci. Rep. 2018, 8, 2359. [Google Scholar] [CrossRef]
- Powell, B.M.; Davis, J.H. Learning structural heterogeneity from cryo-electron sub-tomograms with tomoDRGN. Nat. Methods 2024, 21, 1525–1536. [Google Scholar] [CrossRef]
- Hu, H.; Xiao, A.; Zhang, S.; Li, Y.; Shi, X.; Jiang, T.; Zhang, L.; Zhang, L.; Zeng, J. DeepHINT: Understanding HIV-1 integration via deep learning with attention. Bioinformatics 2019, 35, 1660–1667. [Google Scholar] [CrossRef]
- Peng, X.; Zhu, B. Machine learning identified genetic features associated with HIV sequences in the monocytes. Chin. Med. J. 2023, 136, 3002–3004. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.X.; Pan, X.M. HIV-1 tropism prediction by the XGboost and HMM methods. Sci. Rep. 2019, 9, 9997. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.D.; Ekwunife, O.I.; Mendonca, R.; Kwach, B.; Omollo, V.; Zhang, S.; Ongwen, P.; Hattery, D.; Smedinghoff, S.; Morris, S.; et al. Measuring the performance of computer vision artificial intelligence to interpret images of HIV self-testing results. Front. Public Health 2024, 12, 1334881. [Google Scholar] [CrossRef]
- Turbé, V.; Herbst, C.; Mngomezulu, T.; Meshkinfamfard, S.; Dlamini, N.; Mhlongo, T.; Smit, T.; Cherepanova, V.; Shimada, K.; Budd, J.; et al. Deep learning of HIV field-based rapid tests. Nat. Med. 2021, 27, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.E.; Musinguzi, N.; Bangsberg, D.R.; Bwana, M.B.; Muzoora, C.; Hunt, P.W.; Martin, J.N.; Haberer, J.E.; Petersen, M.L. Super learner analysis of real-time electronically monitored adherence to antiretroviral therapy under constrained optimization and comparison to non-differentiated care approaches for persons living with HIV in rural Uganda. J. Int. AIDS Soc. 2020, 23, e25467. [Google Scholar] [CrossRef] [PubMed]
- Balzer, L.B.; Havlir, D.V.; Kamya, M.R.; Chamie, G.; Charlebois, E.D.; Clark, T.D.; Koss, C.A.; Kwarisiima, D.; Ayieko, J.; Sang, N.; et al. Machine Learning to Identify Persons at High-Risk of Human Immunodeficiency Virus Acquisition in Rural Kenya and Uganda. Clin. Infect. Dis. 2020, 71, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Medland, N.A.; Fairley, C.K.; Wu, J.; Shang, X.; Chow, E.P.F.; Xu, X.; Ge, Z.; Zhuang, X.; Zhang, L. Predicting the diagnosis of HIV and sexually transmitted infections among men who have sex with men using machine learning approaches. J. Infect. 2021, 82, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, W.; Du, T.; Hong, Z.; Lin, J. Targeting HIV/HCV Coinfection Using a Machine Learning-Based Multiple Quantitative Structure-Activity Relationships (Multiple QSAR) Method. Int. J. Mol. Sci. 2019, 20, 3572. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Chen, X.; Zhao, L. HIV-1/HBV Coinfection Accurate Multitarget Prediction Using a Graph Neural Network-Based Ensemble Predicting Model. Int. J. Mol. Sci. 2023, 24, 7139. [Google Scholar] [CrossRef]
- Onywera, H.; Williamson, A.L.; Cozzuto, L.; Bonnin, S.; Mbulawa, Z.Z.A.; Coetzee, D.; Ponomarenko, J.; Meiring, T.L. The penile microbiota of Black South African men: Relationship with human papillomavirus and HIV infection. BMC Microbiol. 2020, 20, 78. [Google Scholar] [CrossRef]
- Namalinzi, F.; Galadima, K.R.; Nalwanga, R.; Sekitoleko, I.; Uwimbabazi, L.F.R. Prediction of precancerous cervical cancer lesions among women living with HIV on antiretroviral therapy in Uganda: A comparison of supervised machine learning algorithms. BMC Womens Health 2024, 24, 393. [Google Scholar] [CrossRef]
- Arrigoni, R.; Santacroce, L.; Ballini, A.; Palese, L.L. AI-Aided Search for New HIV-1 Protease Ligands. Biomolecules 2023, 13, 858. [Google Scholar] [CrossRef]
- Leidner, F.; Kurt Yilmaz, N.; Schiffer, C.A. Target-Specific Prediction of Ligand Affinity with Structure-Based Interaction Fingerprints. J. Chem. Inf. Model. 2019, 59, 3679–3691. [Google Scholar] [CrossRef]
- Kutsal, M.; Ucar, F.; Kati, N. Computational drug discovery on human immunodeficiency virus with a customized long short-term memory variational autoencoder deep-learning architecture. CPT Pharmacomet. Syst. Pharmacol. 2024, 13, 308–316. [Google Scholar] [CrossRef]
- Pham, T.; Ghafoor, M.; Grañana-Castillo, S.; Marzolini, C.; Gibbons, S.; Khoo, S.; Chiong, J.; Wang, D.; Siccardi, M. DeepARV: Ensemble deep learning to predict drug-drug interaction of clinical relevance with antiretroviral therapy. NPJ Syst. Biol. Appl. 2024, 10, 48. [Google Scholar] [CrossRef]
- Rawi, R.; Mall, R.; Shen, C.H.; Farney, S.K.; Shiakolas, A.; Zhou, J.; Bensmail, H.; Chun, T.W.; Doria-Rose, N.A.; Lynch, R.M.; et al. Accurate Prediction for Antibody Resistance of Clinical HIV-1 Isolates. Sci. Rep. 2019, 9, 14696. [Google Scholar] [CrossRef]
- Steiner, M.C.; Gibson, K.M.; Crandall, K.A. Drug Resistance Prediction Using Deep Learning Techniques on HIV-1 Sequence Data. Viruses. 2020, 12, 560. [Google Scholar] [CrossRef]
- Cai, Q.; Yuan, R.; He, J.; Li, M.; Guo, Y. Predicting HIV drug resistance using weighted machine learning method at target protein sequence-level. Mol Divers. 2021, 25, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Blassel, L.; Tostevin, A.; Villabona-Arenas, C.J.; Peeters, M.; Hué, S.; Gascuel, O. UK HIV Drug Resistance Database. Using machine learning and big data to explore the drug resistance landscape in HIV. PLoS Comput. Biol. 2021, 17, e1008873. [Google Scholar] [CrossRef] [PubMed]
- Altamirano-Flores, J.S.; Alvarado-Hernández, L.Á.; Cuevas-Tello, J.C.; Tino, P.; Guerra-Palomares, S.E.; Garcia-Sepulveda, C.A. Identification of Clinically Relevant HIV Vif Protein Motif Mutations through Machine Learning and Undersampling. Cells 2023, 12, 772. [Google Scholar] [CrossRef]
- Li, J.; Hao, Y.; Liu, Y.; Wu, L.; Liang, H.; Ni, L.; Wang, F.; Wang, S.; Duan, Y.; Xu, Q.; et al. Supervised machine learning algorithms to predict the duration and risk of long-term hospitalization in HIV-infected individuals: A retrospective study. Front. Public Health 2024, 11, 1282324. [Google Scholar] [CrossRef]
- Semenova, L.; Wang, Y.; Falcinelli, S.; Archin, N.; Cooper-Volkheimer, A.D.; Margolis, D.M.; Goonetilleke, N.; Murdoch, D.M.; Rudin, C.D.; Browne, E.P. Machine learning approaches identify immunologic signatures of total and intact HIV DNA during long-term antiretroviral therapy. eLife 2024, 13, RP94899. [Google Scholar] [CrossRef] [PubMed]
- Ogishi, M.; Yotsuyanagi, H. Prediction of HIV-associated neurocognitive disorder (HAND) from three genetic features of envelope gp120 glycoprotein. Retrovirology 2018, 15, 12. [Google Scholar] [CrossRef]
- Tu, W.; Johnson, E.; Fujiwara, E.; Gill, M.J.; Kong, L.; Power, C. Predictive variables for peripheral neuropathy in treated HIV type 1 infection revealed by machine learning. AIDS 2021, 35, 1785–1793. [Google Scholar] [CrossRef]
- Paul, R.H.; Cho, K.S.; Belden, A.C.; Mellins, C.A.; Malee, K.M.; Robbins, R.N.; Salminen, L.E.; Kerr, S.J.; Adhikari, B.; Garcia-Egan, P.M.; et al. Machine-learning classification of neurocognitive performance in children with perinatal HIV initiating de novo antiretroviral therapy. AIDS 2020, 34, 737–748. [Google Scholar] [CrossRef]
- Petersen, K.J.; Strain, J.; Cooley, S.; Vaida, F.; Ances, B.M. Machine Learning Quantifies Accelerated White-Matter Aging in Persons With HIV. J. Infect. Dis. 2022, 226, 49–58. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Aouizerat, B.E.; Peng, G.; Marconi, V.C.; Corley, M.J.; Hulgan, T.; Bryant, K.J.; Zhao, H.; Krystal, J.H.; et al. Machine learning selected smoking-associated DNA methylation signatures that predict HIV prognosis and mortality. Clin. Epigenetics. 2018, 10, 155. [Google Scholar] [CrossRef]
- McGowan, E.; Rosenthal, R.; Fiore-Gartland, A.; Macharia, G.; Balinda, S.; Kapaata, A.; Umviligihozo, G.; Muok, E.; Dalel, J.; Streatfield, C.L.; et al. Utilizing Computational Machine Learning Tools to Understand Immunogenic Breadth in the Context of a CD8 T-Cell Mediated HIV Response. Front. Immunol. 2021, 12, 609884. [Google Scholar] [CrossRef]
- Dănăilă, V.R.; Buiu, C. Prediction of HIV sensitivity to monoclonal antibodies using aminoacid sequences and deep learning. Bioinformatics 2022, 38, 4278–4285. [Google Scholar] [CrossRef] [PubMed]
- Montesi, G.; Augello, M.; Polvere, J.; Marchetti, G.; Medaglini, D.; Ciabattini, A. Predicting humoral responses to primary and booster SARS-CoV-2 mRNA vaccination in people living with HIV: A machine learning approach. J. Transl. Med. 2024, 22, 432. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhang, L. AI applications in HIV research: Advances and future directions. Front. Microbiol. 2025, 16, 1541942. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Data Modernization Initiative: Technologies—Artificial Intelligence and Machine Learning. Atlanta (GA): CDC. Available online: https://www.cdc.gov/surveillance/data-modernization/technologies/ai-ml.html (accessed on 24 April 2025).
- European Centre for Disease Prevention and Control. Digital Technologies for Surveillance, Prevention and Control of Infectious Diseases: A Scoping Review. Stockholm: ECDC. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/digital-technologies-surveillance-prevention-and-control-infectious-diseases (accessed on 24 April 2025).
- Yuan, K.; Yoon, C.H.; Gu, Q.; Munby, H.; Walker, A.S.; Zhu, T.; Eyre, D.W. Transformers and large language models are efficient feature extractors for electronic health record studies. Commun. Med. 2025, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Singhal, K.; Azizi, S.; Tu, T.; Mahdavi, S.S.; Wei, J.; Chung, H.W.; Scales, N.; Tanwani, A.; Cole-Lewis, H.; Pfohl, S.; et al. Large language models encode clinical knowledge. Nature 2023, 620, 172–180, Erratum in: Nature 2023, 620, E19. https://doi.org/10.1038/s41586-023-06455-0. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Y.; Liu, X.C.; Nejatian, N.P.; Nasir-Moin, M.; Wang, D.; Abidin, A.; Eaton, K.; Riina, H.A.; Laufer, I.; Punjabi, P.; et al. Health system-scale language models are all-purpose prediction engines. Nature 2023, 619, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.; Wu, J.; Child, R.; Luan, D.; Amodei, D.; Sutskever, I. Language models are unsupervised multitask learners. OpenAI Blog 2019, 1, 9. [Google Scholar]
- Liu, Y.; Whitfield, C.; Zhang, T.; Hauser, A.; Reynolds, T.; Anwar, M. Monitoring COVID-19 pandemic through the lens of social media using natural language processing and machine learning. Health Inf. Sci. Syst. 2021, 9, 25. [Google Scholar] [CrossRef]
- Robotti, C.; Costantini, G.; Saggio, G.; Cesarini, V.; Calastri, A.; Maiorano, E.; Piloni, D.; Perrone, T.; Sabatini, U.; Ferretti, V.V.; et al. Machine Learning-based Voice Assessment for the Detection of Positive and Recovered COVID-19 Patients. J. Voice 2024, 38, 796.e1–796.e13. [Google Scholar] [CrossRef]
- Han, G.R.; Goncharov, A.; Eryilmaz, M.; Ye, S.; Palanisamy, B.; Ghosh, R.; Lisi, F.; Rogers, E.; Guzman, D.; Yigci, D.; et al. Machine learning in point-of-care testing: Innovations, challenges, and opportunities. Nat. Commun. 2025, 16, 3165. [Google Scholar] [CrossRef]
- Ye, X.; Wang, X.; Lin, H. Global Research on Pandemics or Epidemics and Mental Health: A Natural Language Processing Study. J. Epidemiol. Glob. Health 2024, 14, 1268–1280. [Google Scholar] [CrossRef]
- Islam, N.; Mohsin, A.S.M.; Choudhury, S.H.; Shaer, T.P.; Islam, M.A.; Sadat, O.; Taz, N.H. COVID-19 and Pneumonia detection and web deployment from CT scan and X-ray images using deep learning. PLoS ONE 2024, 19, e0302413. [Google Scholar] [CrossRef]
- Shukla, A.K.; Seth, T.; Muhuri, P.K. Artificial intelligence centric scientific research on COVID-19: An analysis based on scientometrics data. Multimed. Tools Appl. 2023, 82, 32755–32787. [Google Scholar] [CrossRef]
- Mahalakshmi, V.; Balobaid, A.; Kanisha, B.; Sasirekha, R.; Ramkumar, R.M. Artificial Intelligence: A Next-Level Approach in Confronting the COVID-19 Pandemic. Healthcare 2023, 11, 854. [Google Scholar] [CrossRef]
- Das, D.; Biswas, S.K.; Bandyopadhyay, S. Perspective of AI system for COVID-19 detection using chest images: A review. Multimed. Tools Appl. 2022, 81, 21471–21501. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.S.; Sircar, S.; Bhat, S.; Ansari, M.I.; Pande, T.; Kumar, P.; Mathapati, B.; Balasubramanian, G.; Kaushik, R.; Natesan, S.; et al. How artificial intelligence may help the Covid-19 pandemic: Pitfalls and lessons for the future. Rev. Med. Virol. 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Kim, T.H.; Chinthaginjala, R.; Srinivasulu, A.; Tera, S.P.; Rab, S.O. COVID-19 health data prediction: A critical evaluation of CNN-based approaches. Sci. Rep. 2025, 15, 9121. [Google Scholar] [CrossRef]
- Garrido, N.J.; González-Martínez, F.; Torres, A.M.; Blasco-Segura, P.; Losada, S.; Plaza, A.; Mateo, J. Role of Artificial Intelligence in Identifying Vital Biomarkers with Greater Precision in Emergency Departments During Emerging Pandemics. Int. J. Mol. Sci. 2025, 26, 722. [Google Scholar] [CrossRef]
- Bowyer, S.; Allen, D.J.; Furnham, N. Unveiling the ghost: Machine learning’s impact on the landscape of virology. J. Gen. Virol. 2025, 106, 002067. [Google Scholar] [CrossRef]
- Zaeri, N. Artificial intelligence and machine learning responses to COVID-19 related inquiries. J. Med. Eng. Technol. 2023, 47, 301–320. [Google Scholar] [CrossRef]
- Hamelin, D.J.; Scicluna, M.; Saadie, I.; Mostefai, F.; Grenier, J.C.; Baron, C.; Caron, E.; Hussin, J.G. Predicting pathogen evolution and immune evasion in the age of artificial intelligence. Comput. Struct. Biotechnol. J. 2025, 27, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Ankolekar, A.; Eppings, L.; Bottari, F.; Pinho, I.F.; Howard, K.; Baker, R.; Nan, Y.; Xing, X.; Walsh, S.L.; Vos, W.; et al. Using artificial intelligence and predictive modelling to enable learning healthcare systems (LHS) for pandemic preparedness. Comput. Struct. Biotechnol. J. 2024, 24, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Badnjević, A.; Pokvić, L.G.; Smajlhodžić-Deljo, M.; Spahić, L.; Bego, T.; Meseldžić, N.; Prnjavorac, L.; Prnjavorac, B.; Bedak, O. Application of artificial intelligence for the classification of the clinical outcome and therapy in patients with viral infections: The case of COVID-19. Technol. Health Care 2024, 32, 1859–1870. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Tsui, J.L.; Chang, S.Y.; Lytras, S.; Khurana, M.P.; Vanderslott, S.; Bajaj, S.; Scheidwasser, N.; Curran-Sebastian, J.L.; Semenova, E.; et al. Artificial intelligence for modelling infectious disease epidemics. Nature 2025, 638, 623–635. [Google Scholar] [CrossRef]
- Anter, J.M.; Yakimovich, A. Artificial Intelligence Methods in Infection Biology Research. Methods Mol. Biol. 2025, 2890, 291–333. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 (General Data Protection Regulation). Off. J. Eur. Union 2016, L119, 1–88. Available online: https://gdpr-info.eu/ (accessed on 22 July 2025).
- Centers for Disease Control and Prevention. Health Insurance Portability and Accountability Act of 1996 (HIPAA). Public Health Law Program. Available online: https://www.cdc.gov/phlp/php/resources/health-insurance-portability-and-accountability-act-of-1996-hipaa.html (accessed on 22 July 2025).
- Cross, J.L.; Choma, M.A.; Onofrey, J.A. Bias in medical AI: Implications for clinical decision-making. PLoS Digit. Health 2024, 3, e0000651. [Google Scholar] [CrossRef]
- Arvai, N.; Katonai, G.; Mesko, B. Health Care Professionals’ Concerns About Medical AI and Psychological Barriers and Strategies for Successful Implementation: Scoping Review. J. Med. Internet Res. 2025, 27, e66986. [Google Scholar] [CrossRef] [PubMed]
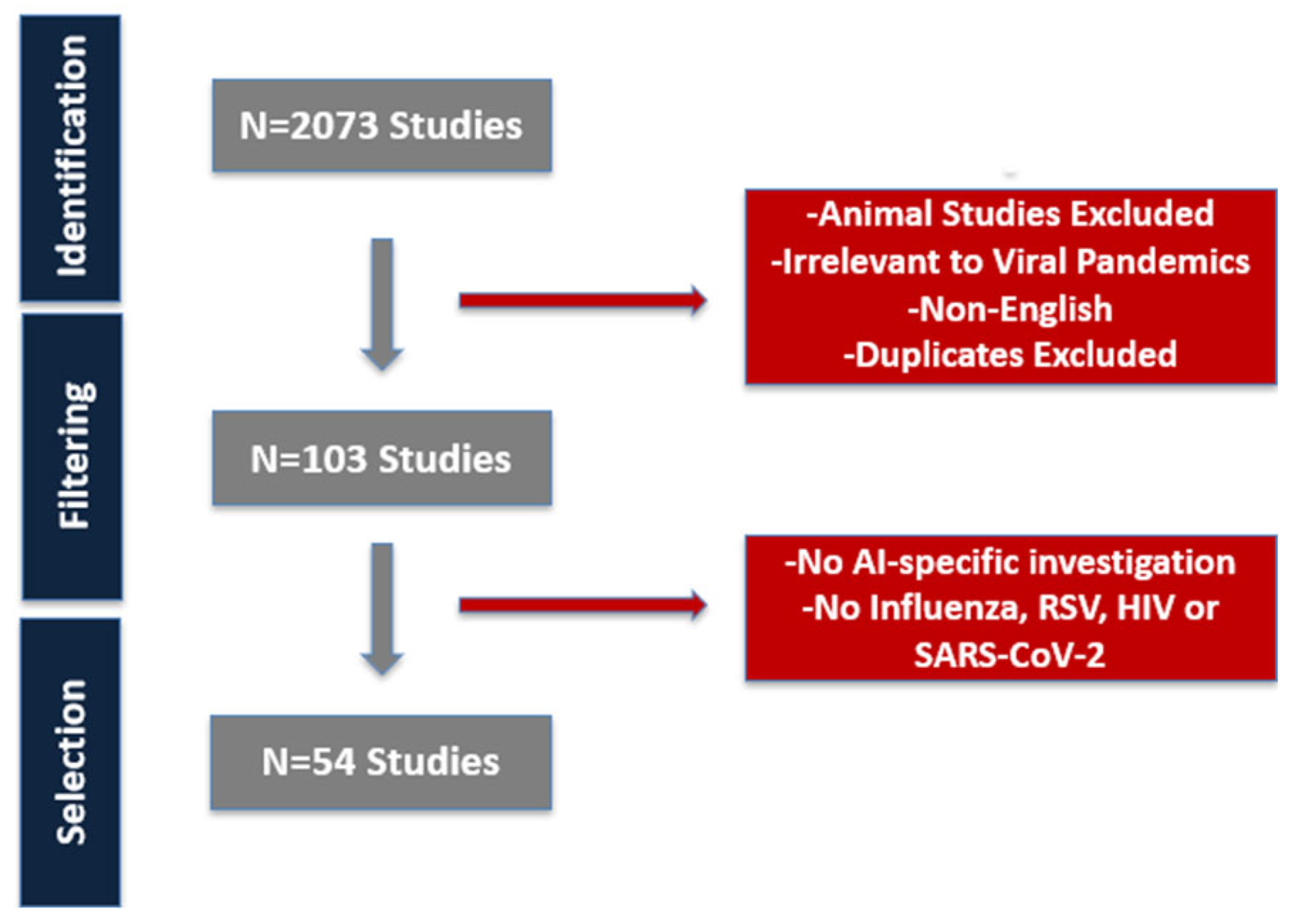
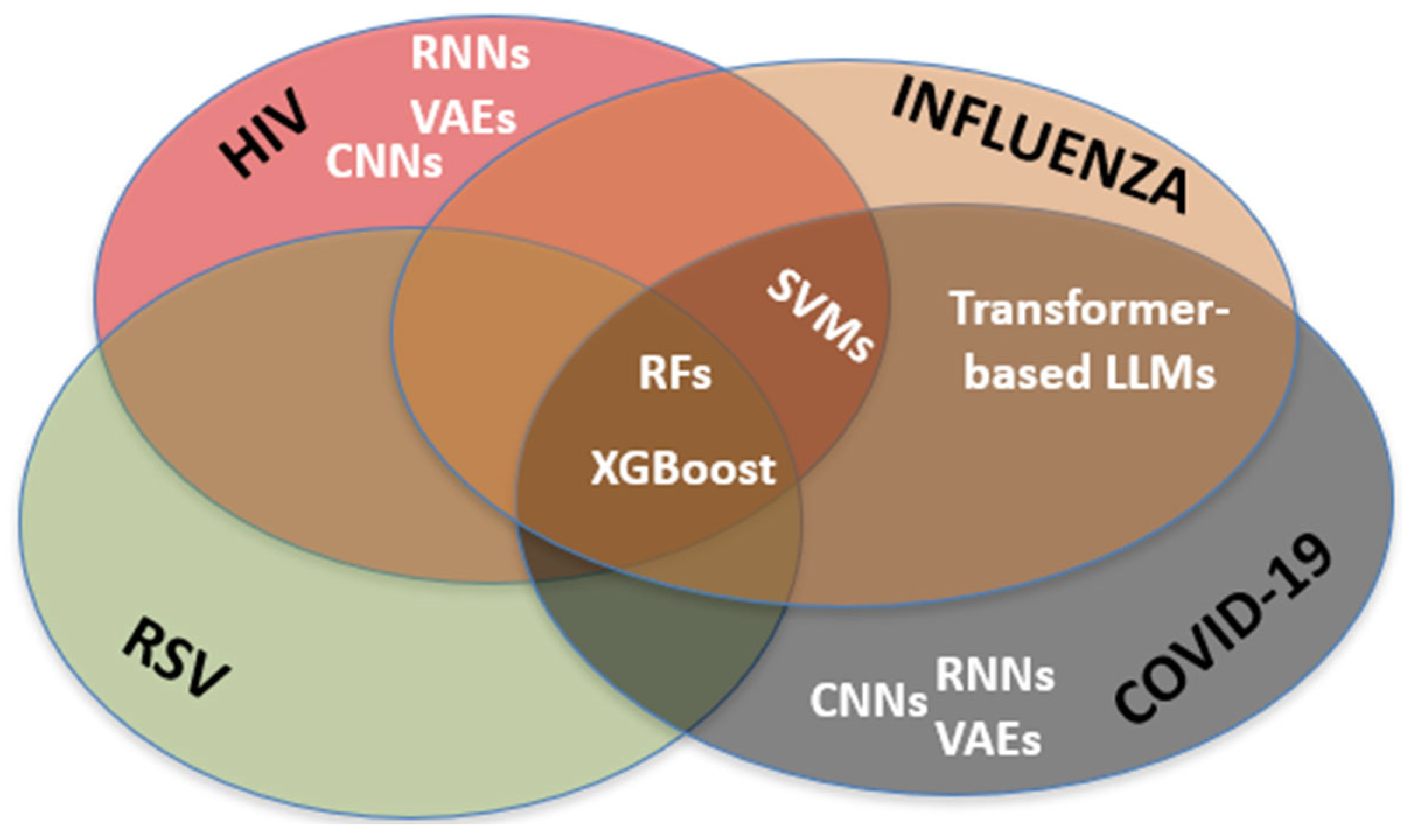
| Author (et al.) (Year) | Disease Studied | AI Method Used | Outcome | Benefit over Traditional Approaches |
|---|---|---|---|---|
| Seasonal Influenza | ||||
| Hu H., et al. (2018) [8] | ILI prediction | AT-optimized BPNN, IAT-BPNN | Delivered MAPE 14.74%, outperforming basic BPNN (MAPE 23.97%) and AT-BPNN (MAPE 20.34%) | Real-time ILI monitoring with near-zero lag; 50–80% error reduction compared to standard BPNN or AT-BPNN; combines social media and official data |
| Reich NG., et al. (2019) [9] | Seasonal influenza outbreaks | Multi-model ensemble approach using stacking (weighted averaging of 21 predictive models) | Highest forecast accuracy during 2017/18 flu season; superior to all individual and CDC baseline ensemble models | Improved forecast accuracy, reduced variability; integrated into CDC public health responses |
| Tomic A., et al. (2019) [7] | Influenza vaccine immune response | SIMON automated ML system (Random Forest, discriminant analysis) | Identified novel T-cell subsets predictive of robust vaccine response (AUROC up to 0.86) | Automated high-performing model selection; discovered novel immune signatures |
| Borkenhagen LK, et al. (2021) [10] | Influenza A virus genotype→phenotype mapping | Random Forest, SVM, neural networks, decision trees, naïve Bayes, k-NN, AdaBoost, logistic regression, Rotation Forest, gradient-boosted trees, hierarchical clustering | RF & SVM consistently highest accuracies; feature-consensus identified critical residue sites | Actionable roadmap for practitioners; feature-consensus map prioritizes experimental follow-up; best-practice checklist enhances model reliability and validation |
| Wolk DM, et al. (2022) [13] | Influenza complications in vaccine-hesitant populations | XGBoost, Shapley values | Developed GFlu-CxFlag model; outperformed traditional models | Improved risk prediction, targeted interventions |
| Marquez E., et al. (2023) [14] | Seasonal influenza diagnosis | Supervised ML (Random Forest, bagging, etc.) | RF: Accuracy 0.86 | Effective in limited-resource settings; reduced unnecessary molecular tests |
| Zeng, et al. (2025) [15] | Influenza | LLM for automated symptom-variable extraction; XGBoost and SHAP interpretability | Prediction model: AUC 0.734 | The data-driven ILI definition significantly outperformed existing WHO, China CDC, and USA CDC definitions |
| New ILI definition (fever ≥ 37.9 °C + cough/rhinorrhea): AUC 0.618 | ||||
| Hung, et al. (2023) [16] | Influenza | XGBoost | AUC 0.82 | Significantly outperforms prior clinical prediction models |
| COVID-19 | ||||
| Tian Y., et al. (2023) [17] | Influenza and COVID-19 detection using social media (Saskatchewan, Canada) | Pretrained transformer-based language models: BERT, BERTweet | CT-BERT had best COVID-19 tweet detection (accuracy = 94.6%), BERTweet-best for flu (accuracy = 92.4%) | Showed social media data can support high-accuracy real-time digital surveillance; domain-Specific models (BERTweet) outperformed general ones; complements traditional public health surveillance tools |
| Keshavarzi Arshadi A., et al. (2020) [1] | Drug and vaccine development | DL, VAE, GCNN, RNN, GAN | Identified drugs targeting SARS-CoV-2 proteins, created comprehensive candidate database CoronaDB-AI for training models | Accelerates discovery through automatic feature extraction, generative models for novel candidates, transfers learned knowledge to overcome data scarcity and improve reliability |
| Arık SÖ., et al. (2021) [11] | COVID-19 epidemiology forecasting | AI-augmented SEIR model (seq-to-seq, quantile regression) | Consistently superior forecasts | Adapted to dynamic policy/behavior; enabled proactive interventions |
| Li L., et al. (2021) [12] | COVID-19 vs. CAP | 3D CNN | COVID-19 AUC: 0.96 CAP AUC: 0.95 | Rapid, precise differentiation between COVID-19 and CAP using CT scans; improved early clinical management |
| Baccega D., et al. (2024) [18] | COVID-19 forecasting precision | Hybrid ML (Prophet) | Accurate forecasts during variant shifts, outbreaks | Better accuracy, interpretability, lower data needs |
| Baik, et al. (2023) [19] | COVID-19 | CNNs for chest X-ray + MLP, XGBoost & RF for EHR data | Early mortality prediction: ensemble AUC 0.8698 | Combining imaging and EHR modalities in a single ensemble markedly improved prognostic accuracy over single-modality models, enabling earlier, data-driven resource allocation |
| Dipaola, et al. (2023) [20] | COVID-19 | TensorFlow combining tabular predictors (age, creatinine, platelets) and NLP-processed text (history, exam, radiology) in a single multimodal model | 30-day mortality: AUC 0.87 | Integrating unstructured text and structured data markedly improved prognostic accuracy |
| Akter, et al. (2024) [21] | COVID-19 | ARIMA for time-series; multilayer NN with increasing hidden layers; SVMs | SVM yielded most accurate mortality-rate forecasting | The SVM approach substantially outperformed classical ARIMA and standard NN models in predictive accuracy |
| Zakariaee, et al. (2023) [22] | COVID-19 | RF on demographics, clinical manifestations, comorbidities & lab results | Accuracy 97.2% | Combining severity scoring with routine patient data and RF delivered near-perfect mortality prediction, surpassing other ML models |
| RSV | ||||
| Kawamoto, et al. (2024) [23] | RSV infection in pediatric outpatients | XGBoost | AUC-ROC 0.811 | Enables remote, non-invasive detection; eliminates need for additional antigen testing in ~75% of patients; reduces discomfort and streamlines diagnosis |
| Soriano-Arandes, et al. (2025) [24] | Pediatric acute respiratory infections (SARS-CoV-2, RSV, influenza A/B, rhinovirus) | Random Forest and boosting models; SHAP value analysis | RSV AUC 0.81; influenza A/B AUC 0.70; SARS-CoV-2 AUC 0.71 | Facilitates early, point-of-care triage based solely on symptoms; reduces reliance on confirmatory laboratory tests; supports rapid, cost-effective decision-making in primary care settings; optimizes resource allocation |
| Tso, et al. (2022) [25] | RSV in pediatric inpatients | XGBoost on routinely collected EHR data | AUROC 0.919 | Provides rapid, non-invasive prediction of RSV positivity using only standard admission data—reducing the need for immediate diagnostic tests and enabling faster infection control measures |
| HIV | ||||
| Mei & Zhao (2018) [26] | HIV-1 and HIV-2 protein classification | SVM; logistic regression; multilayer perceptron | All three models demonstrated superior performance in classifying HIV-1 and HIV-2 proteins | Provided flexible, nonlinear classification, outperforming conventional statistical models |
| Powell & Davis (2024) [27] | Structural heterogeneity of HIV capsid complexes | Low-dimensional continuous representations | Exceptional performance in reconstructing heterogeneous structures | Enabled data-driven reconstruction of structural heterogeneity beyond traditional tomography |
| Hu, et al. (2019) [28] | HIV-1 integration site prediction | Attention-based deep learning | Improved prediction accuracy over conventional models | Automatically learned genomic and epigenetic context, offering mechanistic insights |
| Peng & Zhu (2023) [29] | HIV-1 tropism (monocytes vs. T cells) | Machine learning classification on envelope sequences | Identified five key region features distinguishing proviruses | Enhanced understanding of cell-type tropism beyond basic sequence alignment |
| Chen, et al. (2019) [30] | HIV-1 tropism prediction | XGBoost; hidden Markov model | High-accuracy tropism prediction | Faster, more interpretable prediction than lab-based tropism assays |
| Roche, et al. (2024) [31] | HIV self-test image interpretation | Computer vision AI | Detected four infections missed by human readers, showing higher sensitivity | Surpassed human interpretation, improving reliability of field diagnostics |
| Turbé, et al. (2021) [32] | Rapid HIV field test analysis | Deep learning on test images | Sensitivity 97.8%, outperforming human interpretation | Improved detection of faint lines, reducing false negatives |
| Benitez, et al. (2020) [33] | ART adherence monitoring | Super learner analysis on EAM data | Enhanced prediction of viral load under constrained optimization | More accurate adherence forecasting versus non-differentiated care |
| Balzer, et al. (2020) [34] | HIV acquisition risk | ML-based risk scoring | Improved sensitivity for identifying high-risk individuals | More effective risk stratification than traditional scoring systems |
| Bao, et al. (2021) [35] | HIV and STI risk among MSM | ML on demographic & behavioral data | Promising predictive performance for diagnosis | Early triage using non-lab data, reducing testing burden |
| Wei, et al. (2019) [36] | HIV-1/HCV co-infection | Naive Bayes; SVM | Identified >20 potential multi-target inhibitors, including approved drugs | Virtual multi-target screening accelerates drug discovery compared to sequential assays |
| Wang, et al. (2023) [37] | HIV-1/HBV co-infection | Graph neural network-based ensemble | Discovered six novel compounds targeting both viruses | Demonstrated accurate co-infection virtual screening beyond single-target approaches |
| Onywera, et al. (2020) [38] | HIV/HPV co-infection microbiota | LEfSe analysis on microbiota data | Found increased penile microbiota diversity in co-infected men | Uncovered microbial patterns beyond conventional microbiology methods |
| Namalinzi, et al. (2024) [39] | Cervical cancer risk in women with HIV | Random Forest | Identified key predictors (disease stage, viral load, etc.) | Improved early detection and reduced costs versus standard screening protocols |
| Arrigoni, et al. (2023) [40] | HIV-1 protease inhibitor discovery | AI-aided virtual screening | Novel ligand discovered outside known inhibitor classes validated by docking | Expanded chemical space faster than traditional high-throughput screening |
| Leidner, et al. (2019) [41] | HIV-1 protease ligand potency | Gradient boosting with interaction fingerprints | High accuracy in binding affinity prediction; key interaction features identified | Mechanistic insights into potency beyond conventional QSAR |
| Kutsal, et al. (2024) [42] | HIV candidate compound identification | LSTM; VAE | Accelerated identification of candidate compounds validated by simulations | Cost-effective pipeline compared to manual lead optimization |
| Pham, et al. (2024) [43] | ART drug–drug interaction prediction | Deep-ARV with undersampling & ensemble learning | Prediction of four DDI severity categories | Early identification of high-risk interactions improves safety screening |
| Rawi, et al. (2019) [44] | Resistance to broadly neutralizing antibodies | Gradient boosting machines | Accurate prediction for 33 epitope features identified | Streamlined antibody selection and escape monitoring versus in vitro assays |
| Steiner, et al. (2020) [45] | HIV drug resistance across antiretrovirals | MLP; RNN; CNN | Enhanced resistance prediction for 18 drugs | Combined architectures improve interpretability and accuracy over single-method models |
| Cai, et al. (2021) [46] | HIV drug resistance linked to specific mutations | Random Forest; SVM with various kernels | Impact of 21 mutated residues on resistance elucidated | Insight into mutation effects beyond traditional comparative sequence analysis |
| Blassel, et al. (2021) [47] | HIV RT drug resistance landscape | Machine learning on 55,000 RT sequences | Discovered six novel resistance-associated mutations | Revealed mutation interactions |
| Altamirano-Flores, et al. (2023) [48] | HIV prognosis via protein motifs | MAREV-1 & MAREV-2 undersampling methods | Identified clinically relevant motif mutations | Guided therapeutic strategies beyond standard genetic marker analysis |
| Li, et al. (2024) [49] | HIV mortality risk prediction | Random Survival Forests; SVM meta-analysis | Demonstrated strong ML potential for long-term mortality risk | Improved clinical performance versus standard risk scores |
| Semenova, et al. (2024) [50] | Immunologic signatures of HIV DNA reservoir | Machine learning on immunologic data | Correlated immune cell populations with proviral DNA levels | Provided insights into reservoir dynamics beyond standard virology assays |
| Ogishi & Yotsuyanagi (2018) [51] | HIV-associated neurocognitive disorder | Machine learning on gp120 env gene features | Identified three amino acid positions that have predictive value | Genetic signature identification, surpassing traditional risk factor analysis |
| Tu, et al. (2021) [52] | HIV peripheral neuropathy prediction | Logistic regression + ML techniques | Key predictors (infection duration, peak viral load, age) identified; improved classification | Enhanced early prediction over conventional regression alone |
| Paul, et al. (2020) [53] | Neurocognitive performance in perinatal HIV children | Machine learning classification | Identified children at risk for suboptimal outcomes | Early risk detection, enabling targeted interventions |
| Petersen, et al. (2022) [54] | White matter brain-age gap in persons with HIV | Gaussian process regression on diffusion imaging | +1.5 years brain-age gap per decade with detectable viral load | Quantitative biomarker for accelerated aging versus standard imaging |
| Zhang, et al. (2018) [55] | HIV prognosis and mortality via DNA methylation | ML on methylation data | Smoking-associated methylation signatures predicting mortality | Linked molecular methylation markers to clinical outcomes beyond standard clinical indicators |
| McGowan, et al. (2021) [56] | HIV vaccine antigen identification based on HLA diversity and T cell response | Computational ML tools | Key antigens identified for T cell mediation | Informed vaccine design more precisely than traditional epitope mapping methods |
| Dănăilă & Buiu (2022) [57] | HIV sensitivity to monoclonal antibodies | Deep learning on amino acid sequences | Predicted strain sensitivity to antibodies | Accelerated identification of potent antibody combinations versus wet-lab screening |
| Montesi, et al. (2024) [58] | SARS-CoV-2 mRNA vaccine response in people living with HIV | Random Forest on clinical/demographic data | Predicted humoral response, indicating need for boosters | Enabled tailored booster strategies rather than one-size-fits-all vaccination protocols |
| AdaBoost (Adaptive Boosting), ARIMA (AutoRegressive Integrated Moving Average), ARV (Antiretroviral), AT (Artificial Tree algorithm), AUROC (Area Under the Receiver Operating Characteristic curve), BERT (Bidirectional Encoder Representations from Transformers), BERTweet (BERT for Tweets), BPNN (Back-Propagation Neural Network), CNN (Convolutional Neural Network), CT-BERT (COVID-Twitter BERT), DDI (Drug–Drug Interaction), DL (Deep Learning), EAM (Electronic Adherence Monitoring), EHR (Electronic Health Records), GAN (Generative Adversarial Network), GCNN (Graph Convolutional Neural Network), IAT-BPNN (Improved AT-optimized BPNN), ILI (Influenza-Like Illness), k-NN (k-Nearest Neighbors), LSTM (Long Short-Term Memory), LEfSe (Linear Discriminant Analysis Effect Size), MAPE (Mean Absolute Percentage Error), MAREV (Method for Assessing the Relevance of Each Variable), MLP (Multi-Layer Perceptron), NB (Naïve Bayes), NLP (Natural Language Processing), NN (Neural Networks), OneR (One Rule classifier), PART (Partial decision tree rule learner), RF (Random Forest), RNN (Recurrent Neural Network), SEIR (Susceptible–Exposed–Infectious–Recovered epidemiological model), SHAP (SHapley Additive exPlanations), SIMON (SIMON automated ML system), seq-to-seq (Sequence-to-Sequence model), SMOTE-NC (Synthetic Minority Over-sampling Technique for Nominal and Continuous variables), SVM (Support Vector Machine), VAE (Variational Autoencoders), and XGBoost (eXtreme Gradient Boosting). | ||||
| Disease/Setting (Population) | ML Model | Test-Set AUROC | Key Notes |
|---|---|---|---|
| RSV—outpatient ≤ 24 mo (Japan, 4174 visits) [23] | XGBoost | 0.811 (95% CI 0.784–0.833) | Remote triage from symptom template |
| RSV—in-hospital ≤5 y (USA, 54,413 encounters) [25] | XGBoost | 0.919 (95% CI 0.906–0.932) | Uses vitals + demographics recorded in first 2 h to flag likely positives |
| RSV—primary care (Catalonia, 868 children) [24] | Random Forest + Boosting | 0.81 (sens 0.64; spec 0.77) | Symptom checklist only; SMOTE-NC for class balance |
| SARS-CoV-2—same cohort [24] | Random Forest + Boosting | 0.71 | Best features: absence of wheezing ruled out infection |
| Influenza A/B—same cohort [24] | Random Forest + Boosting | 0.7 | Lower discrimination due to overlapping symptom profile |
| Seasonal Influenza—China, ED/fever clinics (200,135 cases) [15] | XGBoost (Boruta-selected symptoms) | 0.734 (CI 0.710–0.750) | Retrospective 2022–2023; model also yielded new data-driven ILI definition |
| Seasonal Influenza—US & Taiwan, multicenter ED ILI cohort [16] | XGBoost | 0.82 (CI 0.79–0.85) | Prospective 2015–2020; outperformed three classic clinical rules |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livieratos, A.; Kagadis, G.C.; Gogos, C.; Akinosoglou, K. AI Methods Tailored to Influenza, RSV, HIV, and SARS-CoV-2: A Focused Review. Pathogens 2025, 14, 748. https://doi.org/10.3390/pathogens14080748
Livieratos A, Kagadis GC, Gogos C, Akinosoglou K. AI Methods Tailored to Influenza, RSV, HIV, and SARS-CoV-2: A Focused Review. Pathogens. 2025; 14(8):748. https://doi.org/10.3390/pathogens14080748
Chicago/Turabian StyleLivieratos, Achilleas, George C. Kagadis, Charalambos Gogos, and Karolina Akinosoglou. 2025. "AI Methods Tailored to Influenza, RSV, HIV, and SARS-CoV-2: A Focused Review" Pathogens 14, no. 8: 748. https://doi.org/10.3390/pathogens14080748
APA StyleLivieratos, A., Kagadis, G. C., Gogos, C., & Akinosoglou, K. (2025). AI Methods Tailored to Influenza, RSV, HIV, and SARS-CoV-2: A Focused Review. Pathogens, 14(8), 748. https://doi.org/10.3390/pathogens14080748






