Trypanosoma cruzi Growth Is Impaired by Oleoresin and Leaf Hydroalcoholic Extract from Copaifera multijuga in Human Trophoblast and Placental Explants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Cell Culture and Parasite Maintenance
2.3. Human Placenta Explants
2.4. Cell Viability Assay
2.5. Trypomastigote Viability
2.6. T. cruzi Intracellular Proliferation
2.7. Trypomastigote Release Assay
2.8. Invasion and Proliferation of T. cruzi Pretreated with OR and LHE
2.9. Reactive Oxygen Species (ROS) Production
2.10. Morphological Analysis of Trypomastigotes and Amastigotes
2.11. Viability of Human Placenta Explants Treated with OR and LHE
2.12. T. cruzi Proliferation in Human Placenta Explants Treated with OR and LHE
2.13. Cytokine Measurements
2.14. Statistical Analysis
3. Results
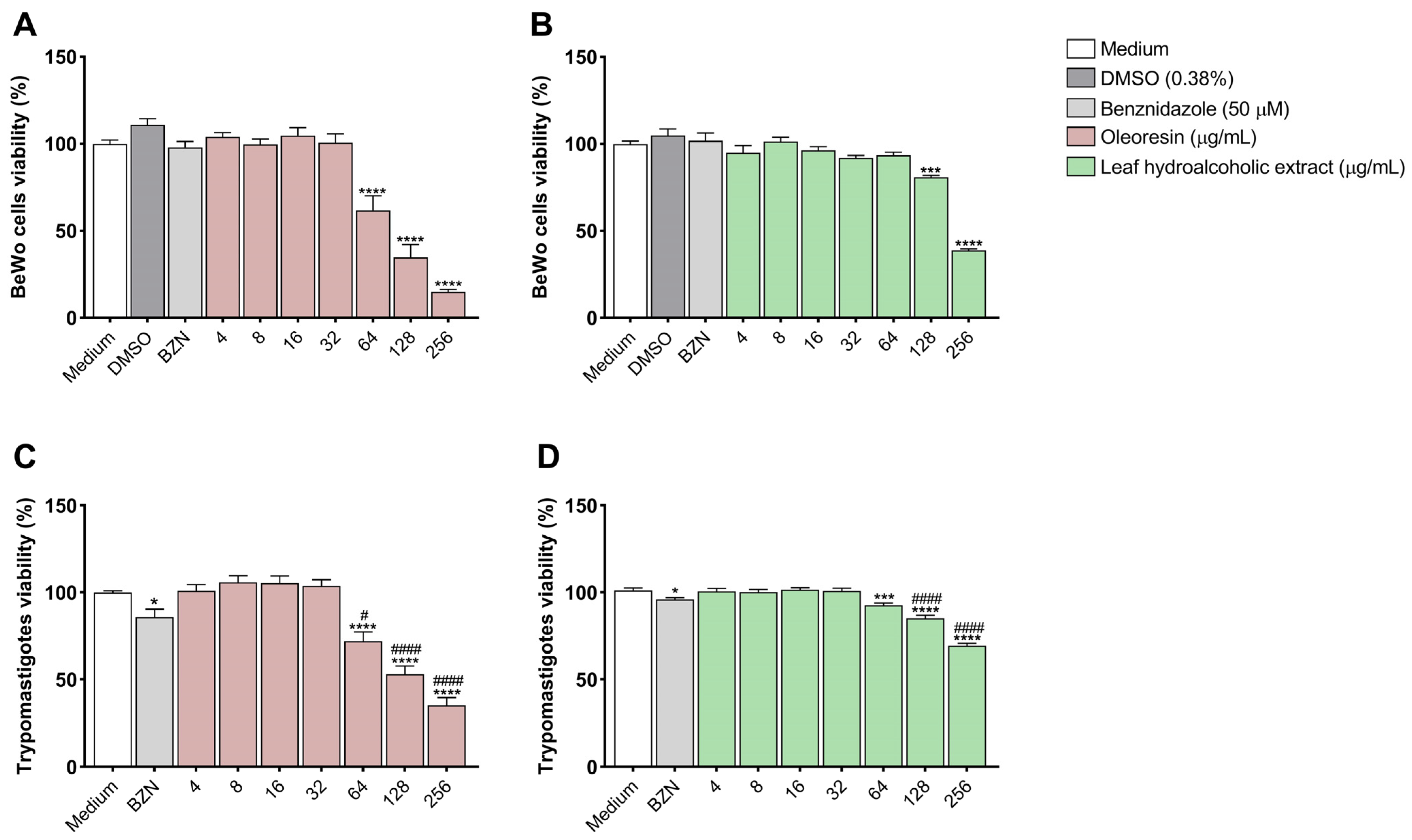
3.1. Oleoresin and Leaf Hydroalcoholic Extract from C. multijuga Are Toxic to BeWo Cells and T. cruzi Trypomastigotes Only at Highest Concentrations
3.2. Oleoresin and Leaf Hydroalcoholic Extract from C. multijuga Decrease T. cruzi Intracellular Proliferation in BeWo Cells
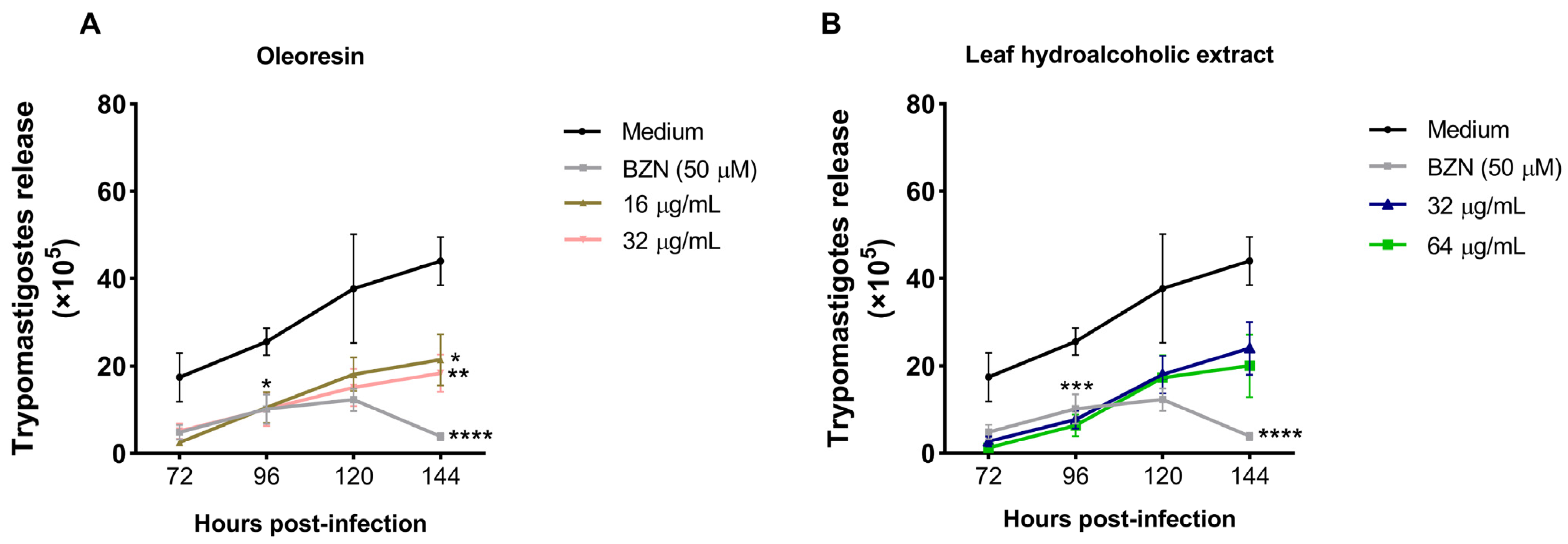
3.3. Oleoresin and Leaf Hydroalcoholic Extract from C. multijuga Decrease the Release of Trypomastigotes into the Supernatant of BeWo Cells
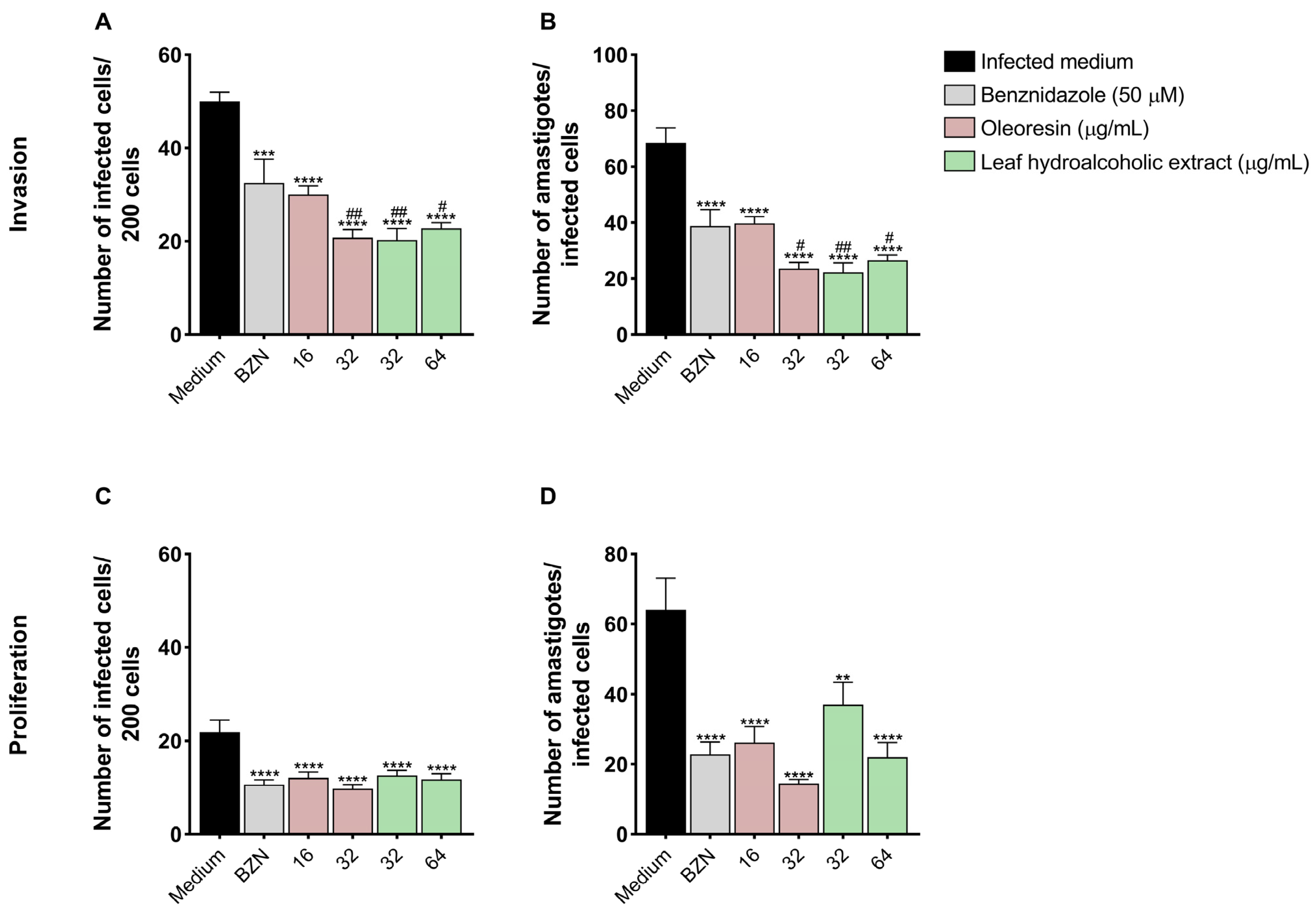
3.4. Pretreatment of T. cruzi Trypomastigotes with Oleoresin and Leaf Hydroalcoholic Extract Compromises Its Ability to Invade and Proliferate in BeWo Cells
3.5. Oleoresin and Leaf Hydroalcoholic Extract from C. multijuga Affect Both Trypomastigote and Amastigote Morphology
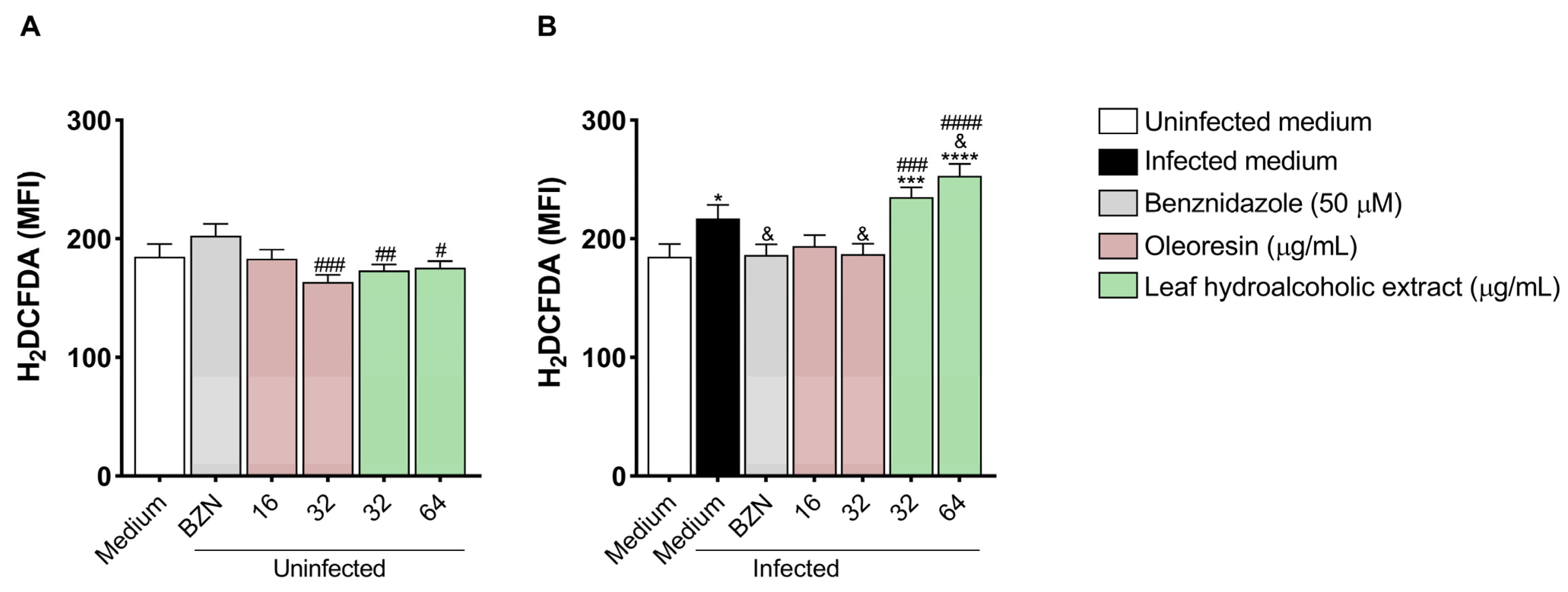
3.6. Oleoresin and Leaf Hydroalcoholic Extract from C. multijuga Modulate Reactive Oxygen Species in BeWo Cells
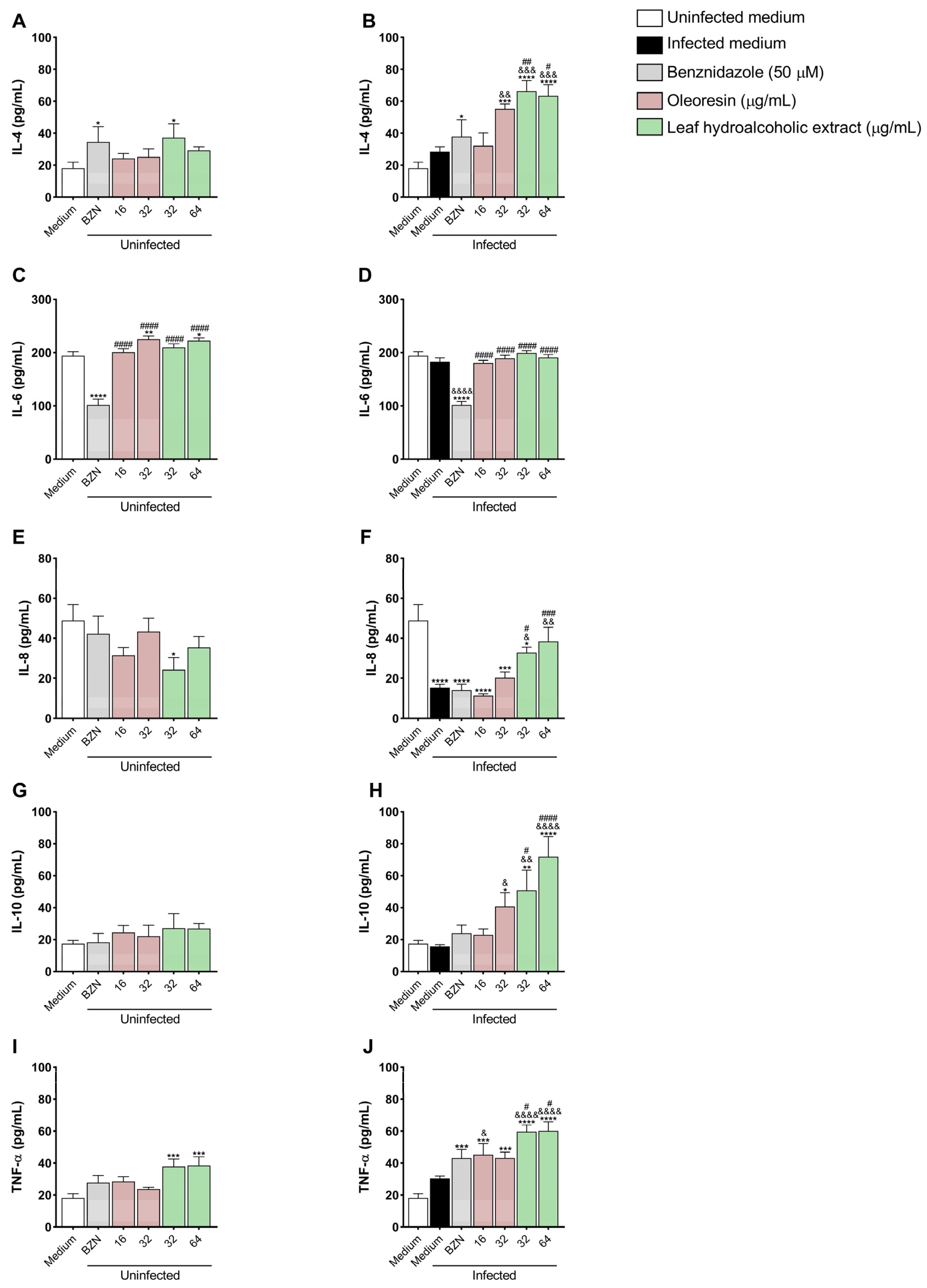
3.7. Oleoresin and Leaf Hydroalcoholic Extract from C. multijuga Increase IL-4, IL-8, IL-10, and TNF-α Production in BeWo Cells
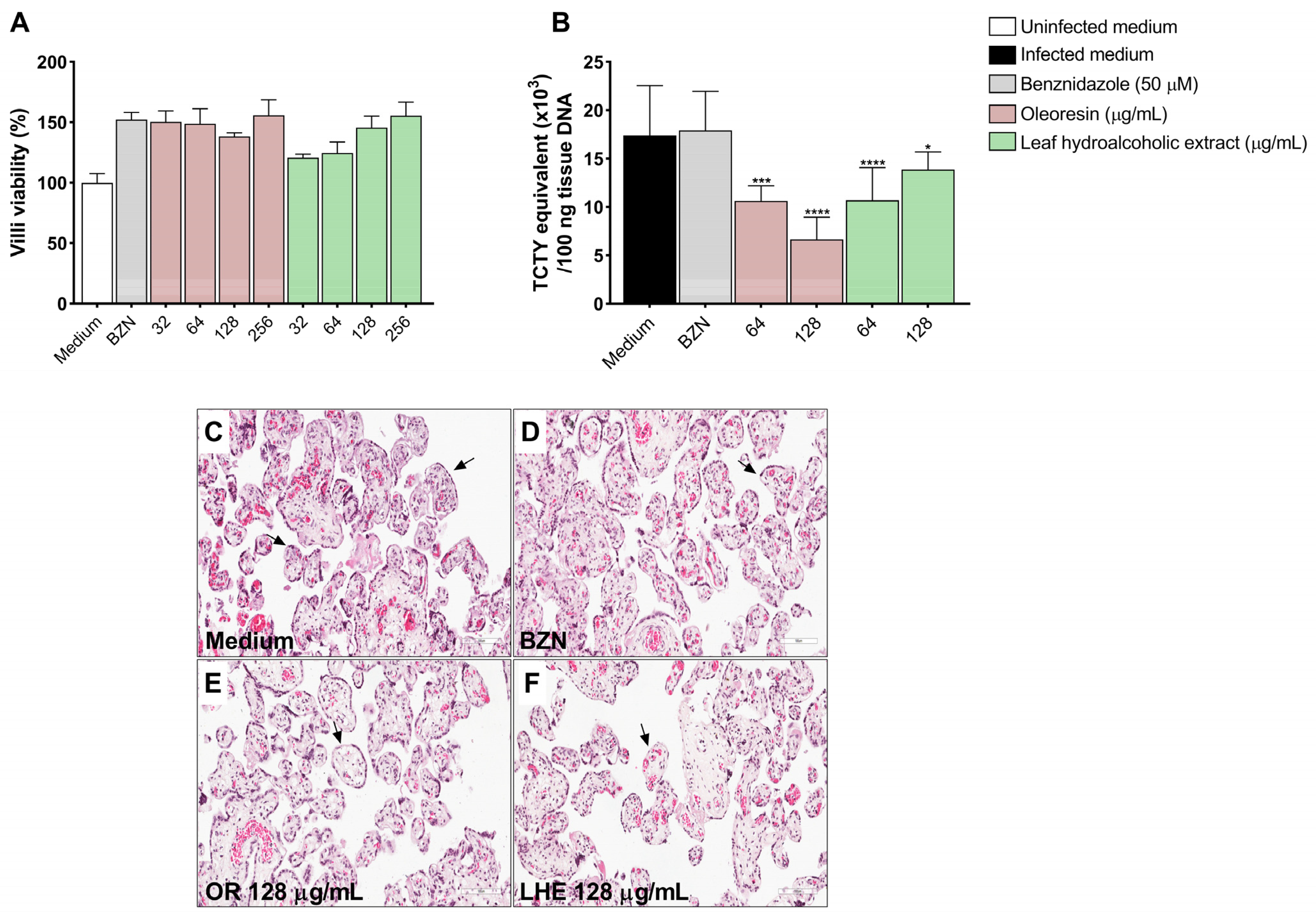
3.8. Oleoresin and Leaf Hydroalcoholic Extract from C. multijuga Reduce T. cruzi Infection in Human Placenta Explants
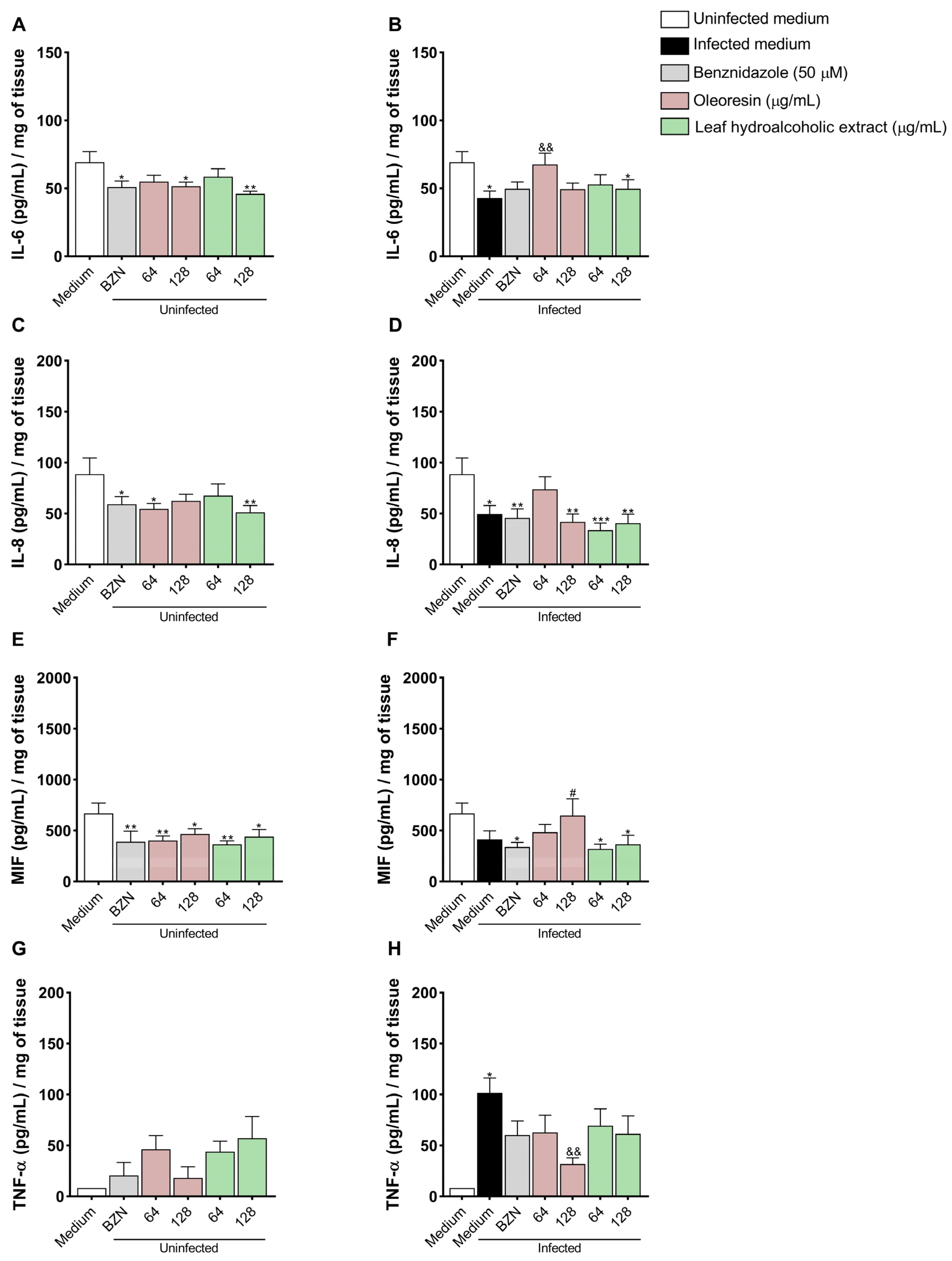
3.9. Oleoresin Increases IL-6 and Decreases TNF-α Production in Human Placenta Explants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Sousa, A.S.; Vermeij, D.; Ramos, A.N., Jr.; Luqueti, A.O. Chagas disease. Lancet 2024, 10422, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Cucunubá, Z.M.; Gutiérrez-Romero, S.A.; Ramírez, J.D.; Velásquez-Ortiz, N.; Ceccarelli, S.; Parra-Henao, G.; Henao-Martínez, A.F.; Rabinovich, J.; Basáñez, M.G.; Nouvellet, P.; et al. The epidemiology of Chagas disease in the Americas. Lancet Reg. Health Am. 2024, 37, 100881. [Google Scholar] [CrossRef]
- Howard, E.J.; Xiong, X.; Carlier, Y.; Sosa-Estani, S.; Buekens, P. Frequency of the congenital transmission of Trypanosoma cruzi: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2014, 1, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Palacios Gil-Antuñano, S.; Gold, S.; Abril, M.; Hernandéz, M.S.; Cancelo-Hidalgo, M.J.; Flores-Chávez, M.; Pelayo-Delgado, I. Mother-to-child Chagas disease transmission: The challenge of detection and prevention in areas without the risk of vectorial transmission. Int. J. Gynaecol. Obstet. 2024, 3, 835–842. [Google Scholar] [CrossRef]
- Messenger, L.A.; Bern, C. Congenital Chagas disease: Current diagnostics, limitations and future perspectives. Curr. Opin. Infect. Dis. 2018, 5, 415–421. [Google Scholar] [CrossRef]
- Antinori, S.; Galimberti, L.; Bianco, R.; Grande, R.; Galli, M.; Corbellino, M. Chagas disease in Europe: A review for the internist in the globalized world. Eur. J. Intern. Med. 2017, 43, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Kemmerling, U.; Osuna, A.; Schijman, A.G.; Truyens, C. Congenital transmission of Trypanosoma cruzi: A review about the interactions between the parasite, the placenta, the maternal and the fetal/neonatal immune responses. Front. Microbiol. 2019, 10, 1854. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.Á. Chagas disease. Lancet 2010, 9723, 1388–1402. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front. Immunol. 2014, 5, 253. [Google Scholar] [CrossRef]
- Todros, T.; Paulesu, L.; Cardaropoli, S.; Rolfo, A.; Masturzo, B.; Ermini, L.; Romagnoli, R.; Ietta, F. Role of the macrophage migration inhibitory factor in the pathophysiology of pre-eclampsia. Int. J. Mol. Sci. 2021, 4, 1823. [Google Scholar] [CrossRef] [PubMed]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojic-Trbojevic, Z.; Dekanski, D.; Krivokuca, M.J. IL-6 and IL-8: An overview of their roles in healthy and pathological pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Muñoz, L.; Carrillo, I.; Liempi, A.; Gallardo, C.; Galanti, N.; Maya, J.D.; Kemmerling, U. Ex vivo infection of human placental chorionic villi explants with Trypanosoma cruzi and Toxoplasma gondii induces different Toll-like receptor expression and cytokine/chemokine profiles. Am. J. Reprod. Immunol. 2017, 78, e12660. [Google Scholar] [CrossRef]
- Haider, S.; Knöfler, M. Human tumour necrosis factor: Physiological and pathological roles in placenta and endometrium. Placenta 2009, 2, 111–123. [Google Scholar] [CrossRef]
- Hamilton, S.T.; Scott, G.; Naing, Z.; Iwasenko, J.; Hall, B.; Graf, N.; Arbuckle, S.; Craig, M.E.; Rawlinson, W.D. Human cytomegalovirus induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS ONE 2012, 12, e52899. [Google Scholar] [CrossRef]
- Droguett, D.; Carrillo, I.; Castillo, C.; Gómez, F.; Negrete, M.; Liempi, A.; Muñoz, L.; Galanti, N.; Maya, J.D.; Kemmerling, U. Trypanosoma cruzi induces cellular proliferation in the trophoblastic cell line BeWo. Exp. Parasitol. 2017, 173, 9–17. [Google Scholar] [CrossRef]
- Liempi, A.; Castillo, C.; Duaso, J.; Droguett, D.; Sandoval, A.; Barahona, K.; Hernández, A.; Galanti, N.; Maya, J.D.; Kemmerling, U. Trypanosoma cruzi induces trophoblast differentiation: A potential local antiparasitic mechanism of the human placenta? Placenta 2014, 12, 1035–1042. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 10115, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Murcia, L.; Simón, M.; Carrilero, B.; Roig, M.; Segovia, M. Treatment of infected women of childbearing age prevents congenital Trypanosoma cruzi infection by eliminating the parasitemia detected by PCR. J. Infect. Dis. 2017, 9, 1452–1458. [Google Scholar] [CrossRef]
- Abras, A.; Ballart, C.; Fernández-Arévalo, A.; Pinazo, M.J.; Gascón, J.; Muñoz, C.; Gállego, M. Worldwide control and management of Chagas disease in a new era of globalization: A close look at congenital Trypanosoma cruzi infection. Clin. Microbiol. Rev. 2022, 2, e0015221. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.G.; Vigliano, C.; Lococo, B.; Bertocchi, G.; Viotti, R. Prevention of congenital Chagas disease by Benznidazole treatment in reproductive-age women. An observational study. Acta Trop. 2017, 174, 149–152. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Malchiodi, E.; Ioset, J.R.; Bivona, A.; Gollob, K.J.; Dutra, W.O. Challenges and advancements in the development of vaccines and therapies against Chagas disease. Lancet Microbe 2024, 10, 100972. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.M.; Senedese, J.M.; Leandro, L.F.; Castro, P.T.; Pereira, D.E.; Carneiro, L.J.; Ambrósio, S.R.; Bastos, J.K.; Tavares, D.C. Copaifera multijuga oleoresin and its constituent diterpene (−)-copalic acid: Genotoxicity and chemoprevention study. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 819, 26–30. [Google Scholar] [CrossRef]
- Gomes, N.M.; de Rezende, C.M.; Fontes, S.P.; Matheus, M.E.; Pinto, A.C.; Fernandes, P.D. Characterization of the antinociceptive and anti-inflammatory activities of fractions obtained from Copaifera multijuga Hayne. J. Ethnopharmacol. 2010, 1, 177–183. [Google Scholar] [CrossRef]
- Pinheiro, J.G.; Tavares, E.A.; Silva, S.S.; Silva, J.F.; Carvalho, Y.M.B.G.; Ferreira, M.R.A.; Araújo, A.A.S.; Barbosa, E.G.; Pedrosa, M.F.F.; Soares, L.A.L.; et al. Inclusion complexes of Copaiba (Copaifera multijuga Hayne) oleoresin and cyclodextrins: Physicochemical characterization and anti-inflammatory activity. Int. J. Mol. Sci. 2017, 11, 2388. [Google Scholar] [CrossRef]
- Teixeira, S.C.; De Souza, G.; Borges, B.C.; Araújo, T.E.; Rosini, A.M.; Aguila, F.A.; Ambrósio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Silva, M.J.B.; et al. Copaifera spp. oleoresins impair Toxoplasma gondii infection in both human trophoblastic cells and human placental explants. Sci. Rep. 2020, 1, 15158. [Google Scholar] [CrossRef]
- Martínez, A.F.F.; Teixeira, S.C.; de Souza, G.; Rosini, A.M.; Lima Júnior, J.P.; Melo, G.N.; Blandón, K.O.E.; Gomes, A.O.; Ambrósio, S.R.; Veneziani, R.C.S.; et al. Leaf hydroalcoholic extract and oleoresin from Copaifera multijuga control Toxoplasma gondii infection in human trophoblast cells and placental explants from third-trimester pregnancy. Front. Cell. Infect. Microbiol. 2023, 13, 1113896. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Rosini, A.M.; de Souza, G.; Martínez, A.F.; Silva, R.J.; Ambrosio, S.R.; Veneziani, R.C.; Bastos, J.K.; Martins, C.H.; Barbosa, B.F.; et al. Polyalthic acid and oleoresin from Copaifera trapezifolia Hayne reduce Toxoplasma gondii growth in human villous explants, even triggering an anti-inflammatory profile. Exp. Parasitol. 2023, 250, 108534. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; Ramos, A.d.S.; Falcão, D.Q.; Ferreira, J.L.P.; Basso, S.L.; Silva, J.R.A.; Amaral, A.C.F. Development of Nanoemulsions to Enhance the Antileishmanial Activity of Copaifera paupera Oleoresins. Biomed. Res. Int. 2018, 2018, 9781724. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.O.D.; Izumi, E.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Veiga-Júnior, V.F.; Nakamura, C.V. Antileishmanial activity of diterpene acids in copaiba oil. Mem. Inst. Oswaldo Cruz. 2013, 1, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.A.G.; Silva, N.C.; Souza, J.; Oliveira, K.R.M.; Fonseca, A.L.; Baratto, L.C.; Oliveira, E.C.P.; Varotti, F.P.; Moraes, W.P. In vitro and in vivo antimalarial potential of oleoresin obtained from Copaifera reticulata Ducke (Fabaceae) in the Brazilian Amazon rainforest. Phytomedicine 2017, 24, 111–118. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Veiga-Junior, V.F.; Nakamura, C.V. Toxicity of oleoresins from the genus Copaifera in Trypanosoma cruzi: A comparative study. Planta Med. 2013, 11, 952–958. [Google Scholar] [CrossRef]
- Lima Júnior, J.P.; Teixeira, S.C.; de Souza, G.; Faria, G.V.; Almeida, M.P.O.; Franco, P.S.; Luz, L.C.; Paschoalino, M.; Dos Santos, N.C.L.; Oliveira, R.M.; et al. Copaifera spp. oleoresins control Trypanosoma cruzi infection in human trophoblast cells (BeWo) and placental explants. Biomed. Pharmacother. 2024, 179, 117425. [Google Scholar] [CrossRef]
- Pimenta, I.P.; Abrão, F.; da Silva, J.J.M.; Oliveira, L.C.; Rogez, H.L.G.; Ambrósio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Martins, C.H.G. Antibacterial profile of Copaifera multijuga oleoresin and hydroalcoholic extract of leaves against oral pathogens. Curr. Dent. 2019, 1, 53–60. [Google Scholar] [CrossRef]
- Costa, I.N.; Ribeiro, M.; Franco, P.S.; Da Silva, R.J.; Araújo, T.E.; Milián, I.C.B.; Luz, L.C.; Guirelli, P.M.; Nakazato, G.; Mineo, J.R.; et al. Biogenic Silver Nanoparticles Can Control Toxoplasma gondii Infection in Both Human Trophoblast Cells and Villous Explants. Front. Microbiol. 2021, 11, 623947. [Google Scholar] [CrossRef]
- De Souza, G.; Teixeira, S.C.; Martínez, A.F.F.; Silva, R.J.; Luz, L.C.; Lima Júnior, J.P.; Rosini, A.M.; Dos Santos, N.C.L.; Oliveira, R.M.; Paschoalino, M.; et al. Trypanosoma cruzi P21 recombinant protein modulates Toxoplasma gondii infection in different experimental models of the human maternal-fetal interface. Front. Immunol. 2023, 14, 1243480. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.A.; Lagoo-Deenadayalan, S.; Whitworth, N.S.; Brackin, M.N.; Hale, E.; Cowan, B.D. Expression and production of interleukin-10 by human trophoblast: Relationship to pregnancy immunotolerance. Early Pregnancy 1997, 3, 190–198. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 1–2, 55–63. [Google Scholar] [CrossRef]
- Kian, D.; Lancheros, C.A.C.; Assolini, J.P.; Arakawa, N.S.; Veiga-Júnior, V.F.; Nakamura, C.V.; Pinge-Filho, P.; Conchon-Costa, I.; Pavanelli, W.R.; Yamada-Ogatta, S.F.; et al. Trypanocidal activity of copaiba oil and kaurenoic acid does not depend on macrophage killing machinery. Biomed. Pharmacother. 2018, 103, 1294–1301. [Google Scholar] [CrossRef]
- Diaz, C.; Nussenzweig, V.; Gonzalez, A. An improved polymerase chain reaction assay to detect Trypanosoma cruzi in blood. Am. J. Trop. Med. Hyg. 1992, 5, 616–623. [Google Scholar] [CrossRef]
- Morilla, M.J.; Romero, E.L. Nanomedicines against Chagas disease: An update on therapeutics, prophylaxis, and diagnosis. Nanomedicine 2015, 3, 465–481. [Google Scholar] [CrossRef]
- Tarleton, R.L. Effective drug discovery in Chagas disease. Trends Parasitol. 2023, 6, 423–431. [Google Scholar] [CrossRef]
- Campos, M.C.O.; Leon, L.L.; Taylor, M.C.; Kelly, J.M. Benznidazole-resistance in Trypanosoma cruzi: Evidence that distinct mechanisms can act in concert. Mol. Biochem. Parasitol. 2015, 201, 83. [Google Scholar] [CrossRef]
- Lima, D.A.; Gonçalves, L.O.; Reis-Cunha, J.L.; Guimarães, P.A.S.; Ruiz, J.C.; Liarte, D.B.; Murta, S.M.F. Transcriptomic analysis of benznidazole-resistant and susceptible Trypanosoma cruzi populations. Parasit. Vectors 2023, 1, 167. [Google Scholar] [CrossRef] [PubMed]
- Vela, A.; Coral-Almeida, M.; Sereno, D.; Costales, J.A.; Barnabé, C.; Brenière, S.F. In vitro susceptibility of Trypanosoma cruzi discrete typing units (DTUs) to benznidazole: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 3, e0009269. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Veiga, V.F., Jr.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 2012, 7, 2994–3001. [Google Scholar] [CrossRef]
- Lazarin-Bidóia, D.; Garcia, F.P.; Ueda-Nakamura, T.; Silva, S.O.; Nakamura, C.V. Natural compounds based chemotherapeutic against Chagas disease and leishmaniasis: Mitochondrion as a strategic target. Mem. Inst. Oswaldo Cruz. 2022, 117, e220396. [Google Scholar] [CrossRef]
- De Moraes, A.R.D.P.; Tavares, G.D.; Rocha, F.J.S.; De Paula, E.; Giorgio, S. Effects of nanoemulsions prepared with essential oils of copaiba- and andiroba against Leishmania infantum and Leishmania amazonensis infections. Exp. Parasitol. 2018, 187, 12–21. [Google Scholar] [CrossRef]
- Maldonado, E.; Rojas, D.A.; Morales, S.; Miralles, V.; Solari, A. Dual and Opposite Roles of Reactive Oxygen Species (ROS) in Chagas Disease: Beneficial on the Pathogen and Harmful on the Host. Oxid. Med. Cell. Longev. 2020, 2020, 8867701. [Google Scholar] [CrossRef]
- Paiva, C.N.; Medei, E.; Bozza, M.T. ROS and Trypanosoma cruzi: Fuel to infection, poison to the heart. PLoS Pathog. 2018, 4, e1006928. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.; Mejía, J.A.A.; Ribeiro, V.P.; Borges, C.H.G.; Martins, C.H.G.; Veneziani, R.C.S.; Ambrósio, S.R.; Bastos, J.K. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus—A review. Biomed. Pharmacother. 2019, 109, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.M.; Vargas, F.S.; Barbosa, P.C.S.; Neves, J.K.O.; Da Silva, J.A.; Veiga-Júnior, V.F. Chemistry and biological activities of terpenoids from copaiba (Copaifera spp.) oleoresins. Molecules 2012, 4, 3866–3869. [Google Scholar] [CrossRef]
- Veiga-Junior, V.F.; Pinto, A.C. The Copaifera L. genus. Quím Nova 2002, 2, 163–169. [Google Scholar] [CrossRef]
- Trindade, R.D.; da Silva, J.K.; Setzer, W.N. Copaifera of the Neotropics: A Review of the Phytochemistry and Pharmacology. Int. J. Mol. Sci. 2018, 5, 1511. [Google Scholar] [CrossRef]
- Martínez, I.; Rivera-Santiago, L.; Rodríguez-Hernández, K.D.; Galván-Hernández, A.; Rodríguez-Fragoso, L.; Díaz-Peralta, L.; Torres-Martínez, L.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Ortega-Blake, I.; et al. A promising amphotericin B derivative induces morphological alterations, mitochondrial damage, and oxidative stress in vitro and prevents mice from death produced by a virulent strain of Trypanosoma cruzi. Microorganisms 2024, 6, 1064. [Google Scholar] [CrossRef]
- Kirkinezos, I.G.; Moraes, C.T. Reactive oxygen species and mitochondrial diseases. Semin. Cell Dev. Biol. 2001, 6, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Lazarin-Bidóia, D.; Desoti, V.C.; Ueda-Nakamura, T.; Dias Filho, B.P.; Nakamura, C.V.; Silva, S.O. Further evidence of the trypanocidal action of eupomatenoid-5: Confirmation of involvement of reactive oxygen species and mitochondria owing to a reduction in trypanothione reductase activity. Free Radic. Biol. Med. 2013, 60, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.B.; Conti, B.J.; Andrade, B.F.M.T.; Silva, J.J.M.; Rogez, H.L.G.; Crevelin, E.J.; Moraes, L.A.B.; Veneziani, R.; Ambrósio, S.R.; Bastos, J.K.; et al. Immunomodulatory action of Copaifera spp oleoresins on cytokine production by human monocytes. Biomed. Pharmacother. 2015, 70, 12–18. [Google Scholar] [CrossRef]
- Torrico, M.C.; Solano, M.; Guzmán, J.M.; Parrado, R.; Suarez, E.; Alonzo-Vega, C.; Truyens, C.; Carlier, Y.; Torrico, F. Estimation of the parasitemia in Trypanosoma cruzi human infection: High parasitemias are associated with severe and fatal congenital Chagas disease. Rev. Soc. Bras. Med. Trop. 2005, 38, 58–61. [Google Scholar]
- Benizio, E.; Moreira-Espinoza, M.J.; Triquell, M.F.; Mezzano, L.; Díaz-Luján, C.M.; Fretes, R.E. Pro-inflammatory cytokines are modified during the multiplication of Trypanosoma cruzi within the placental chorionic villi and are associated with the level of infection via the signaling pathway NF-κB. Am. J. Reprod. Immunol. 2023, 4, e13777. [Google Scholar] [CrossRef] [PubMed]
- Liempi, A.; Castillo, C.; Carrillo, I.; Muñoz, L.; Droguett, D.; Galanti, N.; Maya, J.D.; Kemmerling, U. A local innate immune response against Trypanosoma cruzi in the human placenta: The epithelial turnover of the trophoblast. Microb. Pathog. 2016, 99, 123–129. [Google Scholar] [CrossRef]
- Rojo, G.; Castillo, C.; Duaso, J.; Liempi, A.; Droguett, D.; Galanti, N.; Maya, J.D.; López-Muñoz, R.; Kemmerling, U. Toxic and therapeutic effects of Nifurtimox and Benznidazole on Trypanosoma cruzi ex vivo infection of human placental chorionic villi explants. Acta Trop. 2014, 132, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.G.; Gómez-Hernández, C.; da Silva, M.V.; Rezende-Oliveira, K.; Ferreira, P.T.M.; Oliveira, A.C.M.; Desidério, C.S.; Helmo, F.R.; Carvalho-Costa, T.M.; Dos Santos, I.K.P.; et al. Congenital transmission of Mexican strains of Trypanosoma cruzi TcIa: Interaction between parasite and human placental explants. Parasitology 2021, 3, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Romanowska-Próchnicka, K.; Felis-Giemza, A.; Olesińska, M.; Wojdasiewicz, P.; Paradowska-Gorycka, A.; Szukiewicz, D. The role of TNF-α and anti-TNF-α agents during preconception, pregnancy, and breastfeeding. Int. J. Mol. Sci. 2021, 6, 2922. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, G.d.; Teixeira, C.P.; Lima Júnior, J.P.d.; Almeida, M.P.O.; Paschoalino, M.; Luz, L.C.; dos Santos, N.C.L.; de Oliveira, R.M.; Damasceno, I.S.; Barbosa, M.C.; et al. Trypanosoma cruzi Growth Is Impaired by Oleoresin and Leaf Hydroalcoholic Extract from Copaifera multijuga in Human Trophoblast and Placental Explants. Pathogens 2025, 14, 736. https://doi.org/10.3390/pathogens14080736
Souza Gd, Teixeira CP, Lima Júnior JPd, Almeida MPO, Paschoalino M, Luz LC, dos Santos NCL, de Oliveira RM, Damasceno IS, Barbosa MC, et al. Trypanosoma cruzi Growth Is Impaired by Oleoresin and Leaf Hydroalcoholic Extract from Copaifera multijuga in Human Trophoblast and Placental Explants. Pathogens. 2025; 14(8):736. https://doi.org/10.3390/pathogens14080736
Chicago/Turabian StyleSouza, Guilherme de, Clara Peleteiro Teixeira, Joed Pires de Lima Júnior, Marcos Paulo Oliveira Almeida, Marina Paschoalino, Luana Carvalho Luz, Natália Carine Lima dos Santos, Rafael Martins de Oliveira, Izadora Santos Damasceno, Matheus Carvalho Barbosa, and et al. 2025. "Trypanosoma cruzi Growth Is Impaired by Oleoresin and Leaf Hydroalcoholic Extract from Copaifera multijuga in Human Trophoblast and Placental Explants" Pathogens 14, no. 8: 736. https://doi.org/10.3390/pathogens14080736
APA StyleSouza, G. d., Teixeira, C. P., Lima Júnior, J. P. d., Almeida, M. P. O., Paschoalino, M., Luz, L. C., dos Santos, N. C. L., de Oliveira, R. M., Damasceno, I. S., Barbosa, M. C., Faria, G. V., Ambrosio, M. A. L. V., Veneziani, R. C. S., Bastos, J. K., Gomes, A. O., Alves, R. N., Martins, C. H. G., Teixeira, S. C., Ferro, E. A. V., & Barbosa, B. F. (2025). Trypanosoma cruzi Growth Is Impaired by Oleoresin and Leaf Hydroalcoholic Extract from Copaifera multijuga in Human Trophoblast and Placental Explants. Pathogens, 14(8), 736. https://doi.org/10.3390/pathogens14080736