Abstract
Blastocystis, a common intestinal protozoan in humans, is associated with gastrointestinal disorders, irritable bowel syndrome, urticaria, and colorectal cancer. Its genetic diversity and potential for treatment resistance make it a focus of ongoing research. This study evaluated the in vitro antiprotozoal activity of a postbiotic derived from Pediococcus acidilactici as a natural alternative treatment. P. acidilactici cultures were grown in MRS broth under anaerobic conditions, and the postbiotic was collected and characterized for pH, yield, organic acid composition, and phenolic compound content. Human isolates of Blastocystis subtypes ST1 and ST3 were cultured in Jones’ medium and exposed to varying postbiotic concentrations for 72 h. Viability was assessed microscopically. The cytotoxic effect of the postbiotic-derived P. acidilactici was evaluated by investigating its impact on the viability of HT-29 cells using the Cell Counting Kit 8. The postbiotic showed a 7% yield and a pH of 4.52 ± 0.11. It contained seven different organic acids, predominantly lactic acid, and eleven phenolic compounds, with naringin as the most abundant. At 4.38 mg/mL, the postbiotic achieved over 94% inhibition and 100% inhibition at 8.75 mg/mL and above. A pH analysis confirmed that the inhibition was independent of the culture medium acidity. Cell viability was not affected at the postbiotic concentration showing 100% antiprotozoal activity (8.75 mg/mL). These findings suggest that the P. acidilactici postbiotic is effective on a mixed culture of ST1 and ST3 subtypes and holds promise as a safe, natural antiprotozoal agent. Further in vivo studies are needed to confirm this.
1. Introduction
Blastocystis is an anaerobic, unicellular, and enteric protozoan commonly found in humans. Its life cycle is not fully understood, and its transmission is known to occur via the fecal–oral route through cysts. Given the significant role of contaminated water in its transmission, the World Health Organization (WHO) has classified Blastocystis as a waterborne zoonoses [1]. Its prevalence in humans is estimated to be approximately 10% in developed countries and 50–60% in developing countries [2]. Molecular analysis of the small subunit ribosomal rRNA (SSU-rRNA) gene has identified 44 different subtypes (STs) in humans and animals [3]. Among these, ST1 to ST4 are the most prevalent in humans, accounting for approximately 90% of infections [4]. ST3 is the most frequently reported subtype, followed by ST1; however, the subtype distribution varies across populations and geographic regions [1].
Despite the numerous studies on Blastocystis, its pathogenicity remains one of the most debated issues. Its impact on human health is highly variable, with reports characterizing it as a pathogen, a commensal, and even a beneficial organism [5]. Although many studies have reported associations between Blastocystis and conditions such as gastrointestinal disorders, irritable bowel syndrome [6], urticaria [7], and colorectal cancer [2], its pathogenicity remains controversial. In addition to studies suggesting that its pathogenicity may be influenced by the host’s intestinal microbiota [8], evidence also indicates a potential association with specific Blastocystis subtypes [9].
Another area of controversy in Blastocystis infections is patient management. It is generally accepted that asymptomatic individuals infected with Blastocystis do not require medical treatment. However, emerging evidence supporting the pathogenic potential of Blastocystis underscores the need for treatment in certain cases [10]. For instance, in a reported case of a blastocystosis in a six-year-old child presenting with Stevens–Johnson syndrome, diarrhea, and abdominal pain, clinical symptoms, including skin lesions resolved following antiparasitic treatment [11]. Similarly, in a case report of Blastocystis-associated acute appendicular peritonitis in a nine-year-old child, it was noted that the patient’s C-reactive protein (CRP) levels did not improve without treatment for Blastocystis [12]. These findings underscore the importance of initiating Blastocystis-directed treatment in symptomatic cases where the parasite is identified as the sole infectious agent [13].
Although metronidazole is the first-line treatment for Blastocystis infections, studies have shown that it is not 100% effective, with some cases of blastocystosis persisting despite treatment [14]. On the other hand, alternative natural compounds for common parasitic infections, such as blastocystosis, have emerged as a promising treatment strategy in recent years, driven by the exorbitant production costs and potential adverse effects associated with conventional chemical therapies [10]. One such natural compound is postbiotics. Recent studies have introduced the concept of postbiotics by demonstrating that microorganisms can provide benefits even in their non-viable form [15]. Postbiotics are defined as secreted components and metabolites produced by probiotics that exert biological effects and mediate interactions between symbiotic bacteria and the host [16]. These metabolites contribute to the functional and therapeutic properties traditionally attributed to probiotics. Postbiotics contain a range of macro and micromolecules, including inactivated microbial cells; cell fractions; and metabolites such as organic acids, bacteriocins, and enzymes [15,17,18]. Some postbiotics have also been reported to exhibit antiparasitic effects [19,20].
The aim of this study was to evaluate the in vitro antiprotozoal activity of a postbiotic derived from Pediococcus acidilactici on Blastocystis and to investigate its potential as an alternative natural treatment agent.
2. Materials and Methods
This study was conducted between March and April 2025 at the Parasitology Laboratory of Van Yüzüncü Yıl University Faculty of Medicine. The study was approved by the Van Yüzüncü Yıl University Non-Interventional Clinical Research Ethics Committee (date: 28 February 2025; decision no: 2025/02-29).
2.1. Postbiotic Production Process
P. acidilactici (Lactoferm B-LC-78) culture was obtained from Chr. Hansen Laboratories (Copenhagen, Denmark). The strain was inoculated into De Man, Rogosa and Sharpe (MRS) broth (52.25 mg/mL) (Condalab, Madrid, Spain) and incubated under anaerobic conditions at 37 °C for 48 h. Following incubation, the culture was centrifuged at 4200× g for 10 min at 4 °C, and the supernatant was filtered using 0.45 µm filters (MF-Millipore, Merck KGaA, Darmstadt, Germany) [18]. The pH values were measured at 25 °C using a digital pH meter (EDT. GP 353) to assess the acidity of the postbiotic solution [21]. Subsequently, the freshly obtained postbiotic was lyophilized in a vacuum lyophilizer (Teknosem TRS-2-2, İstanbul, Türkiye) at −80 °C for 24 h. The total yield was calculated based on the difference between the initial and final weights of the sample [21].
2.2. Establishment of Experimental Groups
Three experimental groups were established as follows:
- Metronidazole group: Metronidazole (Nidazol, I.E. Ulagay Pharmaceutical Industry, İstanbul, Türkiye) was used as the reference drug. A stock solution was prepared by dissolving 0.8 mg of metronidazole in 1 mL of distilled water to achieve a final concentration of 0.05 mg/mL in the culture medium.
- Control group: Sterile distilled water was used as a negative control.
- MRS broth group: MRS broth was dissolved in distilled water to prepare a culture medium at a final concentration of 210 mg/mL. The MRS broth medium (210 mg/mL) without added bacteria was centrifuged at 4200× g for 10 min at 4 °C, and the supernatant was used by filtering using 0.45 µm filters. Subsequently, serial dilutions were performed with sterile distilled water to obtain final concentrations of 140, 70, 35, 17.5, and 8.75 mg/mL.
- P. acidilactici group: The fresh P. acidilactici postbiotic obtained after lyophilization was dissolved in sterile distilled water to prepare a stock solution at a concentration of 210 mg/mL. Subsequently, serial dilutions were performed with sterile distilled water to obtain final concentrations of 140, 70, 35, 17.5, and 8.75 mg/mL.
2.3. The Isolation of a Blastocystis Isolate
A Blastocystis isolate was obtained from a stool sample collected from a 58-year-old male patient who presented with diarrhea and abdominal pain at Van Yüzüncü Yıl University Dursun Odabaş Medical Center and tested positive for Blastocystis. A small portion of the stool (approximately the size of a grain of rice) was inoculated into Jones’ medium supplemented with fetal calf serum (FCS) (Capricorn, Ebsdorfergrund, Germany), 100 UI/mL of penicillin, and 100 μg/mL of streptomycin in 1.5 mL Eppendorf tubes supplemented and incubated at 37 °C [4,22]. After 72 h, vacuolar and granular forms of Blastocystis observed under a light microscope were washed with phosphate-buffered saline, and isolates were collected. Blastocystis cells were counted under a light microscope with a 40-times objective using a Thoma slide.
2.4. Antiprotozoal Activity of the Postbiotic on Blastocystis
Jones’ medium supplemented with FCS was dispensed into 1.5 mL Eppendorf tubes and inoculated with Blastocystis at a concentration of 5 × 104 cells/mL. Then, 100 µL of the prepared solutions for each experimental group were added to the cultures, resulting in a final volume of 1600 µL per tube. Postbiotic, medium, and metronidazole concentrations were calculated based on their final concentrations following inoculation. After inoculation, culture tubes were incubated at 37 °C. Viable Blastocystis cells were counted at 24, 48, and 72 h after inoculation. Vital staining with 0.1% eosin and a hemocytometer (Thoma slide) along with a light microscope were used to evaluate antiprotozoal activity. For counting, the culture tubes were gently inverted, and 10 µL of the medium was mixed with 10 µL of the 0.1% eosin solution [22,23]. The preparations were examined under a light microscope using a Thoma slide with a 40-times objective, and the Blastocystis cells were counted. Blastocystis forms that stained orange were considered non-viable, while those that did not stain were considered viable (Figure 1). All processes were triplicated.
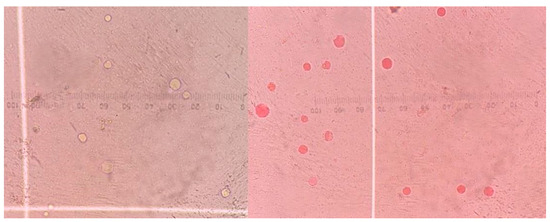
Figure 1.
Thoma slide (400×) image showing viable (unstained) and non-viable (orange-stained) Blastocystis forms.
The percentage reduction of Blastocystis was calculated using the growth inhibition formula:
where A represents the mean number of viable cells in the control group, and B represents the mean number of viable cells in the treated tubes [24].
(A − B/A) × 100,
2.5. pH Measurements of the Culture Media
To assess whether Blastocystis inhibition was attributable to pH changes, the effect of the postbiotic on the pH of the culture medium was evaluated. pH values were measured for both the control group and the postbiotic-supplemented culture medium using a digital pH meter (EDT. GP 353), following the guidelines of the Association of Official Analytical Chemists (AOAC, 1990). Measurements were performed at room temperature by immersing the probe electrode directly into the culture medium. Before each measurement, a two-point calibration was performed using buffer solutions with pH values of 4.01 and 7.00.
2.6. Determination of Blastocystis Subtypes
After 72 h of incubation, 200 µL of a culture medium containing Blastocystis was collected for DNA extraction. DNA was isolated using a stool DNA isolation kit (Norgen Biotek Corp., Thorold, ON, Canada), following the manufacturer’s instructions. To identify Blastocystis subtypes ST1 to ST7, seven subtype-specific primer pairs targeting the SSU rRNA gene were used (Table 1) [25]. PCR reactions were prepared in a total volume of 25 µL, consisting of 5 µL of the Tag 5× Master Mix (containing 12.5 mM of MgCl2), 0.2 µM of each primer, and 2 µL of a template DNA. Amplification was performed using the Applied Biosystems SimpliAmp Thermal Cycler (Applied Biosystems, Foster City, CA, USA). The cycling conditions included 35 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s, and extension at 72 °C for 60 s. In addition to the PCR cycling protocol, an initial denaturation step at 94 °C for 4 min was performed prior to the first cycle, followed by a final extension at 72 °C for 10 min after the last cycle. To visualize the results, 15 µL of the amplified PCR products were subjected to gel electrophoresis and imaged using the UVP Gel documentation system.

Table 1.
Primers used in the identification of Blastocystis subtypes ST1–ST7.
2.7. Postbiotic Characterization
2.7.1. Analysis of the Phenolic Compounds
Quantitative analysis of the phenolic compounds was performed using a high-performance liquid chromatography (HPLC) system equipped with a diode array detector (DAD) (Agilent Technologies, Santa Clara, CA, USA) [26]. The mobile phase consisted of two components: 83% phase A (purified water with 0.1% phosphoric acid) and 17% phase C (100% acetonitrile). The flow rate was maintained at 0.8 mL/min, with the column temperature set to 30 °C and an injection volume of 10 µL. Detection was performed using DAD, with analytes monitored at 300/200 nm and reference wavelengths set to 500/100 nm. The Phenolic Mix20 solution was used as the standard mixture, and quantitation was performed by comparing the peak areas of the identified phenolic compounds against calibration curves. The concentrations are expressed in mg/L based on the derived data.
2.7.2. Analysis of the Organic Acid Content
The organic acid content of the postbiotic sample was determined using HPLC [27]. Separation was performed on a Hi-Plex H-type column (300 × 7.7 mm; PL1170-6830) at a constant temperature of 50 °C. The mobile phase consisted of 0.02 N sulfuric acid in an aqueous solution (100% water). Analyses were conducted under isocratic conditions with a flow rate of 0.6 mL/min and an injection volume of 10 µL. Detection was performed using a DAD set at a wavelength of 210 nm, with a reference wavelength of 400 nm. The resulting chromatographic data were manually integrated and evaluated. An organic acid Mix12 solution was used as the standard mixture, and quantification was performed by comparing the peak areas of the identified organic acids with their respective calibration curves.
2.8. Cell Viability Assay
To evaluate the cytotoxic effect of the postbiotic derived-P. acidilactici, its impact on the viability of HT-29 cells was investigated. Cell viability was measured using the Cell Counting Kit 8 (CCK-8) (Abbkine, Atlanta, Georgia, USA, Cat. No. KTA1020). HT-29 cells were seeded into 96-well plates at a density of 1 × 105 cells/well. Absorbance at 450 nm was measured 72 h after treatment with postbiotics (0.55 mg/mL–52.5 mg/mL) using a microplate reader (Infinite PRO 200; Tecan Austria GmbH, Grodig, Austria). The experiment was repeated three times. Cell viability is expressed as a percentage relative to the control group (% control).
2.9. Statistical Analysis
All the experiments were conducted in two independent replicates, and the results are expressed as the mean ± standard error of the mean. Descriptive statistics were used to summarize the characterization data of the postbiotic. Analysis of variance (ANOVA) was used to evaluate differences in Blastocystis viability among the treatment groups. Multiple comparisons were performed using Tukey’s test, with statistical significance set at p < 0.05. The pH values of the culture media at different postbiotic concentrations were compared using the Kruskal–Wallis test. All statistical analyses were performed with IBM SPSS Statistics for Windows 21.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Postbiotic Production
The postbiotic was obtained with a yield of 7% and had a pH of 4.52 ± 0.11.
3.2. Antiprotozoal Activity of the Postbiotic on Blastocystis
The P. acidilactici postbiotic exhibited inhibitory effects on the in vitro growth of Blastocystis. The minimum concentration that inhibits 90% of organisms (MIC90) was determined to be 4.38 mg/mL, at which point inhibition of more than 94% was observed. Complete (100%) inhibition was achieved at concentrations of 8.75 and 13.13 mg/mL (Table 2).

Table 2.
Percentage (%) inhibition of Blastocystis by the P. acidilactici postbiotic over time and at varying concentrations.
Evaluation of the effects of the postbiotic concentration and incubation time on Blastocystis inhibition revealed statistically significant differences in the inhibition rates depending on both factors (p < 0.05). At a concentration of 2.19 mg/mL, the inhibition rate was 75.47% at 24 h, increasing to 84.06% at 48 and 87.21% at 48 and 72 h. Similarly, at 1.09 mg/mL, inhibition increased from 55.35% at 24 h to 83.85% at 72 h. The time-dependent increase in inhibition was statistically significant at both concentrations (p < 0.05) (Figure 2). These findings suggest that at lower concentrations, the antiprotozoal efficacy of the postbiotic increases over time, highlighting the importance of the incubation duration.
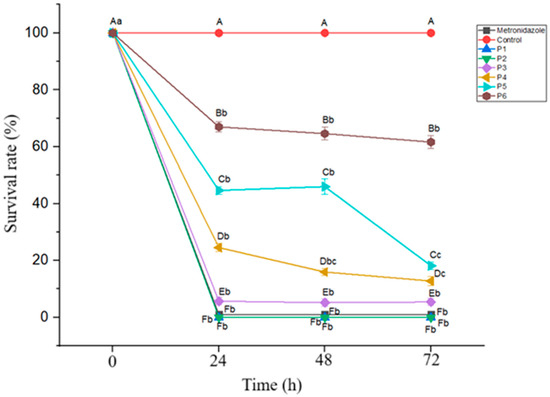
Figure 2.
Blastocystis survival rates at different postbiotic concentrations over a 72 h incubation period. Mean values marked with different lowercase letters (a–c) indicate significant differences across the time points (p < 0.05), while different uppercase letters (A–F) indicate significant differences between the postbiotic concentrations (p < 0.05). (P: postbiotic, P1: 13.13 mg/mL, P2: 8.75 mg/mL, P3: 4.38 mg/mL, P4: 2.19 mg/mL, P5: 1.09 mg/mL, and P6: 0.55 mg/mL).
3.3. pH Measurement of the Culture Medium
The average pH of the culture pH medium in the control group was 7.43 ± 0.11. In the cultures containing postbiotic concentrations ranging from 0.55 to 4.38 mg/mL, the pH values remained above 7 (Table 3).

Table 3.
pH values of the culture medium at different postbiotic concentrations.
3.4. Blastocystis Subtype Identification
A PCR analysis revealed that the patient was co-infected with Blastocystis ST1 and ST3. Accordingly, both subtypes were simultaneously propagated in the culture medium (Figure 3).
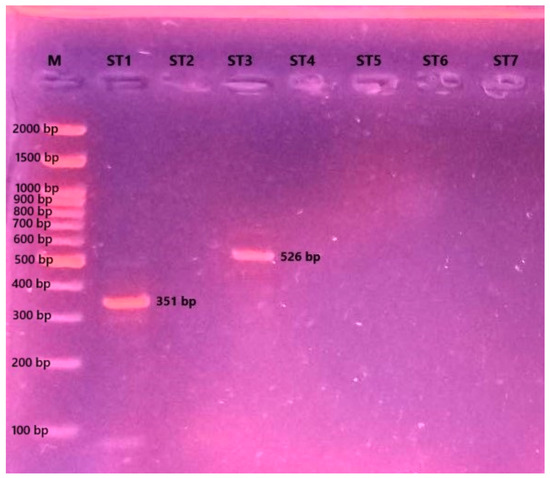
Figure 3.
Agarose gel electrophoresis image showing the Blastocystis subtype-specific PCR products (M: molecular marker (Grisp Brand, Porto, Portugal); ST1-ST7: Blastocystis subtypes).
3.5. Characterization of the Postbiotic
The analytical results indicate that the postbiotic was rich in organic acids, with seven distinct acids identified, of which lactic acid was the most abundant. The types and concentrations of organic acids present in the postbiotic are summarized in Table 4, and the corresponding chromatographic spectrum is presented in Figure 4.

Table 4.
Composition and concentrations of the organic acids identified in the postbiotic sample.
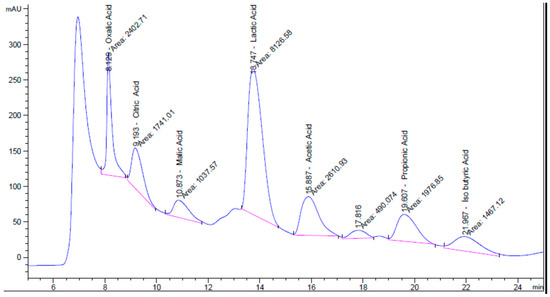
Figure 4.
HPLC chromatogram showing the separation and detection of organic acids in the postbiotic sample.
In the phenolic compound analysis, eleven distinct phenolic compounds were identified in the postbiotic, with naringin, vanillin, chlorogenic acid, and caffeic acid being the most prominent. The compounds detected in the highest concentrations were naringin, vanillin, chlorogenic acid, o-coumaric acid, and caffeic acid, respectively. The types and concentrations of phenolic compounds present in the postbiotic are listed in Table 5, and the corresponding chromatographic spectrum is presented in Figure 5.

Table 5.
Composition and concentrations of phenolic compounds identified in the postbiotic sample.
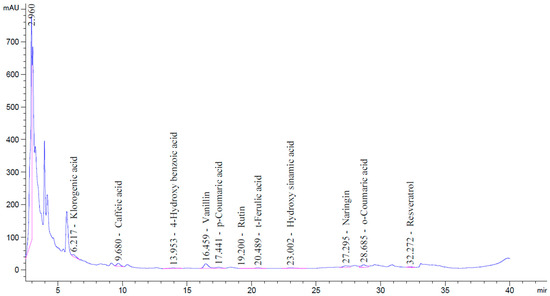
Figure 5.
HPLC chromatogram showing the phenolic compounds identified in the postbiotic sample.
3.6. Cytotoxic Effect of the Postbiotic
No significant decrease in the viability rates of HT-29 cells was observed in postbiotic applications at concentrations of 0.55, 1.09, 2.19, 4.38, and 8.75 mg/mL. However, a 34.76% decrease in cell viability was observed at a concentration of 13.13 mg/mL (p = 0.001). The findings reveal that cell viability was not affected at the concentration showing 100% antiprotozoal activity (8.75 mg/mL) and that the LD50 value determined for HT-29 cells was well above the concentrations showing 100% antiprotozoal activity (Figure 6).
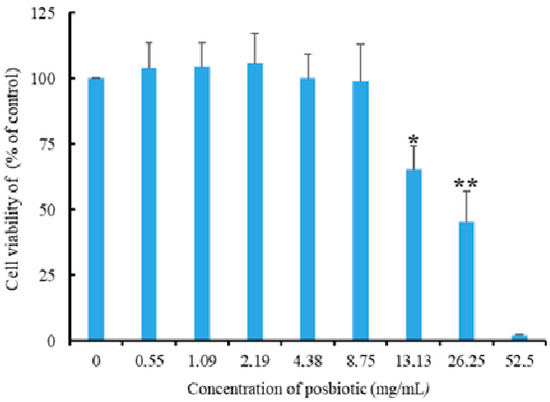
Figure 6.
The effect of Pediococcus acidilactici-derived postbiotics on the viability of HT-29 cells (** and *: statistically significant difference compared to other groups; p = 0.001).
4. Discussion
The efficiency of postbiotic products is closely linked to the production process and the metabolic capacity of the microbial species used. Literature reports indicate that the efficiency of postbiotic production can vary depending on the microbial strain, medium composition, and fermentation conditions. However, yields typically fall within the range of 5–10% [21,28,29]. The 7% yield obtained in the present study is consistent with values reported in the literature. The acidic pH of the postbiotic indicates successful organic acid production and suggests high metabolic activity of the microbial culture [16]. The pH of the postbiotic produced in this study was 4.52 ± 0.11. Additionally, the P. acidilactici postbiotic was rich in organic acids and phenolic compounds. While the overall composition was comparable to previous studies [21,30], minor differences were observed. These variations are likely attributable to differences in the postbiotic production conditions, microbial strains, and analysis methods.
The surface layer protein SlpA, found in certain probiotics, plays a protective role in maintaining the gastrointestinal microbiota and the integrity of the intestinal mucosal barrier [31,32]. The most commonly used probiotics in clinical practice include bacterial strains from the genera Lactobacillus, Bifidobacterium, and Streptococcus, as well as the yeast Saccharomyces. One study reported that Lactobacillus rhamnosus, L. lactis, and Enterococcus faecium showed potential for use as prophylactic agents against Blastocystis colonization or as adjuvants in combination with standard drug therapies [33]. Another study found that L. acidophilus may be used as an adjuvant treatment alongside metronidazole in the management of blastocystosis [34].
Although the use of probiotics in the treatment of blastocystosis has been reported [33,34], their efficacy and safety, particularly in high-risk patients, remain controversial. As a result, postbiotics, which are bioactive metabolites produced by probiotics, have emerged as a promising alternative therapeutic approach [35]. Postbiotics offer several advantages over probiotics, including their safe use in the elderly and immunocompromised individuals without the risk of infection. Additionally, because they do not rely on viability, postbiotics retain their functionality even when administered alongside antibiotics [36]. In general, strains belonging to Lactobacillus, Lactococcus, Enterococcus, Pediococcus, Staphylococcus, Leuconostoc, and Streptococcus species are commonly used for postbiotic production [30].
Pediococcus spp., a member of the family Lactobacillaceae, and particularly P. acidilactici, are widely used in the food industry, animal husbandry, and medicine. Bacteriocins produced by P. acidilactici have been reported to inhibit the growth of pathogenic microorganisms in the host and function as signal-regulating peptides that modulate host health [37]. They have also been reported to play regulatory roles in maintaining intestinal flora balance [38].
Postbiotics have been reported to be effective in the treatment of protozoan infections, such as giardiasis, trypanosomiasis, and leishmaniasis [19]. Cuellar-Guevara et al. [20] demonstrated that postbiotics derived from Lactobacillus spp. inhibited the growth of Entamoeba histolytica protozoan under axenic conditions and induced morphometric changes in the cell membrane of the trophozoites. However, no studies to date have reported the effect of any bacterially derived postbiotic on Blastocystis viability. The current study is the first to demonstrate that postbiotics produced by P. acidilactici can inhibit the viability of Blastocystis. The P. acidilactici postbiotic exceeded the MIC90 threshold at a concentration of 4.38 mg/mL in the culture medium and achieved 100% inhibition of Blastocystis at 8.75 mg/mL. Consistent with previous reports [22], metronidazole at a concentration of 0.05 mg/mL also resulted in complete (100%) inhibition of Blastocystis in the present study. Although the inhibition activity of the postbiotic was observed at higher concentrations compared to metronidazole, which is currently used as the reference drug, the P. acidilactici-derived postbiotic appears promising as a natural therapeutic alternative, particularly given the well-documented side effects of metronidazole [39,40] and the emergence of metronidazole-resistant Blastocystis strains [41].
The growth of Blastocystis in Jones’ medium is strongly influenced by the pH of the culture environment [42]. Haziqah et al. [42] investigated the effect of pH on the viability of Blastocystis isolates from both humans and birds and reported that growth was suppressed at pH values below 3 for human isolates, below 4 for bird isolates, and below 5 in both types of isolates. Therefore, for in vitro studies, it is important to consider how the tested substance influences the pH of the culture medium when evaluating anti-Blastocystis activity. Substances that exert their inhibitory effect by lowering the pH of the culture medium may not be therapeutically effective in vivo, as they are unlikely to significantly alter the pH of the intestinal environment. In the present study, the pH value of the culture medium at the MIC90 concentration was above 7 and was similar to that of the control group. This suggests that the anti-Blastocystis effect of the P. acidilactici postbiotic is attributable to its bioactive compounds, independent of pH alteration.
Some studies have reported that the anti-Blastocystis activity of plant extracts varies depending on the Blastocystis subtype [4]. Accordingly, it is plausible that the effectiveness of postbiotics may also differ among Blastocystis subtypes. In the current study, the postbiotic derived from P. acidilactici was found to be effective against ST1, which is commonly associated with pathogenicity, and ST3, which is the most prevalent subtype in humans. Its observed effectiveness against a mixed culture of subtypes ST1 and ST3 supports the potential of this postbiotic as a therapeutic agent, although subtype-specific responses require further investigation.
The postbiotic produced in this study was found to be rich in organic acids, with seven distinct types identified. Organic acids are known to exert inhibitory effects on the growth of enteropathogenic microorganisms [43,44]. Indeed, organic acids have been widely reported to serve as acidifiers in animal feed, where they help modulate the intestinal microbiota and promote animal health through their antimicrobial activity [45]. Similarly, organic acids have been reported to exert inhibitory effects on Blastocystis infections [43,44]. However, no studies to date have examined the specific effects of the organic acids present in the postbiotic produced in this study on Blastocystis viability. On the other hand, oxalic [46], acetic, citric, lactic [47], and malic [48] acids have all been reported to exhibit antimicrobial activity. Propionic acid is a registered fungicide and bactericide commonly used in hay, grain storage, and drinking water for livestock and poultry [49]. Based on these findings, it is reasonable to suggest that the organic acids present in the postbiotic contribute to its anti-Blastocystis effect.
It should also be considered that the phenolic compounds present in the postbiotic may contribute to its anti-Blastocystis effect. Previous studies have reported that phenolic compounds suppress protozoan viability by disrupting cell membrane permeability [50]. Méabed et al. [51] reported that phenolic compounds in plant extracts exerted significant antiparasitic effects on Blastocystis, supporting the findings of the present study. In the present study, chlorogenic acid was identified as one of the predominant phenolic compounds in the postbiotic, and it has also been reported as a major component in certain plants known to exert anti-Blastocystis effects [51]. Moreover, naringenin, vanillin, o-coumaric acid, caffeic acid, and p-coumaric acid have also been reported to exhibit antiprotozoal activity. Specifically, both naringenin and o-coumaric acid have demonstrated leishmanicidal effects [52,53], vanillin has shown anti-Toxoplasma activity [54], caffeic acid has exhibited anti-malarial properties [55], and p-coumaric acid has exhibited anti-amoebic activity [56]. Based on these findings, it is plausible to suggest that the phenolic profile of the postbiotic contributes to its observed anti-Blastocystis activity.
Although postbiotics derived from P. acidilactici have been found to exhibit antiprotozoal activity, it is important to investigate their potential effects on the proliferation and viability of intestinal epithelial cells in order to evaluate their suitability for use as therapeutic agents. In one study, postbiotics derived from P. acidilactici were reported to increase the levels of short-chain fatty acids (SCFAs), such as acetic acid, propionic acid, and butyric acid in the intestinal tract, thereby supporting gut epithelial function, enhancing mucosal integrity, and maintaining intestinal homeostasis [57]. In this study, it was determined that the 8.75 mg/mL concentration, which was found to exhibit 100% antiprotozoal activity, did not have a negative effect on HT-29 cell viability. These findings suggest that P. acidilactici postbiotics may not only act against pathogens but also positively influence host gut health.
5. Conclusions
This study demonstrated that the postbiotic derived from P. acidilactici had strong inhibitory properties, achieving over 94% inhibition at a concentration of 4.38 mg/mL and complete inhibition at concentrations of 8.75 mg/mL and above. Importantly, these effects were independent of changes in the pH of the culture medium, suggesting that the antimicrobial activity was due to the bioactive components, such as organic acids and phenolic compounds, present in the postbiotic. Given the resistance issues and side effects associated with metronidazole, these findings highlight the potential of P. acidilactici-derived postbiotics as a promising natural alternative for the treatment of Blastocystis infections. However, in vivo studies are essential to validate these findings and explore the postbiotic’s mechanisms of action, bioavailability, and safety profile under physiological conditions.
Author Contributions
Conceptualization, S.A., M.E.A. and E.G.; Methodology, S.A., Y.E.A., M.E.A., Y.Y. and A.E.; Formal analysis, S.A., Y.E.A. and F.B.; Investigation, S.A., Y.E.A., M.E.A., F.B., E.G. and A.E.; Resources, Y.Y.; Data curation, S.A., Y.E.A., F.B. and E.G.; Writing – original draft, S.A., M.E.A., F.B., Y.Y. and A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aydemir, S.; Barlik, F.; Yurekturk, S.; Saygin, M.; Unlu, A.H.; Ekici, A.; Yilmaz, H. Prevalence of Blastocystis infection in humans in Turkiye: A systematic review and meta-analysis. Microb. Pathog. 2024, 195, 106876. [Google Scholar] [CrossRef] [PubMed]
- Sulzyc-Bielicka, V.; Kolodziejczyk, L.; Adamska, M.; Skotarczak, B.; Jaczewska, S.; Safranow, K.; Bielicki, P.; Kładny, J.; Bielicki, D. Colorectal cancer and Blastocystis sp. infection. Parasites Vectors 2021, 14, 200. [Google Scholar] [CrossRef]
- Matovelle, C.; Quilez, J.; Tejedor, M.T.; Beltran, A.; Chueca, P.; Monteagudo, L.V. Subtype distribution of Blastocystis spp. in patients with gastrointestinal symptoms in northern Spain. Microorganisms 2024, 12, 1084. [Google Scholar] [CrossRef]
- Mokhtar, A.B.; Ahmed, S.A.; Eltamany, E.E.; Karanis, P. Anti-Blastocystis activity in vitro of Egyptian herbal extracts (Family: Asteraceae) with emphasis on Artemisia judaica. Int. J. Environ. Res. Public Health 2019, 16, 1555. [Google Scholar] [CrossRef] [PubMed]
- Rudzinska, M.; Sikorska, K. Epidemiology of Blastocystis infection: A review of data from Poland in relation to other reports. Pathogens 2023, 12, 1050. [Google Scholar] [CrossRef]
- Abedi, S.H.; Fazlzadeh, A.; Mollalo, A.; Sartip, B.; Mahjour, S.; Bahadory, S.; Taghipour, A.; Rostami, A. The neglected role of Blastocystis sp. and Giardia lamblia in development of irritable bowel syndrome: A systematic review and meta-analysis. Microb. Pathog. 2022, 162, 105215. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, F.; Babaei, E.; Badirzadeh, A.; Riabi, T.R.; Abdoli, A. Blastocystis, urticaria, and skin disorders: Review of the current evidences. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1027–1042. [Google Scholar] [CrossRef]
- Deng, L.; Wojciech, L.; Gascoigne, N.R.J.; Peng, G.N.; Tan, K.S.W. New insights into the interactions between Blastocystis, the gut microbiota, and host immunity. PLoS Pathog. 2021, 17, e1009253. [Google Scholar] [CrossRef]
- Deng, L.; Lee, J.W.J.; Tan, K.S.W. Infection with pathogenic Blastocystis ST7 is associated with decreased bacterial diversity and altered gut microbiome profiles in diarrheal patients. Parasites Vectors 2022, 15, 312. [Google Scholar] [CrossRef]
- Ahmed, S.A.A.; Schou, C.; Mokhtar, A.B.; Karanis, P.; Gad, S.E.M. Blastocystis species growth inhibition in vitro by plant extracts. Microb. Pathog. 2024, 196, 106970. [Google Scholar] [CrossRef]
- Singh, A.; Priyadarshi, K.; Rai, T.; Banerjee, T. A case report of Blastocystis infection and Stevens—Johnson syndrome. Trop. Biomed. 2019, 36, 987–992. [Google Scholar] [PubMed]
- Montero, J.A.; Álvarez, S.S.; Anaut, M.B.; Medrano, R.L.; Esteras, M.A.R.; Ruiz, M.R.; García, F.M.I. Blastocystis hominis-associated acute appendicular peritonitis in a 9-year-old boy: A case report and a comprehensive review of the literature. Pediatr. Infect. Dis. J. 2024, 43, 327–330. [Google Scholar] [CrossRef]
- Kurt, Ö.; Dogruman Al, F.; Tanyüksel, M. Eradication of Blastocystis in humans: Really necessary for all? Parasitol. Int. 2016, 65, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Cobuccio, L.G.; Laurent, M.; Gardiol, C.; Wampfler, R.; Poppert, S.; Senn, N.; Eperon, G.; Genton, B.; Locatelli, I.; Valliere, S. Should we treat Blastocystis sp.? A double-blind placebo-controlled randomized pilot trial. J. Travel Med. 2023, 30, 143. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; Cruz, A.G.; Pereira, E.; da Costa, W.K.A.; Rocha, R.d.S.; Pedrosa, G.T.d.S.; Rocha, C.d.S.; Alves, J.M.; Alvarenga, V.O.; Sant’aNa, A.S.; et al. Postbiotics: An overview of concepts, inactivation technologies, health effects, and driver trends. Trends Food Sci. Technol. 2023, 138, 199–214. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Incili, G.K.; Karatepe, P.; Akgöl, M.; Kaya, B.; Kanmaz, H.; Hayaloglu, A.A. Characterization of Pediococcus acidilactici postbiotic and impact of postbiotic-fortified chitosan coating on the microbial and chemical quality of chicken breast fillets. Int. J. Biol. Macromol. 2021, 184, 429–437. [Google Scholar] [CrossRef]
- Ramesh, L.; Dharumadurai, D. Antiprotozoan activity of postbiotics. In Postbiotics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 207–210. [Google Scholar]
- Cuellar-Guevara, F.L.; Barron-Gonzalez, M.P.; Menchaca-Arredondo, J.L. Effect of Lactobacillus postbiotics on Entamoeba histolytica trophozoites. Rev. Investig. Clin. 2019, 71, 402–407. [Google Scholar] [CrossRef]
- Incili, G.; Akgöl, M.; Karatepe, P.; Kanmaz, H.; Kaya, B.; Tekin, A.; Hayaloğlu, A.A. Inhibitory effect of bioactive compounds derived from freeze-dried paraprobiotic of Pediococcus acidilactici against food-borne pathogens: In vitro and food model studies. Food Res. Int. 2023, 170, 113045. [Google Scholar] [CrossRef]
- Yıldız, S.; Aydemir, S.; Ekici, A.; Deniz, N.Y.; Yilmaz, H. Inhibitory effect of thymoquinone and capsaicin on Blastocystis grown in vitro. Indian J. Exp. Biol. 2024, 62, 423–428. [Google Scholar]
- El-Sayed, N.M. Evaluation of the in vitro effects of ethanol extracts of Ocimum basilicum and Thymus vulgaris for anti-Blastocystis hominis activity. Egypt J. Med. Sci. 2009, 30, 1229–1243. [Google Scholar]
- Aykur, M.; Karakavuk, E.; Karakavuk, M.; Akıl, M.; Can, H.; Döşkaya, M.; Gürüz, Y.; Dağcı, H. Inhibitory effect of Tunceli garlic (Allium tuncelianum) on Blastocystis subtype 3 grown in vitro. Expert Opin. Orphan Drugs. 2020, 8, 489–496. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Wu, Z.; Kimata, I.; Iseki, M.; Ali, I.K.M.D.; Hossain, M.B.; Zaman, V.; Haque, R.; Takahashi, Y. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol. Res. 2004, 92, 22–29. [Google Scholar] [PubMed]
- Başar, Y.; Yiğit, A.; Tunç, A.K.; Sarıtaş, B.M. Lavandula stoechas extract; synthesis of silver nanoparticles (nature-friendly green synthesis method), characterization, antimicrobial activity and in silico molecular docking study. Curr. Perspect. Med. Aromat. Plants 2023, 7, 24–33. [Google Scholar] [CrossRef]
- Aksoy, A.; Altunatmaz, S.S.; Aksu, F.; Tokatlı Demirok, N.; Yazıcı, K.; Yıkmış, S. Bee bread as a functional product: Phenolic compounds, amino acid, sugar, and organic acid profiles. Foods 2024, 13, 795. [Google Scholar] [CrossRef]
- Ooi, M.F.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Rahim, R.A.; Ariff, A. A refined medium to enhance the antimicrobial activity of postbiotic produced by Lactiplantibacillus plantarum RS5. Sci. Rep. 2021, 11, 7617. [Google Scholar] [CrossRef]
- Amiri, S.; Mokarram, R.R.; Khiabani, M.S.; Bari, M.R.; Alizadeh, M. Optimization of food-grade medium for co-production of bioactive substances by Lactobacillus acidophilus LA-5 for explaining pharmabiotic mechanisms of probiotic. J. Food Sci. Technol. 2021, 58, 1–12. [Google Scholar] [CrossRef]
- Incili, G.K.; Karatepe, P.; Akgol, M.; Gungoren, A.; Koluman, A.; Ilhak, O.I.; Kanmaz, H.; Kaya, B.; Hayaloğlu, A.A. Characterization of lactic acid bacteria postbiotics: Evaluation of in vitro antibacterial effect, microbial and chemical quality on chicken drumsticks. Food Microbiol. 2022, 104, 104001. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, X.; Huang, L.; Shen, R.; Qin, H. Gut microbiota alteration after long-term consumption of probiotics in the elderly. Probiotics Antimicrob. Proteins 2019, 11, 655–666. [Google Scholar] [CrossRef]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef] [PubMed]
- Lepczynska, M.; Dzika, E. The influence of probiotic bacteria and human gut microorganisms causing opportunistic infections on Blastocystis ST3. Gut Pathog. 2019, 11, 6. [Google Scholar] [CrossRef]
- Alkady, S.F.H.; Naggar, H.M.A.E.; Thabet, H.S.; El-Sayed, H.S.; Magdy, M.; Fahmy, I.A.; Abou-Seri, H.M. Assessment of Lactobacillus acidophilus (L. acidophilus) therapeutic and prophylactic role in rats experimentally infected with Blastocystis subtype 3 (ST3). Parasitol. Res. 2025, 124, 11. [Google Scholar] [CrossRef]
- Szajewska, H.; Kolodziej, M.; Gieruszczak-Bialek, D.; Skorka, A.; Ruszczynski, M.; Shamir, R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—A 2019 update. Aliment. Pharmacol. Ther. 2019, 49, 1376–1384. [Google Scholar] [CrossRef]
- Warda, A.K.; Clooney, A.G.; Ryan, F.; Bettio, P.H.d.A.; Di Benedetto, G.; Ross, R.P.; Hill, C.; Ercolini, D. A postbiotic consisting of heat-treated lactobacilli has a bifidogenic effect in pure culture and in human fermented fecal communities. Appl. Environ. Microbiol. 2021, 87, e02459-20. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Saravanakumar, K.; Daliri, E.B.; Kim, J.H.; Lee, J.K.; Jo, H.Y.; Kim, S.H.; Ramakrishnan, S.R.; Madar, I.H.; Wei, S.; et al. Unveiling the potentials of bacteriocin (Pediocin L50) from Pediococcus acidilactici with antagonist spectrum in a Caenorhabditis elegans model. Int. J. Biol. Macromol. 2020, 143, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Yue, Y.; Wang, X.; Zeng, X.; Guo, Q.; Yan, X.; Du, G.; Yuan, Y.; Yue, T. Enrichment and distribution of selenium in Pediococcus acidilactici MRS-7: Impact on its biochemical composition, microstructure, and gastrointestinal survival. J. Agric. Food Chem. 2022, 70, 14877–14885. [Google Scholar] [CrossRef] [PubMed]
- Ayinde, O.; Ross, J.D. The frequency and duration of side-effects associated with the use of oral metronidazole: A prospective study of VITA trial participants. Int. J. STD AIDS 2023, 34, 897–902. [Google Scholar] [CrossRef]
- Ceruelos, A.H.; Romero-Quezada, L.; Ledezma, J.R.; Contreras, L.L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar]
- Rajamanikam, A.; Hooi, H.S.; Kudva, M.; Samudi, C.; Kumar, S. Resistance towards metronidazole in Blastocystis sp.: A pathogenic consequence. PLoS ONE 2019, 14, e0212542. [Google Scholar] [CrossRef]
- Farah Haziqah, M.T.; Chandrawathani, P.; Douadi, B.; Suresh, K.; Wilson, J.J.; Mohd Khalid, M.K.N.; Rajamanikam, A.; Lewis, J.W.; Mohd Zain, S.N. Impact of pH on the viability and morphology of Blastocystis isolates. Trop. Biomed. 2018, 35, 501–510. [Google Scholar] [PubMed]
- Abdel-Hafeez, E.; Ahmed, A.; Abdellatif, M.; Kamal, A.; Toni, M. The efficacy of pomegranate (Punica granatum) peel extract on experimentally infected rats with Blastocystis spp. J. Infect. Dis. Prev. Med. 2016, 4, 131. [Google Scholar] [CrossRef]
- Girish, S.; Kumar, S.; Aminudin, N. Eurycoma longifolia (Tongkat Ali): A possible therapeutic candidate against Blastocystis sp. Parasites Vectors 2015, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In vitro antimicrobial activities of organic acids and their derivatives on several species of Gram-negative and Gram-positive bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef]
- Kwak, A.M.; Lee, I.K.; Lee, S.Y.; Yun, B.S.; Kang, H.W. Oxalic acid from Lentinula edodes culture filtrate: Antimicrobial activity on phytopathogenic bacteria and qualitative and quantitative analyses. Mycobiology 2016, 44, 338–342. [Google Scholar] [CrossRef]
- In, Y.W.; Kim, J.J.; Kim, H.J.; Oh, S.W. Antimicrobial activities of acetic acid, citric acid and lactic acid against Shigella species. J. Food Saf. 2013, 33, 79–85. [Google Scholar] [CrossRef]
- Borah, H.J.; Borah, A.; Yadav, A.; Hazarika, S. Extraction of malic acid from Dillenia indica in organic solvents and its antimicrobial activity. Sep. Sci. Technol. 2023, 58, 314–325. [Google Scholar] [CrossRef]
- Haque, M.; Chowdhury, R.; Islam, K.; Akbar, M. Propionic acid is an alternative to antibiotics in poultry diet. Bangladesh J. Anim. Sci. 2009, 38, 115–122. [Google Scholar] [CrossRef]
- Palomo-Ligas, L.; Estrada-Camacho, J.; Garza-Ontiveros, M.; Vargas-Villanueva, J.R.; Gutiérrez-Gutiérrez, F.; Nery-Flores, S.D.; Montoya, J.A.C.; Ascacio-Valdés, J.; Campos-Muzquiz, L.G.; Rodriguez-Herrera, R. Polyphenolic extract from Punica granatum peel causes cytoskeleton-related damage on Giardia lamblia trophozoites in vitro. PeerJ 2022, 10, e13350. [Google Scholar] [CrossRef]
- Méabed, E.M.; El-Sayed, N.M.; Abou-Sreea, A.I.; Roby, M.H. Chemical analysis of aqueous extracts of Origanum majorana and Foeniculum vulgare and their efficacy on Blastocystis spp. cysts. Phytomedicine 2018, 43, 158–163. [Google Scholar] [CrossRef]
- Saha, S.; Srivastava, R.; Sarma, P.; Bhatt, T.K.; Prakash, A.; Kumar, D. Identification of potential inhibitors of Leishmania donovani sterol 24-C-methyltransferase: In silico and in vitro studies. Mol. Simul. 2023, 49, 1311–1323. [Google Scholar] [CrossRef]
- Monzote, L.; Córdova, W.H.P.; García, M.; Piñón, A.; Setzer, W.N. In vitro and in vivo activities of phenolic compounds against cutaneous leishmaniasis. Rec. Nat. Prod. 2016, 10, 269–276. [Google Scholar]
- Oliveira, C.B.; Meurer, Y.S.; Oliveira, M.G.; Medeiros, W.M.; Silva, F.O.; Brito, A.C.; Pontes, D.D.L.; Andrade-Neto, V.F. Comparative study on the antioxidant and anti-Toxoplasma activities of vanillin and its resorcinarene derivative. Molecules 2014, 19, 5898–5912. [Google Scholar] [CrossRef] [PubMed]
- Alson, S.G.; Jansen, O.; Cieckiewicz, E.; Rakotoarimanana, H.; Rafatro, H.; Degotte, G.; Francotte, P.; Frederich, M. In vitro and in vivo antimalarial activity of caffeic acid and some of its derivatives. J. Pharm. Pharmacol. 2018, 70, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Aldaba-Muruato, L.R.; Ventura-Juárez, J.; Perez-Hernandez, A.M.; Hernández-Morales, A.; Muñoz-Ortega, M.H.; Martínez-Hernández, S.L.; Alvarado, B.; Macías, J.R. Therapeutic perspectives of p-coumaric acid: Anti-necrotic, anti-cholestatic and anti-amoebic activities. World Acad. Sci. J. 2021, 3, 47. [Google Scholar] [CrossRef]
- Ren, L.; Wang, S.; Liu, S.; Prasanthi, H.A.C.; Li, Y.; Cao, J.; Zhong, F.; Guo, L.; Lu, F.; Luo, X. Postbiotic of Pediococcus acidilactici GQ01, a novel probiotic strain isolated from natural fermented wolfberry, attenuates hyperuricaemia in mice through modulating uric acid metabolism and gut microbiota. Foods 2024, 13, 923. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).