Evaluating Entomopathogenic Nematodes as Biocontrol Agents Against Two Major Cockroach Species, Blattella germanica and Periplaneta americana, in Antalya, Türkiye
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Cockroaches
2.2. Tested Nematodes
2.3. Nematode Efficacy Testing on Cockroaches
2.4. Statistical Analysis
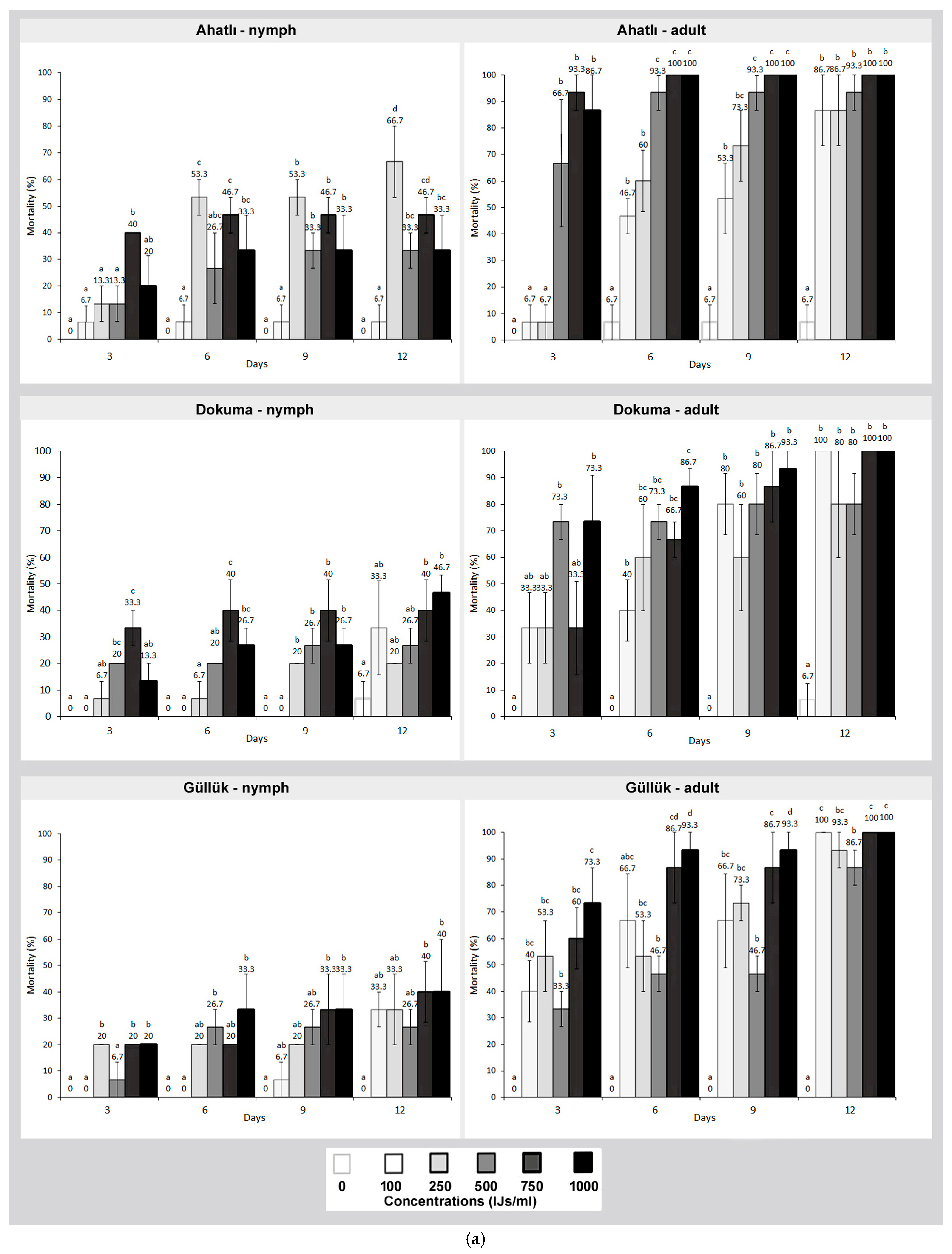
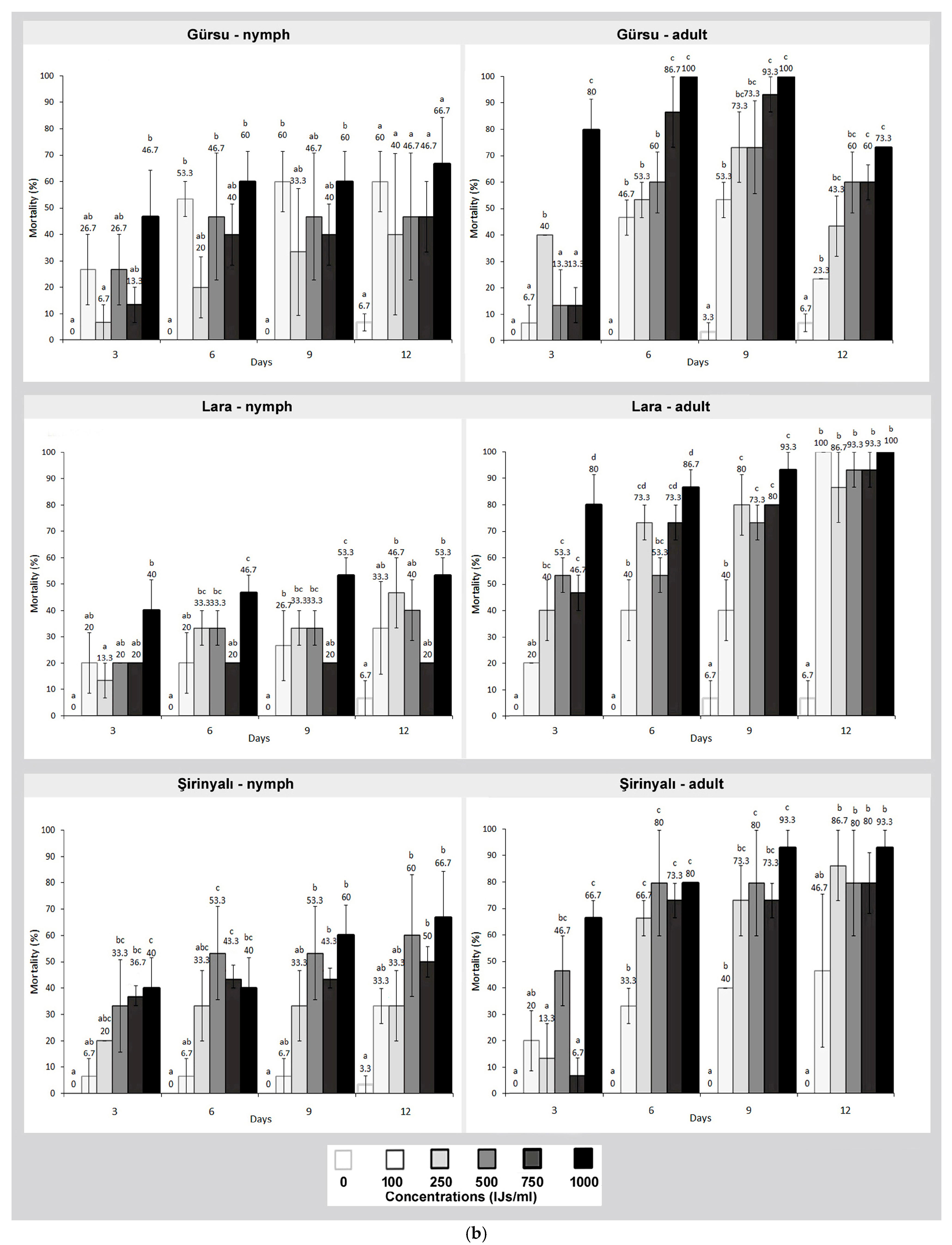
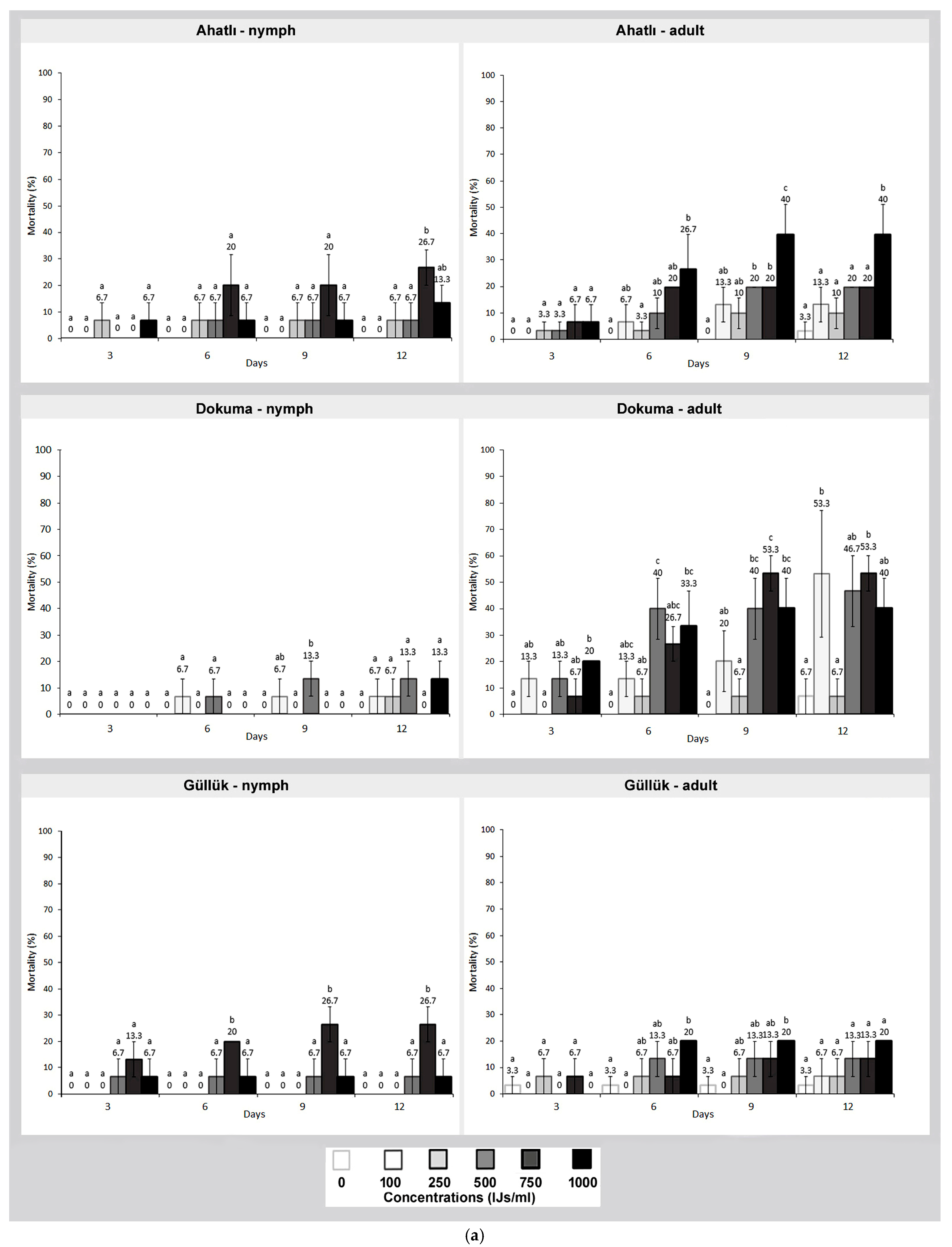
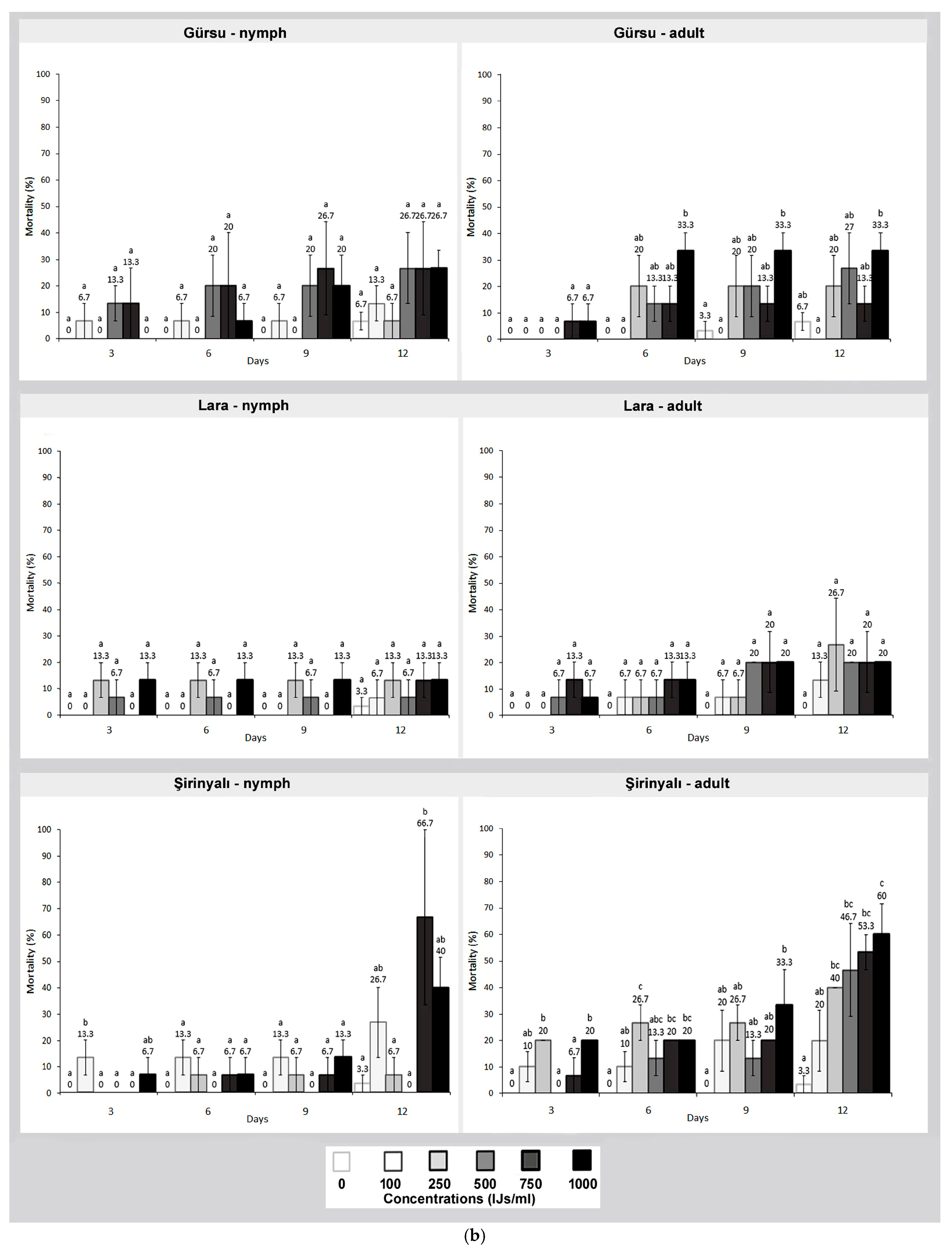
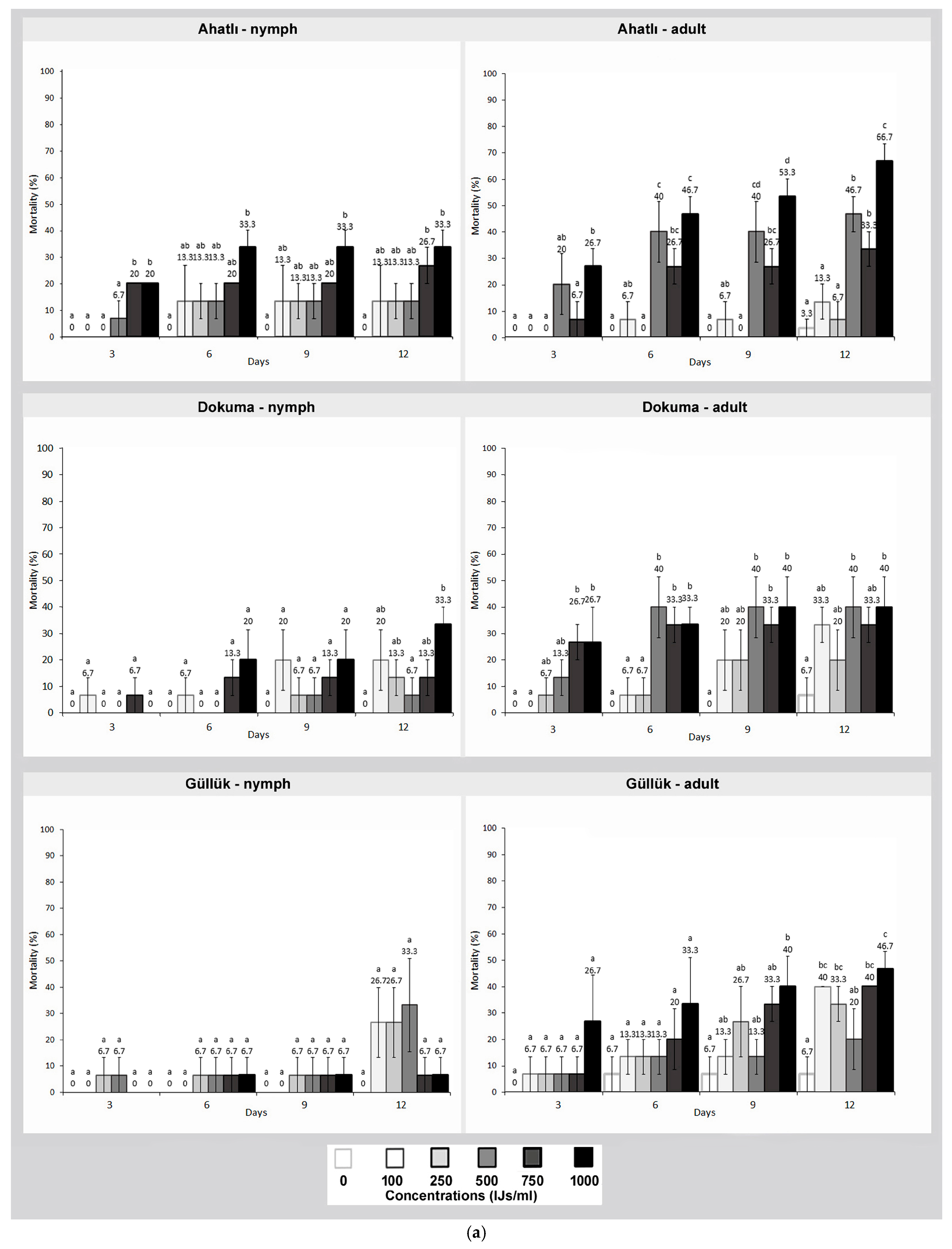
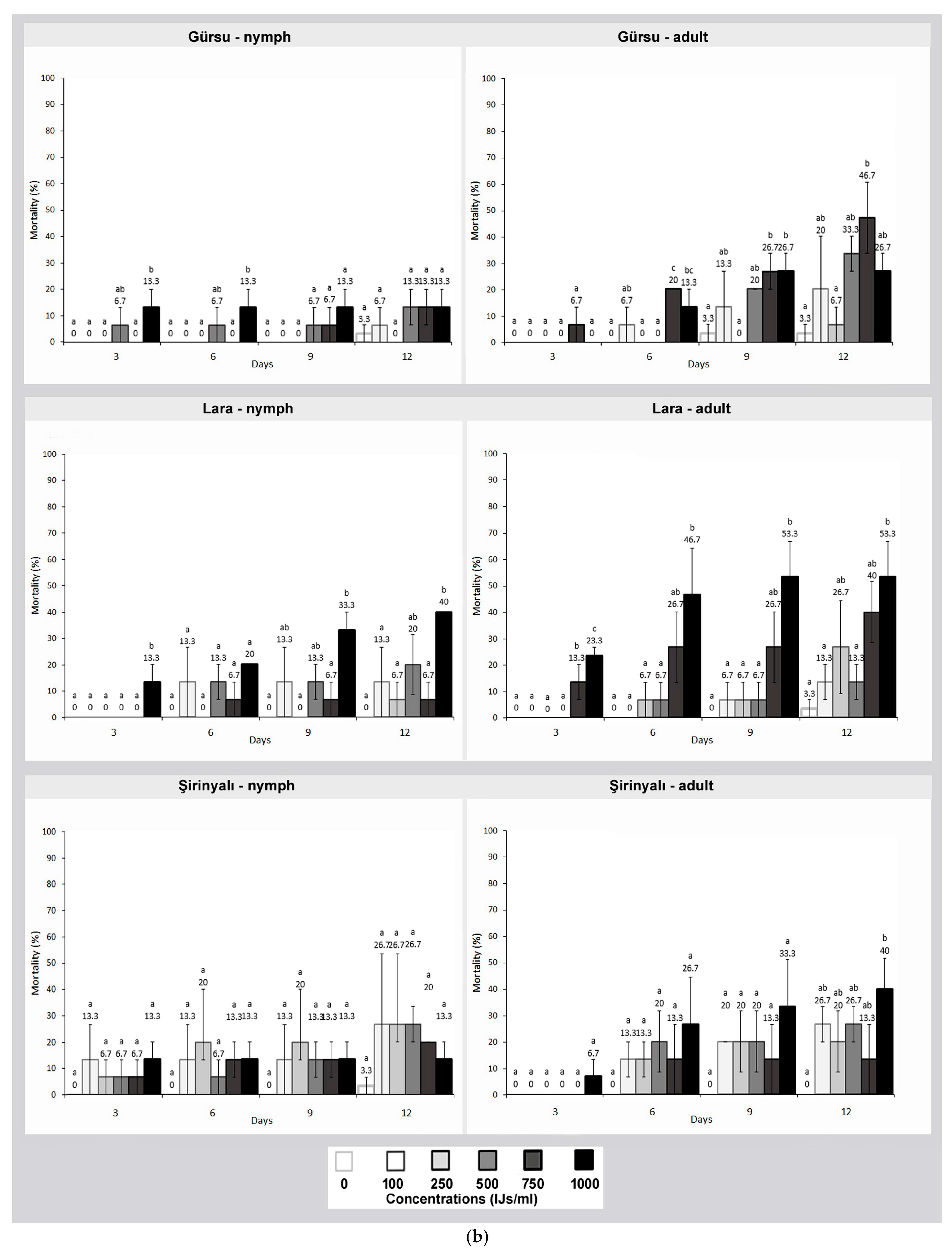
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ± | Plus–Minus |
| % | Percent |
| ~ | Tilde (Approximately) |
| ≤ | Less than or Equal to |
| ® | Registered Trademark Symbol |
| ANOVA | Analysis of Variance |
| cm | Centimeters |
| cm2 | Square Centimeters |
| °C | Degree Celsius |
| df | Degrees of freedom |
| e.g., | Exempli Gratia (For Example) |
| EPNs | Entomopathogenic Nematodes |
| F | F distribution |
| HB | Heterorhabditis bacteriophora |
| LC50 | Lethal Concentration |
| LD50 | Lethal Dose |
| LT50 | Lethal Time |
| IJs | Infective Juveniles |
| IPM | Integrated Pest Management |
| mL | Milliliters |
| nth | Ordinal Numbers |
| N105 | N105 strain of Steinernema rarum |
| OLI | OLI strain of Steinernema rarum |
| p | Probability |
| SPSS | Statistical Package for the Social Sciences |
| SC | Steinernema carpocapsae |
| SF | Steinernema feltiae |
References
- Bell, W.J.; Roth, L.M.; Nalepa, C.A. Cockroaches: Ecology, Behavior, and Natural History; Johns Hopkins University Press: Baltimore, MD, USA, 2007; 248p. [Google Scholar]
- Nasirian, H. Infestation of cockroaches (Insecta: Blattaria) in the human dwelling environments: A systematic review and meta-analysis. Acta Trop. 2017, 167, 86–98. [Google Scholar] [CrossRef]
- Mullen, G.R.; Durden, L.A. Medical and Veterinary Entomology, 3rd ed.; Academic Press: London, UK, 2018; 792p. [Google Scholar]
- McPherson, S.; Wada-Katsumata, A.; Hatano, E.; Silverman, J.; Schal, C. Comparison of Diet Preferences of Laboratory-Reared and Apartment-Collected German Cockroaches. J. Econ. Entomol. 2021, 114, 2189–2197. [Google Scholar] [CrossRef]
- Wang, C.; Lee, C.-Y.; Rust, M.K. Biology and Management of the German Cockroach; CSIRO Publishing: Clayton South, Australia, 2021; p. 320. [Google Scholar] [CrossRef]
- Bell, W.J. The Laboratory Cockroach: Experiments in Cockroach Anatomy, Physiology and Behavior; Kansas University: Lawrence, KS, USA, 1981; 161p. [Google Scholar]
- Solomon, F.; Belayneh, F.; Kibru, G.; Ali, S. Vector Potential of Blattella germanica (L.) (Dictyoptera: Blattidae) for Medically Important Bacteria at Food Handling Establishments in Jimma Town, Southwest Ethiopia. BioMed Res. Int. 2016, 2016, 3490906. [Google Scholar] [CrossRef]
- Ismael, B.; Wilson, M.; Miller, D.; Pietri, J.E. Differences in Salmonella typhimurium infection and excretion among laboratory and field strains of the German cockroach suggest a genomic basis for vector competence. Infect. Genet. Evol. 2024, 123, 105624. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Feng, L.; Lin, C.; Ye, C.; Liu, J.; Li, H.; Hao, L.; Liu, H. Intestinal pathogens detected in cockroach species within different food-related environments in Pudong, China. Sci. Rep. 2024, 14, 1947. [Google Scholar] [CrossRef]
- Hamu, H.; Debalke, S.; Zemene, E.; Birlie, B.; Mekonnen, Z.; Yewhalaw, D. Isolation of intestinal parasites of public health importance from cockroaches (Blattella germanica) in Jimma Town, Southwestern Ethiopia. J. Parasitol. Res. 2014, 2014, 186240. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Son, M.; Lee, J.H.; Hong, C.S.; Park, J.W. Allergenic characterization of a novel allergen, homologous to chymotrypsin, from German cockroach. Allergy Asthma Immunol. Res. 2015, 7, 283–289. [Google Scholar] [CrossRef]
- Zarchi, A.A.; Vatani, H. A Survey on Species and Prevalence Rate of Bacterial Agents Isolated from Cockroaches in Three Hospitals. Vector-Borne Zoonotic Dis. 2009, 9, 197–200. [Google Scholar] [CrossRef]
- Adenusi, A.A.; Akinyemi, M.I.; Akinsanya, D. Domiciliary cockroaches as carriers of human intestinal parasites in Lagos Metropolis, Southwest Nigeria: Implications for public health. J. Arthropod-Borne Dis. 2018, 12, 141–151. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, R.K.; Kumar, R.; Spalgais, S.; Mavi, A.K.; Singh, K. Cockroach exposure and its allergy sensitization in asthma patients. Monaldi Arch. Chest Dis. 2021, 91. [Google Scholar] [CrossRef]
- Nasirian, H.; Salehzadeh, A. Control of Cockroaches (Blattaria) in Sewers: A Practical Approach Systematic Review. J. Med. Entomol. 2019, 56, 181–191. [Google Scholar] [CrossRef]
- Hemingway, J.; Monro, A.G.; Small, G.J. Pyrethroid Resistance in German Cockroaches (Dictyoptera: Blattelidae): Resistance Levels and Underlying Mechanisms. J. Econ. Entomol. 1993, 86, 1931–1938. [Google Scholar] [CrossRef]
- Gondhalekar, A.D.; Scharf, M.E. Mechanisms underlying fipronil resistance in a multiple insecticide-resistant strain of the German cockroach (Blattodea: Blattellidae). J. Med. Entomol. 2012, 49, 122–131. [Google Scholar] [CrossRef]
- Oz, E.; Cetin, H.; Yanikoglu, A. Investigation of resistance to synthetic pyrethroids in Blattella germanica L., 1767 (Blattodea: Ectobiidae) and Periplaneta americana L., 1758 (Blattodea: Blattidae) populations in Turkey. Turk. J. Entomol. 2021, 45, 361–370. [Google Scholar] [CrossRef]
- Chai, R.Y.; Lee, C.Y. Insecticide resistance profiles and synergism in field populations of the German cockroach (Dictyoptera: Blattellidae) from Singapore. J. Econ. Entomol. 2010, 103, 460–471. [Google Scholar] [CrossRef]
- Heudorf, U.; Butte, W.; Schulz, C.; Angerer, J. Reference values for metabolites of pyrethroid and organophosphorous insecticides in urine for human biomonitoring in environmental medicine. Int. J. Hyg. Environ. Health 2006, 209, 293–299. [Google Scholar] [CrossRef]
- Koureas, M.; Tsakalof, A.; Tsatsakis, A.; Hadjichristodoulou, C. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol. Lett. 2012, 210, 155–168. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.Y. Entomopathogenic nematodes in insect pest biocontrol: Diversity and function of excretory/secretory proteins. J. Invertebr. Pathol. 2024, 207, 108205. [Google Scholar] [CrossRef]
- Bhat, A.; Chaubey, A.; Askary, T. Global distribution of entomopathogenic nematodes, Steinernema and Heterorhabditis. Egypt. J. Biol. Pest Control 2020, 30, 31. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I. Entomopathogenic Nematodes and Insect Management. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 781–784. [Google Scholar] [CrossRef]
- Göze Özdemir, F.G.; Uzun, A.; Demirözer, O. Determination of the efficacy of Steinernema feltiae Filipjev and Heterorhabditis bacteriophora Poinar, 1976 on Leptinotarsa decemlineata (Say, 1824). JAF 2021, 16, 115–121. [Google Scholar]
- Partal, E.; Kaşkavalcı, G. Determination of the efficacy of some entomopathogenic nematodes on Spodoptera littoralis (Boisduval, 1833) (Lepidoptera: Noctuidae) in laboratory conditions. Turk. J. Entomol. 2024, 48, 251–259. [Google Scholar] [CrossRef]
- Polat, B.; Cengiz, A.; Koç, S.; Kahraman Kökten, S.; Gültekin, Z.N.; Çalışkan, C.; Kocaoğlu Cenkci, S.; Yıldırım, T.; Tufan Çetin, O.; Çetin, H. Efficacy of Steinernema feltiae nematode against lesser mealworm (Alphitobius diaperinus) populations from poultry farms in Türkiye. Vet. Sci. 2024, 11, 567. [Google Scholar] [CrossRef]
- Morton, A.; García-del-Pino, F. Sex-related differences in the susceptibility of Periplaneta americana and Capnodis tenebrionis to the entomopathogenic nematode Steinernema carpocapsae. J. Invertebr. Pathol. 2013, 112, 203–207. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, G.A.; El-Bahrawy, A.F.; El-Sharabasy, H.M.; El-Badry, Y.S.; El-Ashry, R.M.A.; Mahmoud, M.F. Pathogenicity and reproduction of the entomopathogenic nematode, Steinernema carpocapsae (Wieser) in the German cockroach, Blattella germanica L. (Dictyoptera: Blattellidae). Egypt. J. Biol. Pest Control 2014, 24, 133–138. [Google Scholar]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Appel, A.G.; Benson, E.P.; Ellenberger, J.M.; Manweiler, S.A. Laboratory and field evaluations of an entomogenous nematode (Nematoda: Steinernematidae) for German cockroach (Dictyoptera: Blattellidae) control. J. Econ. Entomol. 1993, 86, 777–784. [Google Scholar] [CrossRef]
- El-Kady, G.A.; El-Badry, Y.S.; El-Bahrawy, A.F.; El-Sharabasy, H.M.; Mahmoud, M.F. Evaluation of entomopathogenic nematode, Steinernema carpocapsae against the German cockroach, Blattella germanica (L.) under laboratory conditions. Egypt. J. Biol. Pest Control 2015, 25, 355–358. [Google Scholar]
- Maketon, M.; Hominchan, A.; Hotaka, D. Control of the American cockroach (Periplaneta americana) and German cockroach (Blattella germanica) by entomopathogenic nematodes. Rev. Colomb. Entomol. 2010, 36, 249–253. [Google Scholar] [CrossRef]
- Cutler, J.; Hughes, K.; Rae, R. Susceptibility of cockroaches (Gromphadorhina portentosa, Nauphoeta cinerea, and Blaptica dubia) exposed to entomopathogenic nematodes. Biocontrol Sci. Technol. 2017, 27, 556–564. [Google Scholar] [CrossRef]
- Baker, N.; Ali, H.B.; Ali, H.B.; Gowen, S. Reproduction of entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora on the German cockroach Blattella germanica at different temperatures. Iraqi J. Sci. 2012, 53, 505–512. [Google Scholar] [CrossRef]
- Varela, A.D.A.; Bertolotti, M.A.; Cagnolo, S.R. Susceptibility of Periplaneta americana (Blattodea: Blattidae) to two isolates of Steinernema rarum (Rhabditida: Steinernematidae) of the province of Córdoba, Argentina. Rev. Soc. Entomol. Argent. 2021, 80, 40–47. [Google Scholar] [CrossRef]
- Özdemir, E.; Evlice, E. Assessment of the Susceptibility of the Turkestan Cockroach, Blatta lateralis, to Turkish Isolates of Entomopathogenic Nematodes. Turk. Bull. Entomol. 2020, 11, 129–137. [Google Scholar] [CrossRef]
- Grewal, P.S.; Gaugler, R.; Selvan, S. Host recognition by entomopathogenic nematodes: Behavioral response to contact with host feces. J. Chem. Ecol. 1993, 19, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Parle, E.; Herbaj, S.; Sheils, F.; Larmon, H.; Taylor, D. Buckling failures in insect exoskeletons. Bioinspir. Biomim. 2015, 11, 016003. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.G.; Strong, C.A.; Patterson, R.S.; Valles, S.M. Differential susceptibility of German cockroach (Dictyoptera: Blattellidae) sexes and nymphal age classes to insecticides. J. Econ. Entomol. 1993, 86, 785–792. [Google Scholar] [CrossRef]
- Ladonni, H. Evaluation of three methods for detecting permethrin resistance in adult and nymphal Blattella germanica (Dictyoptera: Blattellidae). J. Econ. Entomol. 2001, 94, 694–697. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cengiz, A.; Polat, B.; Kokten, S.K.; Aslan Bıckı, U.; Calıskan, C.; Koc, S.; Oz, E.; Kocaoglu-Cenkci, S.; Tufan-Cetin, O.; Cetin, H. Evaluating Entomopathogenic Nematodes as Biocontrol Agents Against Two Major Cockroach Species, Blattella germanica and Periplaneta americana, in Antalya, Türkiye. Pathogens 2025, 14, 655. https://doi.org/10.3390/pathogens14070655
Cengiz A, Polat B, Kokten SK, Aslan Bıckı U, Calıskan C, Koc S, Oz E, Kocaoglu-Cenkci S, Tufan-Cetin O, Cetin H. Evaluating Entomopathogenic Nematodes as Biocontrol Agents Against Two Major Cockroach Species, Blattella germanica and Periplaneta americana, in Antalya, Türkiye. Pathogens. 2025; 14(7):655. https://doi.org/10.3390/pathogens14070655
Chicago/Turabian StyleCengiz, Aysegul, Burak Polat, Sevval Kahraman Kokten, Ummuhan Aslan Bıckı, Cansu Calıskan, Samed Koc, Emre Oz, Serap Kocaoglu-Cenkci, Ozge Tufan-Cetin, and Huseyin Cetin. 2025. "Evaluating Entomopathogenic Nematodes as Biocontrol Agents Against Two Major Cockroach Species, Blattella germanica and Periplaneta americana, in Antalya, Türkiye" Pathogens 14, no. 7: 655. https://doi.org/10.3390/pathogens14070655
APA StyleCengiz, A., Polat, B., Kokten, S. K., Aslan Bıckı, U., Calıskan, C., Koc, S., Oz, E., Kocaoglu-Cenkci, S., Tufan-Cetin, O., & Cetin, H. (2025). Evaluating Entomopathogenic Nematodes as Biocontrol Agents Against Two Major Cockroach Species, Blattella germanica and Periplaneta americana, in Antalya, Türkiye. Pathogens, 14(7), 655. https://doi.org/10.3390/pathogens14070655





