Growth Inhibition and Additive Effect to Antimalarial Drugs of Brucea javanica Extracts on Asexual Blood-Stage Plasmodium falciparum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Identification
2.2. Preparation of B. javanica Extract
2.3. In Vitro Culture of P. falciparum
2.4. Antimalarial Activity Test of B. javanica Extracts by Growth Inhibition Assay
2.5. Co-Incubation of B. javanica Extracts with Antimalarial Drugs
2.6. Microscopic Analysis of P. falciparum from Growth Inhibition and Co-Incubation Assay
2.7. Statistical Analysis
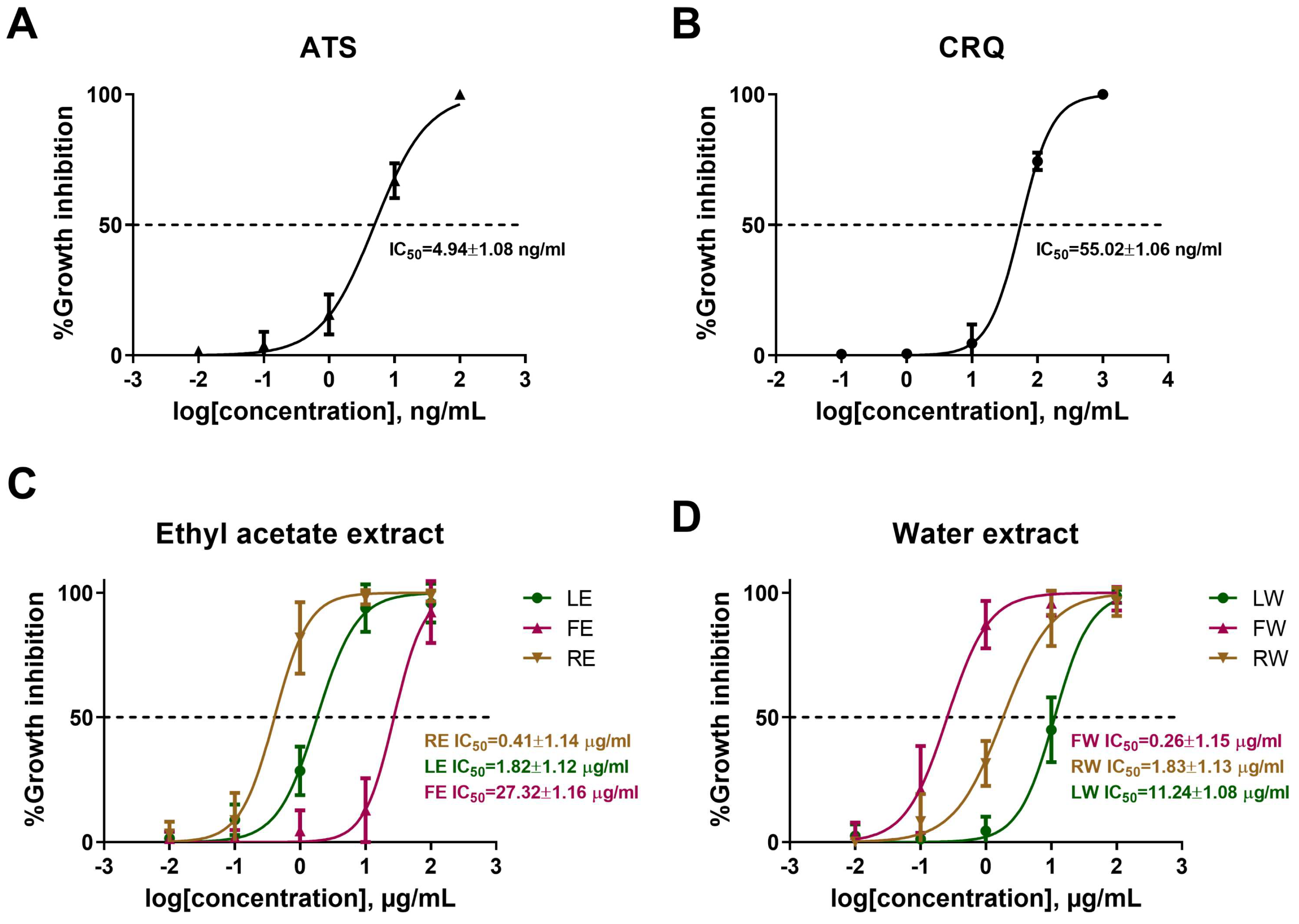
3. Results
3.1. B. javanica Extracts Inhibit P. falciparum Survival
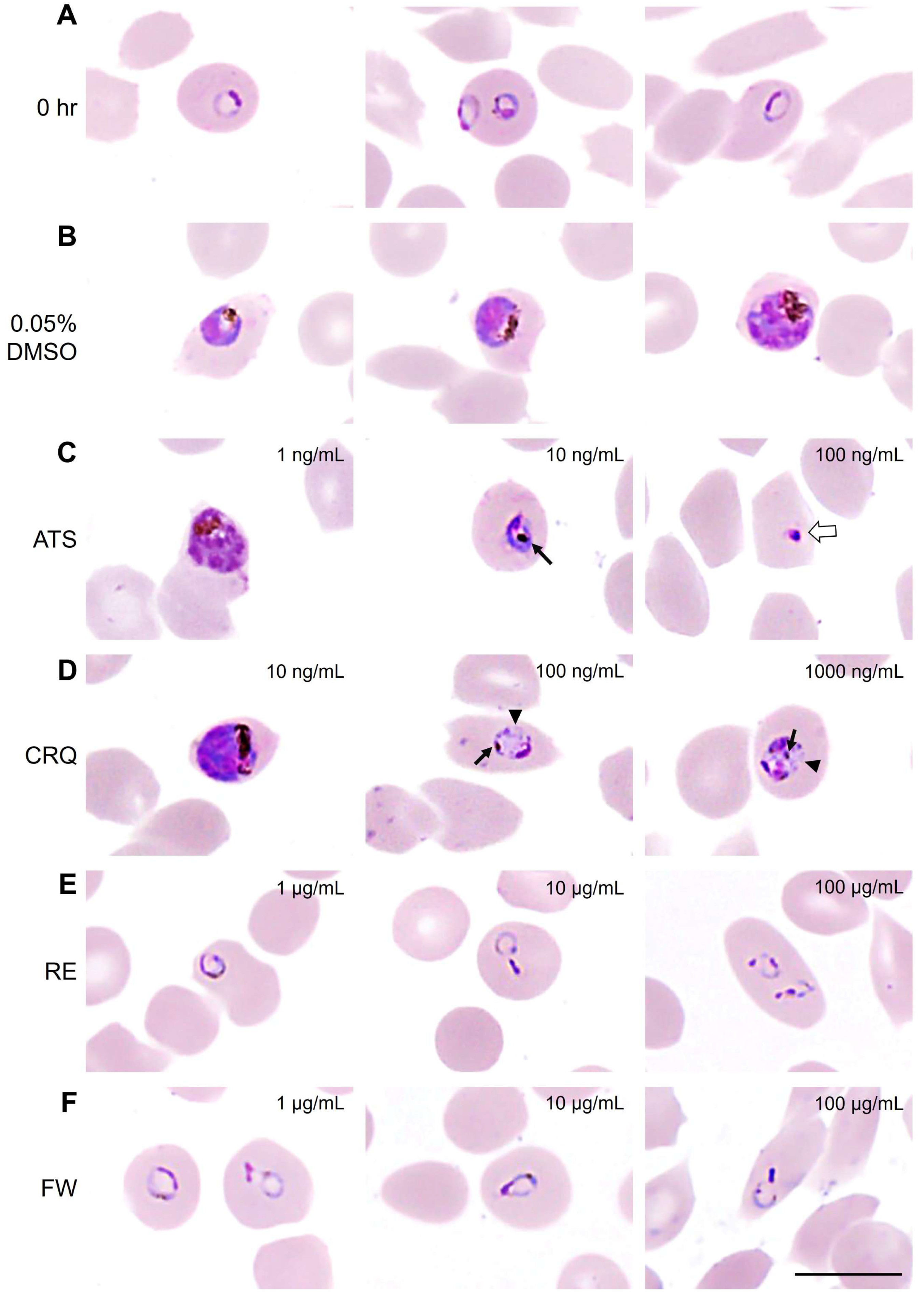
3.2. B. javanica Extracts Arrested the Development of P. falciparum
3.3. Morphological Changes in Malaria Parasite After Treatments with Antimalarial Drugs and B. javanica Extracts
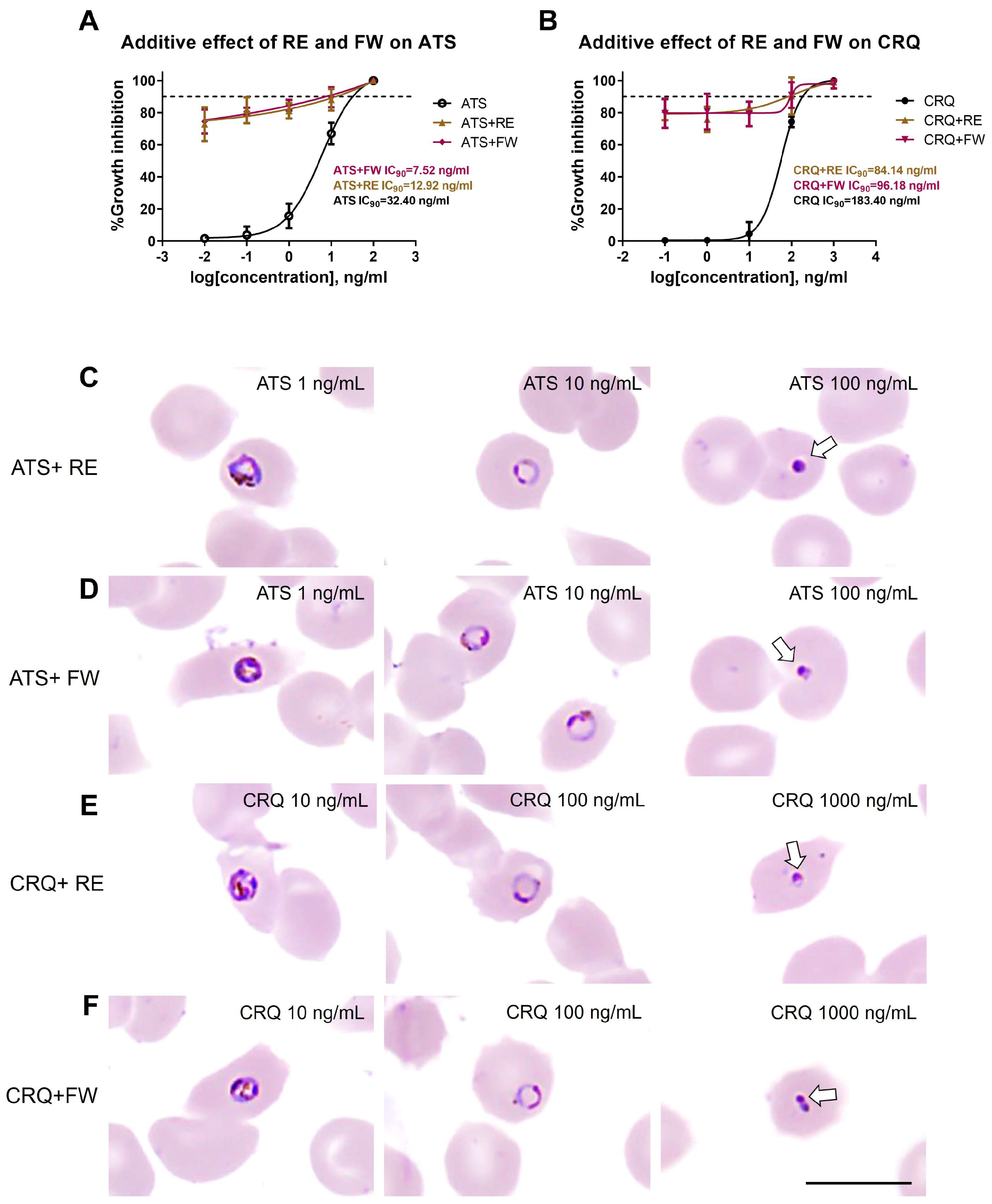
3.4. Additive Effect of B. javanica Extracts on Antimalarial Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poespoprodjo, J.R.; Douglas, N.M.; Ansong, D.; Kho, S.; Anstey, N.M. Malaria. Lancet 2023, 402, 2328–2345. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2024: Addressing Inequity in the Global Malaria Response; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Miller, L.H.; Ackerman, H.C.; Su, X.Z.; Wellems, T.E. Malaria biology and disease pathogenesis: Insights for new treatments. Nat. Med. 2013, 19, 156–167. [Google Scholar] [CrossRef]
- Mawson, A.R. The pathogenesis of malaria: A new perspective. Pathog. Glob. Health 2013, 107, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Plewes, K.; Turner, G.D.H.; Dondorp, A.M. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr. Opin. Infect. Dis. 2018, 31, 69–77. [Google Scholar] [CrossRef]
- Martviset, P.; Kitvatanachai, S.; Tarasuk, M.; Muhamad, P.; Na-Bangchang, K. Pretreatment gametocyte carriage in symptomatic patients with Plasmodium falciparum and Plasmodium vivax infections on the Thai-Myanmar border. J. Vector Borne Dis. 2021, 58, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Cowman, A.F. Moving in and renovating: Exporting proteins from Plasmodium into host erythrocytes. Nat. Rev. Microbiol. 2010, 8, 617–621. [Google Scholar] [CrossRef]
- Tuikue Ndam, N.; Moussiliou, A.; Lavstsen, T.; Kamaliddin, C.; Jensen, A.T.R.; Mama, A.; Tahar, R.; Wang, C.W.; Jespersen, J.S.; Alao, J.M.; et al. Parasites causing cerebral falciparum malaria bind multiple endothelial receptors and express EPCR and ICAM-1-binding PfEMP1. J. Infect. Dis. 2017, 215, 1918–1925. [Google Scholar] [CrossRef]
- Albrecht-Schgoer, K.; Lackner, P.; Schmutzhard, E.; Baier, G. Cerebral Malaria: Current Clinical and Immunological Aspects. Front. Immunol. 2022, 13, 863568. [Google Scholar] [CrossRef]
- Thu, A.M.; Phyo, A.P.; Landier, J.; Parker, D.M.; Nosten, F.H. Combating multidrug-resistant Plasmodium falciparum malaria. FEBS J. 2017, 284, 2569–2578. [Google Scholar] [CrossRef]
- Ippolito, M.M.; Moser, K.A.; Kabuya, J.B.; Cunningham, C.; Juliano, J.J. Antimalarial Drug Resistance and Implications for the WHO Global Technical Strategy. Curr. Epidemiol. Rep. 2021, 8, 46–62. [Google Scholar] [CrossRef]
- Sidhu, A.B.; Verdier-Pinard, D.; Fidock, D.A. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 2002, 298, 210–213. [Google Scholar] [CrossRef]
- Amambua-Ngwa, A.; Button-Simons, K.A.; Li, X.; Kumar, S.; Brenneman, K.V.; Ferrari, M.; Checkley, L.A.; Haile, M.T.; Shoue, D.A.; McDew-White, M.; et al. Chloroquine resistance evolution in Plasmodium falciparum is mediated by the putative amino acid transporter AAT1. Nat. Microbiol. 2023, 8, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Pousibet-Puerto, J.; Salas-Coronas, J.; Sánchez-Crespo, A.; Molina-Arrebola, M.A.; Soriano-Pérez, M.J.; Giménez-López, M.J.; Vázquez-Villegas, J.; Cabezas-Fernández, M.T. Impact of using artemisinin-based combination therapy (ACT) in the treatment of uncomplicated malaria from Plasmodium falciparum in a non-endemic zone. Malar. J. 2016, 15, 339. [Google Scholar] [CrossRef]
- Mutabingwa, T.K. Artemisinin-based combination therapies (ACTs): Best hope for malaria treatment but inaccessible to the needy! Acta Trop. 2005, 95, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Imwong, M.; Jindakhad, T.; Kunasol, C.; Sutawong, K.; Vejakama, P.; Dondorp, A.M. An outbreak of artemisinin resistant falciparum malaria in Eastern Thailand. Sci. Rep. 2015, 5, 17412. [Google Scholar] [CrossRef]
- Noisang, C.; Prosser, C.; Meyer, W.; Chemoh, W.; Ellis, J.; Sawangjaroen, N.; Lee, R. Molecular detection of drug resistant malaria in Southern Thailand. Malar. J. 2019, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Boonyalai, N.; Thamnurak, C.; Sai-ngam, P.; Ta-aksorn, W.; Arsanok, M.; Uthaimongkol, N.; Sundrakes, S.; Chattrakarn, S.; Chaisatit, C.; Praditpol, C.; et al. Plasmodium falciparum phenotypic and genotypic resistance profile during the emergence of Piperaquine resistance in Northeastern Thailand. Sci. Rep. 2021, 11, 13419. [Google Scholar] [CrossRef] [PubMed]
- Rahmasari, F.V.; Asih, P.B.S.; Dewayanti, F.K.; Rotejanaprasert, C.; Charunwatthana, P.; Imwong, M.; Syafruddin, D. Drug resistance of Plasmodium falciparum and Plasmodium vivax isolates in Indonesia. Malar. J. 2022, 21, 354. [Google Scholar] [CrossRef]
- Rosenthal, P.J.; Asua, V.; Conrad, M.D. Emergence, transmission dynamics and mechanisms of artemisinin partial resistance on malaria parasite in Africa. Nat. Rev. Microbiol. 2024, 22, 373–384. [Google Scholar] [CrossRef]
- White, N.J.; Chotivanich, K. Artemisinin-resistant malaria. Clin. Microbiol. Rev. 2024, 37, e00109-24. [Google Scholar] [CrossRef]
- Graf, E. Chinese Drugs of Plant Origin. Chemistry, Pharmacology, and Use in Traditional and Modern Medicine. Von W. Tang und G. Eisenbrand. Springer-Verlag Berlin etc. 1992, X, 1056, S., 41 Abb. gebd. DM 248,00. Pharm. Unserer Zeit 1992, 21, 281. [Google Scholar] [CrossRef]
- Su, Z.; Ma, Z.; Liu, K.; Li, T.; Zhou, B. Quassilactones A and B, structural characterization of a new class of norquassinoids from Brucea javanica. J. Nat. Med. 2020, 74, 599–605. [Google Scholar] [CrossRef]
- Su, Z.; Hao, J.; Xu, Z.; Huang, R.; Zhang, N.; Qiu, S. A new quassinoid from fruits of Brucea javanica. Nat. Prod. Res. 2013, 27, 2016–2021. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, J.F.; Wang, Y.M.; Chen, W.H.; Qian, S.; Zhou, Z.G.; Xu, M. Brucea javanica oil emulsion suppresses tumor growth in human cervical cancer cells through inhibition of the E6 oncogene and induction of apoptosis. Transl. Cancer Res. 2020, 9, 918–929. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.X.; Dou, Y.X.; Huang, Q.H.; Xian, Y.F.; Lin, Z.X. Major Constituents From Brucea javanica and Their Pharmacological Actions. Front. Pharmacol. 2022, 13, 853119. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, Z.; Guo, R.; Ren, F. Nrf2 inhibitor, brusatol in combination with trastuzumab exerts synergistic antitumor activity in HER2-positive cancers by inhibiting Nrf2/HO-1 and HER2-AKT/ERK1/2 pathways. Oxid. Med. Cell Longev. 2020, 2020, 9867595. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, J.; Balasubramaniam, J.; Sellamuthu, A.; Ravi, A. An in vitro study on the reversal of epithelial to mesenchymal transition by brusatol and its synergistic properties in triple-negative breast cancer cells. J. Pharm. Pharmacol. 2021, 73, 749–757. [Google Scholar] [CrossRef]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M.; et al. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.M.; Bai, L.L.; Hu, S.Z.; Tian, H.Y.; Ruan, L.J.; Tan, Y.F.; Hu, L.P.; Ye, W.C.; Zhang, D.M.; Jiang, R.W. Isolation, chemotaxonomic significance and cytotoxic effects of quassinoids from Brucea javanica. Fitoterapia 2015, 105, 66–72. [Google Scholar] [CrossRef]
- Shen, J.G.; Zhang, Z.K.; Wu, Z.J.; Ouyang, M.A.; Xie, L.H.; Lin, Q.Y. Antiphytoviral activity of bruceine-D from Brucea javanica seeds. Pest. Manag. Sci. 2008, 64, 191–196. [Google Scholar] [CrossRef]
- Dou, Y.X.; Zhou, J.T.; Wang, T.T.; Huang, Y.F.; Chen, V.P.; Xie, Y.L.; Lin, Z.X.; Gao, J.S.; Su, Z.R.; Zeng, H.F. Self-nanoemulsifying drug delivery system of bruceine D: A new approach for anti-ulcerative colitis. Int. J. Nanomed. 2018, 13, 5887–5907. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Zhang, Y.; Wang, P.; Gao, W. Brusatol inhibits amyloid-β-induced neurotoxicity in U-251 cells via regulating the Nrf2/HO-1 pathway. J. Cell Biochem. 2019, 120, 10556–10563. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, F.; Ding, Q.; Cai, Y.; Hao, Y.; Tang, B. Bruceine D elevates Nrf2 activation to restrain Parkinson’s disease in mice through suppressing oxidative stress and inflammatory response. Biochem. Biophys. Res. Commun. 2020, 526, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Pavanand, K.; Nutakul, W.; Dechatiwongse, T.; Yoshihira, K.; Yongvanitchit, K.; Scovill, J.P.; Flippen-Anderson, J.L.; Gilardi, R.; George, C.; Kanchanapee, P.; et al. In vitro antimalarial activity of Brucea javanica against multi-drug resistant Plasmodium falciparum. Planta Med. 1986, 52, 108–111. [Google Scholar] [CrossRef]
- Sakaki, T.; Yoshimura, S.; Tsuyuki, T.; Takahashi, T.; Honda, T.; Nakanishi, T. Structures of Yadanziosides K, M, N, and O, New Quassinoid Glycosides from Brucea javanica (L.) Merr. Bull. Chem. Soc. Jpn. 2006, 59, 3541–3546. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Bray, D.H.; Boardman, P.; Chan, K.L.; Phillipson, J.D.; Warhurst, D.C.; Peters, W. Plants as sources of antimalarial drugs, Part 4: Activity of Brucea javanica fruits against chloroquine-resistant Plasmodium falciparum in vitro and against Plasmodium berghei in vivo. J. Nat. Prod. 1987, 50, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sornwatana, T.; Roytrakul, S.; Wetprasit, N.; Ratanapo, S. Brucin, an antibacterial peptide derived from fruit protein of Fructus Bruceae, Brucea javanica (L.) Merr. Lett. Appl. Microbiol. 2013, 57, 129–136. [Google Scholar] [CrossRef]
- Henny, H.; Idha, S. Phytochemical tested and in vitro screening anti- malaria activity of belilik (Brucea javanica (L)). MERR against Plasmodium falciparum. Berk. Penelit. Hayati 2014, 19, 1–4. [Google Scholar]
- Sriwilaijaroen, N.; Kondo, S.; Nanthasri, P.; Auparakkitanon, S.; Suzuki, Y.; Wilairat, P. Antiplasmodial effects of Brucea javanica (L.) Merr. and Eurycoma longifolia Jack extracts and their combination with chloroquine and quinine on Plasmodium falciparum in culture. Trop. Med. Health 2010, 38, 61–68. [Google Scholar] [CrossRef]
- Cranmer, S.L.; Magowan, C.; Liang, J.; Coppel, R.L.; Cooke, B.M. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 363–365. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, D. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes D. Invertebr. Surviv. J. 2007, 4, 92–94. [Google Scholar]
- Olasehinde, G.I.; Ojurongbe, O.; Adeyeba, A.O.; Fagade, O.E.; Valecha, N.; Ayanda, I.O.; Ajayi, A.A.; Egwari, L.O. In vitro studies on the sensitivity pattern of Plasmodium falciparum to anti-malarial drugs and local herbal extracts. Malar. J. 2014, 13, 63. [Google Scholar] [CrossRef]
- van der Pluijm, R.W.; Amaratunga, C.; Dhorda, M.; Dondorp, A.M. Triple Artemisinin-Based Combination Therapies for Malaria—A New Paradigm? Trends Parasitol. 2021, 37, 15–24. [Google Scholar] [CrossRef]
- Angelina, M.; Dewijanti, I.; Yuliani, T. Acute toxicity studies of Brucea javanica Merril leaves extract on mice. J. Trop. Life Sci. 2012, 2, 29–31. [Google Scholar] [CrossRef]
- Lemma, M.T.; Ahmed, A.M.; Elhady, M.T.; Ngo, H.T.; Vu, T.L.; Sang, T.K.; Campos-Alberto, E.; Sayed, A.; Mizukami, S.; Na-Bangchang, K.; et al. Medicinal plants for in vitro antiplasmodial activities: A systemic review of literature. Parasitol. Int. 2017, 66, 713–720. [Google Scholar] [CrossRef]
- Lu, Y.H.; Yang, Y.Y.; Pan, I.H. Anti-proliferative Effect of Brucea javanica Fruit Extract Against Human Hepatocarcinoma Cell Lines and Its Mechanism. Planta Med. 2011, 77, PM56. [Google Scholar] [CrossRef]
- Bissinger, R.; Modicano, P.; Alzoubi, K.; Honisch, S.; Faggio, C.; Abed, M.; Lang, F. Effect of saponin on erythrocytes. Int. J. Hematol. 2014, 100, 51–59. [Google Scholar] [CrossRef]
- Wongtanachai, J.; Silamut, K.; Day, N.P.; Dondorp, A.; Chaisri, U. Effects of antimalarial drugs on movement of Plasmodium falciparum. Southeast Asian J. Trop. Med. Public Health 2012, 43, 1–9. [Google Scholar]
- Thommen, B.T.; Passecker, A.; Buser, T.; Hitz, E.; Voss, T.S.; Brancucci, N.M.B. Revisiting the Effect of Pharmaceuticals on Transmission Stage Formation in the Malaria Parasite Plasmodium falciparum. Front. Cell Infect. Microbiol. 2022, 12, 802341. [Google Scholar] [CrossRef] [PubMed]
- Yipsirimetee, A.; Chiewpoo, P.; Tripura, R.; Lek, D.; Day, N.P.J.; Dondorp, A.M.; Pukrittayakamee, S.; White, N.J.; Chotivanich, K. Assessment In Vitro of the Antimalarial and Transmission-Blocking Activities of Cipargamin and Ganaplacide in Artemisinin-Resistant Plasmodium falciparum. Antimicrob. Agents Chemother. 2022, 66, e0148121. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kangwanrangsan, N.; Niramolyanun, G.; Praikongkatham, C.; Chantree, P.; Martviset, P.; Pankao, V. Growth Inhibition and Additive Effect to Antimalarial Drugs of Brucea javanica Extracts on Asexual Blood-Stage Plasmodium falciparum. Pathogens 2025, 14, 646. https://doi.org/10.3390/pathogens14070646
Kangwanrangsan N, Niramolyanun G, Praikongkatham C, Chantree P, Martviset P, Pankao V. Growth Inhibition and Additive Effect to Antimalarial Drugs of Brucea javanica Extracts on Asexual Blood-Stage Plasmodium falciparum. Pathogens. 2025; 14(7):646. https://doi.org/10.3390/pathogens14070646
Chicago/Turabian StyleKangwanrangsan, Niwat, Gamolthip Niramolyanun, Chonnipa Praikongkatham, Pathanin Chantree, Pongsakorn Martviset, and Viriya Pankao. 2025. "Growth Inhibition and Additive Effect to Antimalarial Drugs of Brucea javanica Extracts on Asexual Blood-Stage Plasmodium falciparum" Pathogens 14, no. 7: 646. https://doi.org/10.3390/pathogens14070646
APA StyleKangwanrangsan, N., Niramolyanun, G., Praikongkatham, C., Chantree, P., Martviset, P., & Pankao, V. (2025). Growth Inhibition and Additive Effect to Antimalarial Drugs of Brucea javanica Extracts on Asexual Blood-Stage Plasmodium falciparum. Pathogens, 14(7), 646. https://doi.org/10.3390/pathogens14070646






