Cases of Lungworm in Cats from Southern Poland in the Autopsy and Cytological Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Laboratory Analysis
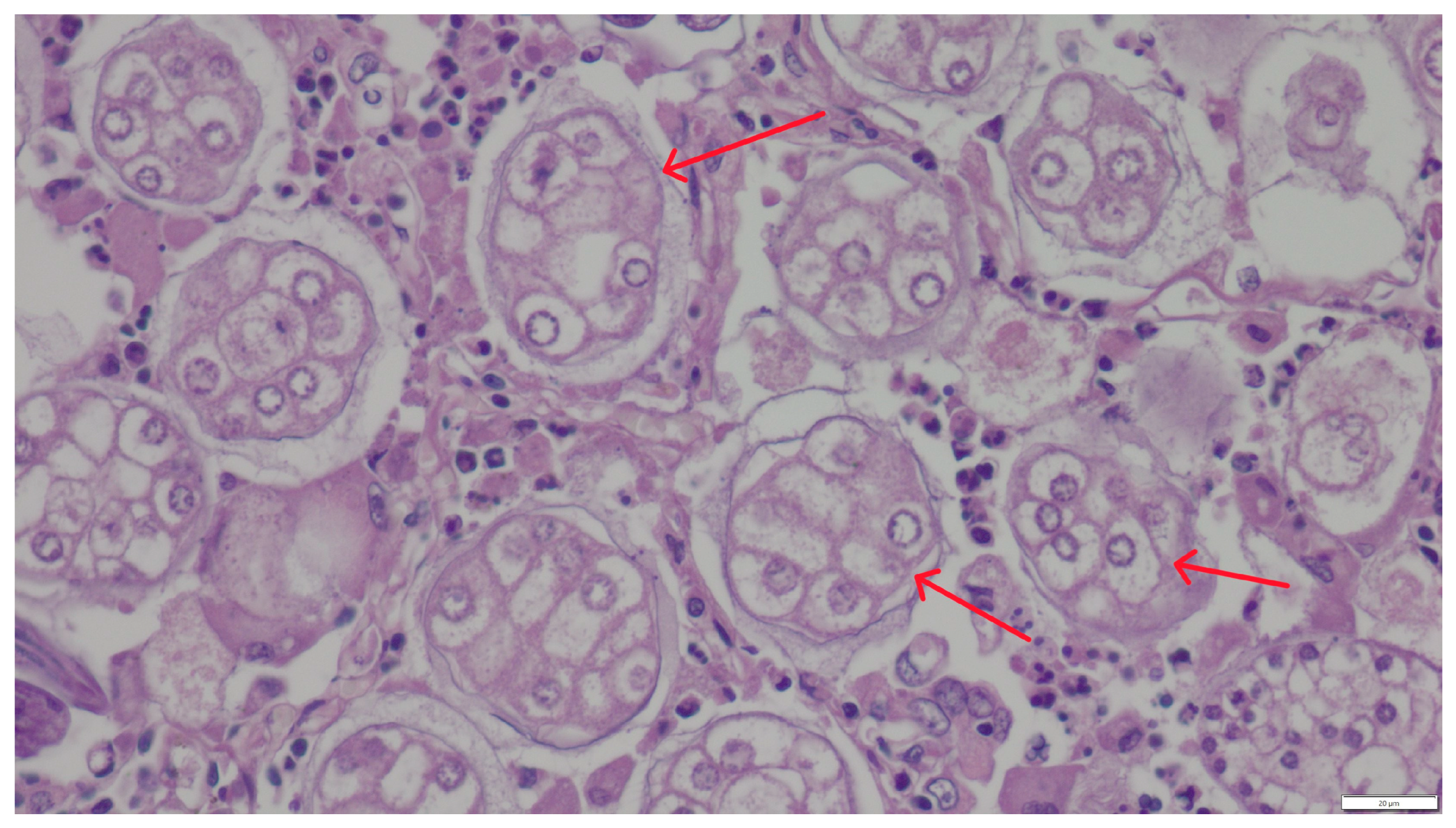
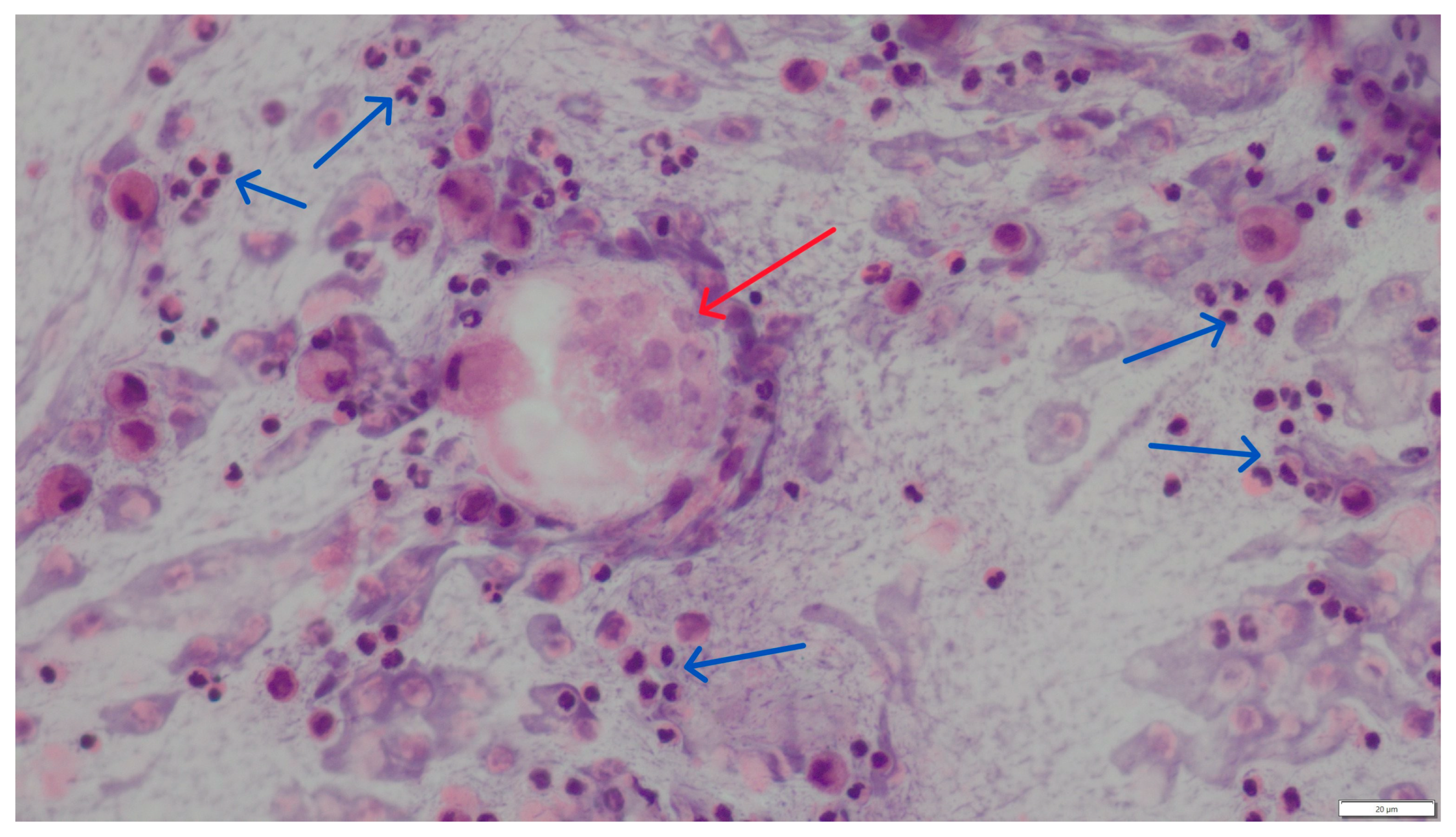
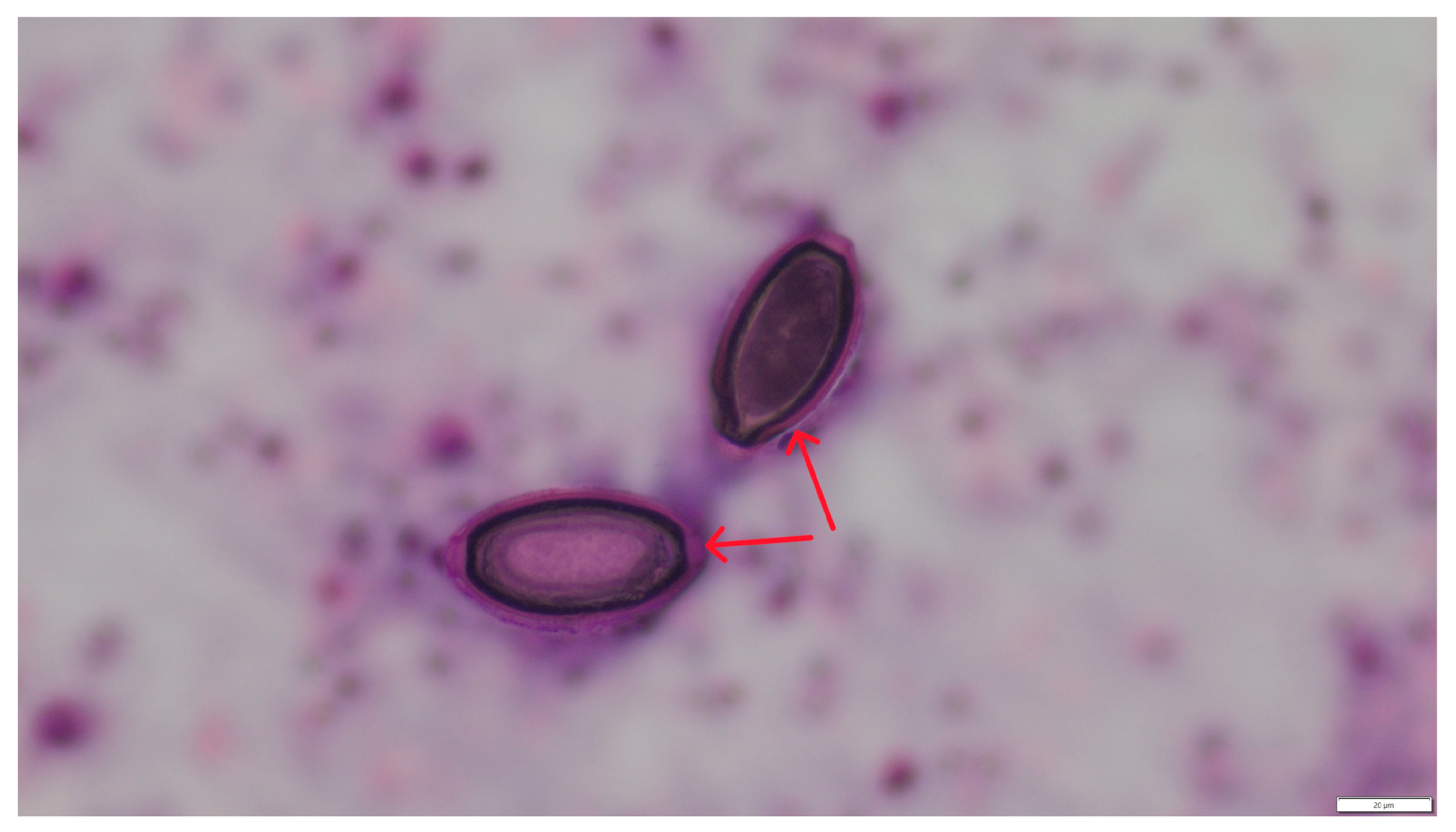
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAL | Bronchoalveoral lavage |
References
- Giannelli, A.; Capelli, G.; Joachim, A.; Hinney, B.; Losson, B.; Kirkova, Z.; René-Martellet, M.; Papadopoulos, E.; Farkas, R.; Napoli, E.; et al. Lungworms and gastrointestinal parasites of domestic cats: A European perspective. Int. J. Parasitol. Parasites Wildl. 2017, 47, 517–528. [Google Scholar] [CrossRef]
- Gerdin, J.A.; Slater, M.R.; Makolinski, K.V.; Looney, A.L.; Appel, L.D.; Martin, M.N.; McDonough, S.P. Post-mortem findings in 54 cases of anesthetic associated death in cats from two spay-neuter programs in New York State. J. Feline Med. Surg. 2011, 13, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Osario, S.; Navarro-Ruiz, J.L.; Rave, A.; Taubert, A.; Hermosilla, C.; Chaparro-Gutierrez, J.J. Aelurostrongylus abstrusus Infections in Domestic cats (Felis silvestris catus) from Antioquia, Colombia. Pathogens 2021, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Dzimira, S.; Popiołek, M. Case of aelurostrongylosis in a domestic cat. Med. Weter. 2005, 8, 894–895. [Google Scholar]
- Szczęsna, J.; Popiołek, M.; Schmidt, K.; Kowalczyk, R. The first record of Aelurostrongylus abstrusus (Angistrongylidae: Nematoda) in Eurasian lynx (Lynx lynx L.) from Poland, based on fecal analysis. Wiad. Parazytol. 2006, 52, 321–322. [Google Scholar]
- Studzińska, M.B.; Demkowska-Kutrzepa, M.; Komsta, R.; Tomczuk, K.; Kruczel, A.; Junkuszew, A.; Dudko, P. Aelurostrongylus abstrusus—A cause of respiratory disesase. Med. Weter. 2017, 73, 739–742. [Google Scholar] [CrossRef]
- Wierzbowska, A.I.; Kornaś, S.; Piontek, A.M.; Rola, K. The prevalence of endoparasites of free ranging cats (Felis catus) from urban habitats in Southern Poland. Animals 2020, 10, 748. [Google Scholar] [CrossRef]
- Anderson, R.C. Nematode Parasites of Vertebrates. Their Development and Transmission; CABI Publishing: Wallingford, UK, 2000; p. 672. [Google Scholar]
- Gundłach, J.L.; Sadzikowski, A.B. Parazytologia i Parazytozy Zwierząt; PWRiL: Warszawa, Poland, 2004. [Google Scholar]
- Traversa, D.; Di Cesare, A. Diagnosis and management of lungworm infections in cats. Cornerstones, dilemmas and new avenues. J. Feline Med. Surg. 2016, 18, 7–20. [Google Scholar] [CrossRef]
- Foreyt, W.J. Diagnostic parasitology. Vet. Clin. N. Am. Small Anim. Pract. 1989, 19, 979–1000. [Google Scholar] [CrossRef]
- Traversa, D.; Lia, R.P.; Iorio, R.; Boari, A.; Paradies, P.; Capelli, G.; Avolio, S.; Otranto, D. Diagnosis and risk factors of Aelurostrongylus abstrusus (Nematoda, Strongylida) infection in cats from Italy. Vet. Parsitol. 2008, 153, 182–186. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A. Feline lungworms: What a dilemma. Trends Parasitol. 2013, 29, 423–429. [Google Scholar] [CrossRef]
- Di Cesare, A.; Di Francesco, G.; Frangipane di Regalbono, A.; Eleni, C.; De Liberato, C.; Marruchella, G.; Iorio, R.; Malatesta, D.; Romanucci, M.R.; Bongiovanni, L.; et al. Retrospective study on the occurrence of the feline lungworms Aelurostrongylus abstrusus and Troglostrongylus spp. in endemic areas of Italy. Vet. J. 2015, 203, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, K.; Leśniak, P.; Studzińska, M.; Roczeń-Karczmarz, M.; Demkowska-Kutrzepa, M.; Junkuszew, A.; Tomczuk, K. Occurence of larvae of Metastrongylidae in faeces of cats from southwestern Poland. Med. Weter. 2019, 75, 605–608. [Google Scholar] [CrossRef]
- Mircean, V.; Titilincu, A.; Vasile, C. Prevalence of endoparasites in household cat (Felis catus) populations from Transylvania (Romania) and association with risk factors. Vet. Parasitol. 2010, 171, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Barutzki, D.; Schaper, R. Occurrence and regional distribution of Aelurostrongylus abstrusus in cats in Germany. Parasitol. Res. 2012, 112, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.C.; Rohen, M.; Epe, C.; Schnieder, T. Prevalence of endoparasites in stray and fostered dogs and cats in Northern Germany. Parasitol. Res. 2012, 111, 849–857. [Google Scholar] [CrossRef]
- Kiszely, S.; Gyurkovszky, M.; Solymosi, N.; Farkas, R. Survey of lungworm infection of domestic cats in Hungary. Acta Vet. Hun 2019, 67, 407–417. [Google Scholar] [CrossRef]
- Knaus, M.; Kusi, I.; Rapti, D.; Xhaxhiu, D.; Winter, R.; Visser, M.; Rehbein, S. Endoparasites of cats from the Tirana area and the first report on Aelurostrongylus abstrusus (Railliet, 1898) in Albania. Wien. Klin. Wochenschr. 2011, 123, 31–35. [Google Scholar] [CrossRef]
- Salling Olsen, C.; Willesen, J.L.; Pipper, C.B.; Mejer, H. Occurrence of Aelurostrongylus abstrusus (Railliet, 1898) in Danish cats: A modified lung digestion method for isolating adult worms. Vet. Parasitol. 2015, 210, 32–39. [Google Scholar] [CrossRef]
- Grandi, G.; Comin, A.; Ibrahim, O.; Schaper, R.; Forshell, U.; Osterman Lind, E. Prevalence of helminth and coccidian parasites in Swedish outdoor cats and the first report of Aelurostrongylus abstrusus in Sweden: A coprological investigation. Acta Vet. Scan 2017, 59, 19. [Google Scholar] [CrossRef]
- Waap, H.; Gomes, J.; Nunes, T. Parasite communities in stray cat populations from Lisbon, Portugal. J. Helminthol. 2014, 88, 389–395. [Google Scholar] [CrossRef]
- Tull, A.; Moks, E.; Saarma, U. Endoparasite prevalence and infection risk factors among cats in an animal shelter in Estonia. Folia Parasitol. 2021, 68, 010. [Google Scholar] [CrossRef] [PubMed]
- Payo-Puente, P.; Botelho-Dinis, M.; Carvaja Urueña, A.M.; Payo-Puente, M.; Gonzalo-Orden, J.M.; Rojo-Vazquez, F.A. Prevalence study of the lungworm Aelurostrongylus abstrusus in stray cats of Portugal. J. Feline Med. Surg. 2008, 10, 242–246. [Google Scholar] [CrossRef]
- Carruth, A.J.; Buch, J.S.; Braff, J.C.; Chandrashekar, R.; Bowman, D.D. Distribution of the feline lungworm Aelurostrongylus abstrusus in the USA based on fecal testing. J. Feline Med. Surg. Open Rep. 2019, 5, 2055116919869053. [Google Scholar] [CrossRef]
- Willard, M.D.; Roberts, R.E.; Allison, N.; Grieve, R.B.; Escher, K. Diagnosis of Aelurostrongylus abstrusus and Dirofilaria immitis infections in cats from a human shelter. J. Am. Vet. Med. Assoc. 1988, 192, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Elsheikha, H.M.; Schnyder, M.; Traversa, D.; Di Cesare, A.; Wright, I.; Lacher, D.W. Updates on feline aelurostrongylosis and research priorities for the next decade. Parasit. Vectors 2016, 9, 389. [Google Scholar] [CrossRef]
- Hawley, M.M.; Johnson, L.; Traversa, D.; Bucy, D.; Vernau, K.W.; Vernau, W. Respiratory distress associated with lungworm infection in a kitten. J. Feline Med. Surg. Open Rep. 2016, 2, 2055116916675801. [Google Scholar] [CrossRef] [PubMed]
- Khatat, S.E.; Rosenberg, D.; Benchekroun, G.; Polack, P. Lungworm Eucoleus aerophilus (Capillaria aerophila) infection in a feline immunodeficiency virus-positive cat in France. J. Feline Med. Surg. Open Rep. 2016, 2, 2055116916651649. [Google Scholar] [CrossRef]
- Napoli, E.; Pugliese, M.; Basile, A.; Passantino, A.; Brianti, E. Clinical, Radiological, and Echocardiographic Findings in Cats Infected by Aelurostrongylus abstrusus. Pathogens 2023, 12, 273. [Google Scholar] [CrossRef]
- Vezzosi, T.; Perrucci, S.; Parisi, F.; Morelli, S.; Maestrini, M.; Mennuni, G.; Traversa, D.; Poli, A. Fatal pulmonary hypertension and right-sided congestive heart failure in a cat infected with Aelurostrongylus abstrusus. Animals 2020, 10, 2263. [Google Scholar] [CrossRef]
- Barrs, V.R.; Swinney, G.R.; Martin, P.; Nicoll, R.G. Concurrent Aelurostrongylus abstrusus infection and salmonellosis in a kitten. Aust. Vet. J. 1999, 77, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Crisi, P.E.; Aste, G.; Traversa, D.; Di Cesare, A.; Febo, E.; Vignoli, M.; Santori, D.; Luciani, A.; Boari, A. Single and mixed feline lungworm infections: Clinical, radiographic and therapeutic features of 26 cases (2013–2015). J. Feline Med. Surg. 2017, 19, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, K.; Tomczuk, K.; Adaszek, Ł. Parazytologia w Praktyce. Pasożyty Wewnętrzne Psów i Kotów; Elamed: Katowice, Poland, 2025. [Google Scholar]
- Schnyder, M.; Tanner, I.; Webster, P.; Barutzki, D.; Deplazes, P. An ELISA for sensitive and specific detection of circulating antigen of Angiostrongylus vasorum in serum samples of naturally and experimentally infected dogs. Vet. Parasitol. 2011, 179, 152–158. [Google Scholar] [CrossRef] [PubMed]


| No. | Country | Study Methods/Techniques | Number of Cats in Study | Number and Percent of Positive Cats | References |
|---|---|---|---|---|---|
| 1 | Poland (southwestern region) | Autopsy/histopathology | 1 (case report) | 1/(100%) A | Dzimira et al., 2005 [4] |
| 2 | Italy (central and southern regions) | floatations with sugar and zinc sulphate solutions and a Baermann technique | 227 | 8/(17.3%) A 12/(18.5%) A | Traversa et al., 2008 [12] |
| 3 | Portugal | Baermann-Wetzel method | 97 | 17/(17.4%) A | Payo-Puente et al. 2008 [25] |
| 4 | Romania | Sodium chloride flotation | 414 | 23/(5.6%) A 13/(3.1%) C | Mircean et al. 2010 [16] |
| 5 | Albania | Autopsy/histopathology | 18 | 9/(50%) A 3/(16.7%) C | Knaus et al., 2011 [20] |
| 6 | Germany | zinc chloride/sodium chloride flotation and Baermann technique | 391 | 24/(6.6%) A | Barutzki et al., 2012 [17] |
| 7 | Germany | Baermann-Wetzel method | 837 | 8/(1%) A | Becker et al., 2012 [18] |
| 8 | Portugal | Flotation and sedimentation techniques | 162 | (12.4%) A (0.6%) C | Waap et al., 2014 [23] |
| 9 | Denmark | modified Baermann technique | 147 | 17/(13.6%) A | Salling Olsen et al., 2015 [21] |
| 10 | Poland (southeastern region) | flotation, decantation technique, and Baermann’s larvoscopic method. | 716 | 7/(0.98%) A | Szczepaniak et al., 2017 [15] |
| 11 | 12 European countriess (Austria, Belgium, Bulgaria, France, Greece, Hungary, Portugal, Romania, Spain, Italy, Switzerland, Great Britain) | The McMaster technique and a quantitative Baermann-Wetzel method | 1990 | 210/(10.6%) CO | Gianelli et al., 2017 [1] |
| 12 | Sweden | Baermann’s larvoscopic method. | 205 | 1/(0.48%) A | Grandi et al., 2017 [22] |
| 13 | Hungary | Baermann-Wetzel method | 303 | 60/(19.8%) A | Kiszely et al., 2019 [19] |
| 14 | Poland (southern region) | McMaster technique | 81 | 2/(2.47%) C | Wierzbowska et al., 2020 [7] |
| 15 | Estonia | flotation technique | 290 | 6/(2.1%) C | Tull et al., 2021 [24] |
| 16 | Poland (south and southwestern region) | Autopsy/histopathology | 420 | 4/(0.95%) A | Our study |
| 17 | Poland (south and southwestern region) | Cytology (BAL) | 310 | 2/(0.64%) CO | Our study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzimira, S.; Kandefer-Gola, M.; Ciaputa, R.; Demkowska-Kutrzepa, M. Cases of Lungworm in Cats from Southern Poland in the Autopsy and Cytological Material. Pathogens 2025, 14, 630. https://doi.org/10.3390/pathogens14070630
Dzimira S, Kandefer-Gola M, Ciaputa R, Demkowska-Kutrzepa M. Cases of Lungworm in Cats from Southern Poland in the Autopsy and Cytological Material. Pathogens. 2025; 14(7):630. https://doi.org/10.3390/pathogens14070630
Chicago/Turabian StyleDzimira, Stanisław, Małgorzata Kandefer-Gola, Rafał Ciaputa, and Marta Demkowska-Kutrzepa. 2025. "Cases of Lungworm in Cats from Southern Poland in the Autopsy and Cytological Material" Pathogens 14, no. 7: 630. https://doi.org/10.3390/pathogens14070630
APA StyleDzimira, S., Kandefer-Gola, M., Ciaputa, R., & Demkowska-Kutrzepa, M. (2025). Cases of Lungworm in Cats from Southern Poland in the Autopsy and Cytological Material. Pathogens, 14(7), 630. https://doi.org/10.3390/pathogens14070630









