3-Bromopyruvate Impairs Mitochondrial Function in Trypanosoma cruzi
Abstract
1. Introduction
2. Materials and Methods
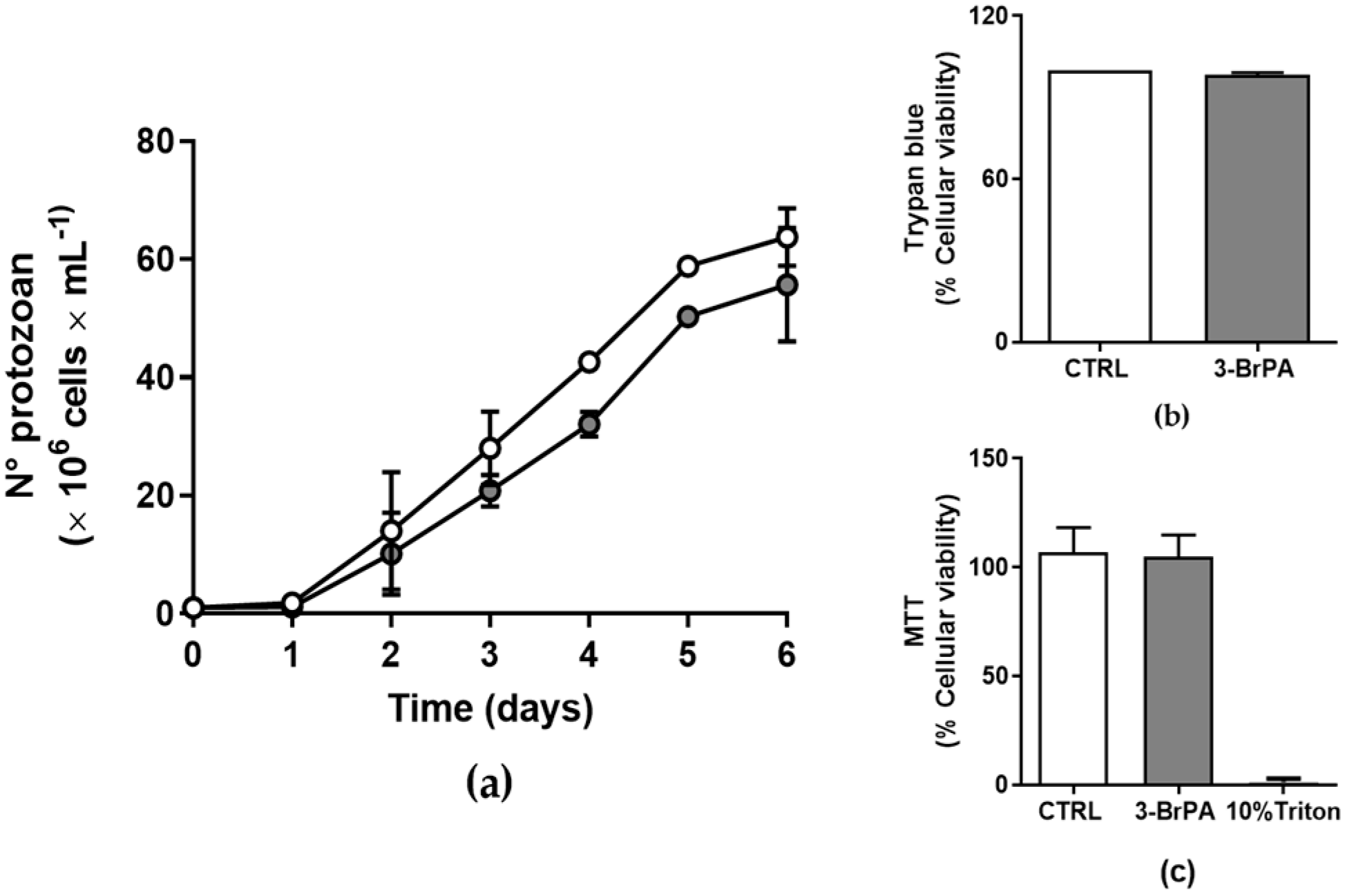
2.1. Cell Culture and Proliferation Profile
2.2. Cell Viability
2.3. Hypoxic Induction
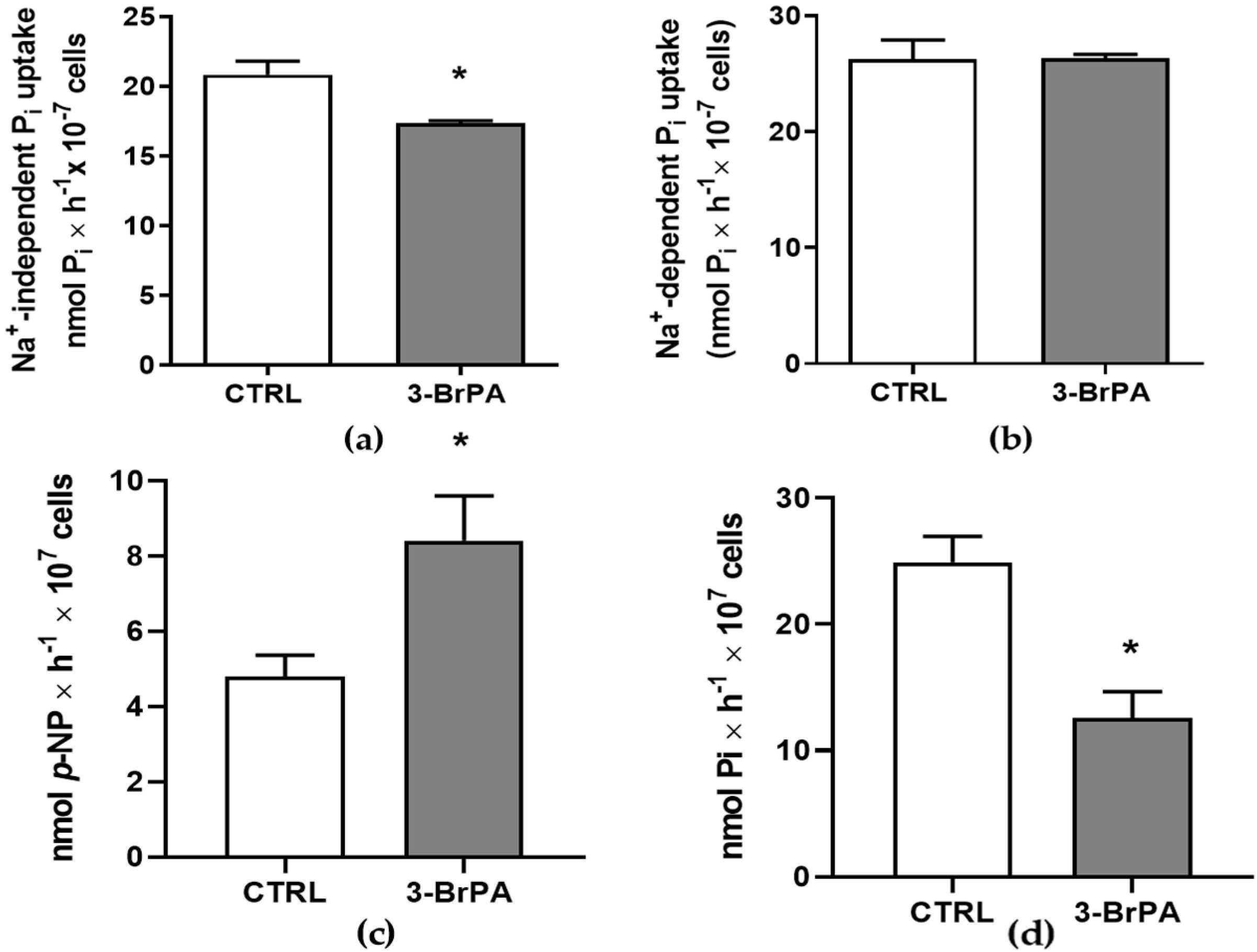
2.4. Inorganic Phosphate Transport
2.5. Ecto-Phosphatase Activity
2.6. Ecto-5′-Nucleotidase Activity
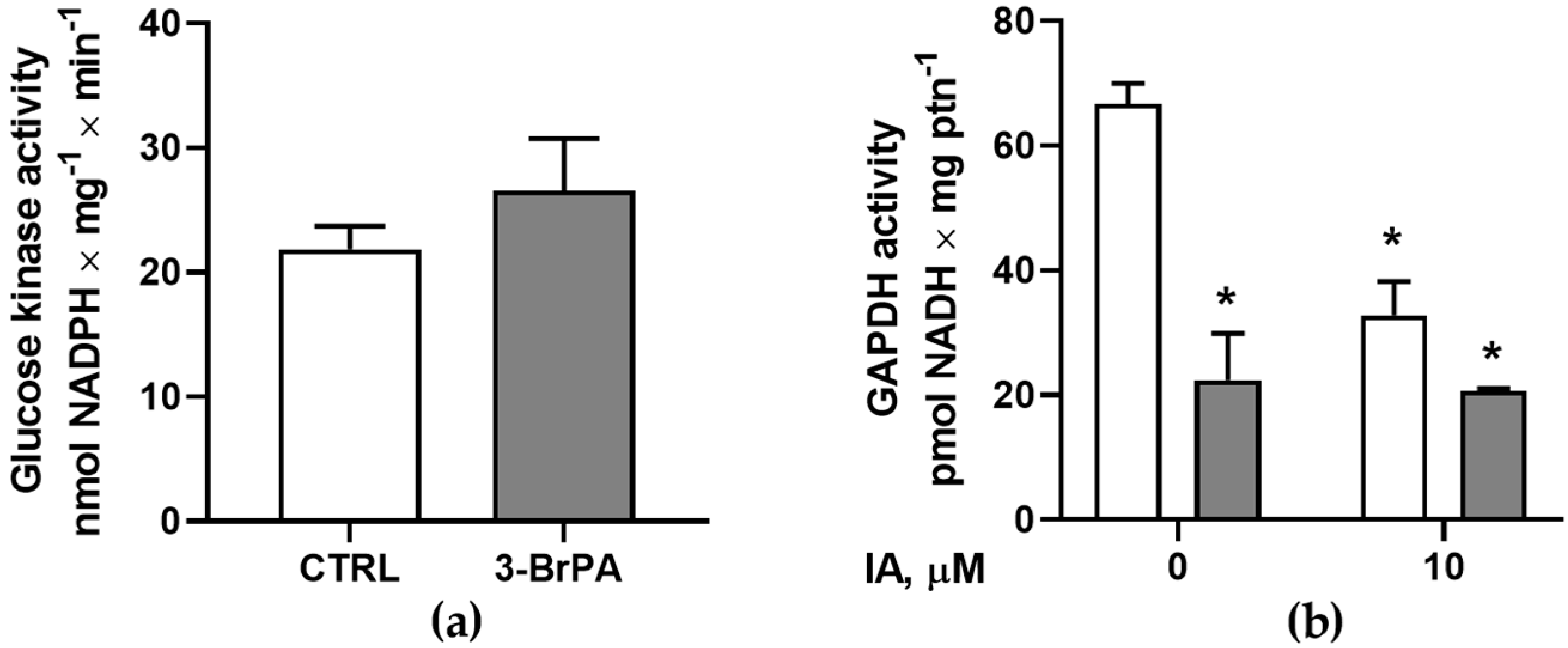
2.7. Glucokinase Activity
2.8. Total Glyceraldehyde 3-Phosphate Dehydrogenase Activity
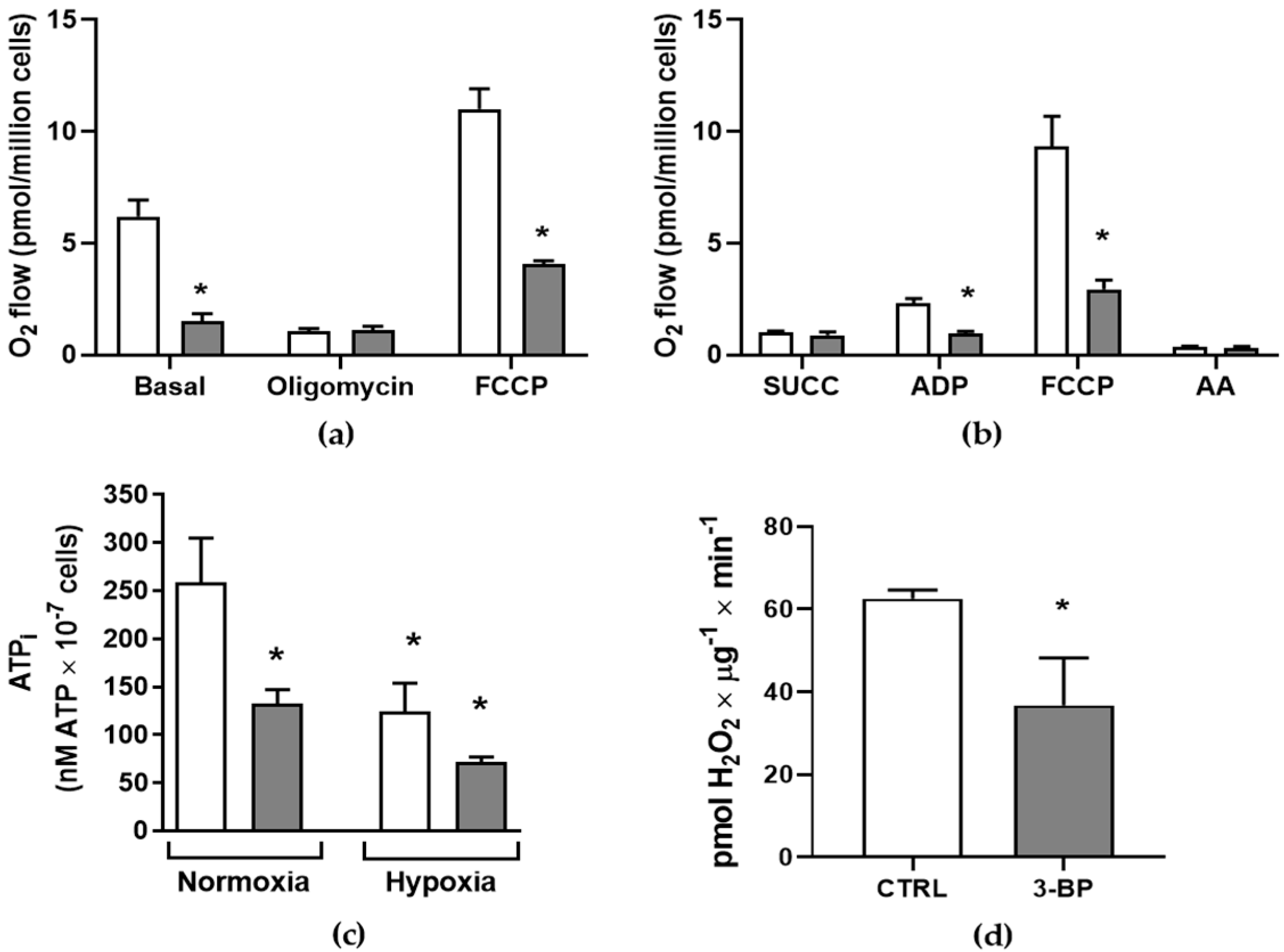
2.9. Intracellular ATP Measurement
2.10. High-Resolution Respirometry
2.11. Amplex® Red Peroxidase Assay
2.12. Immunofluorescence Microscopy
2.13. Statistical Analysis
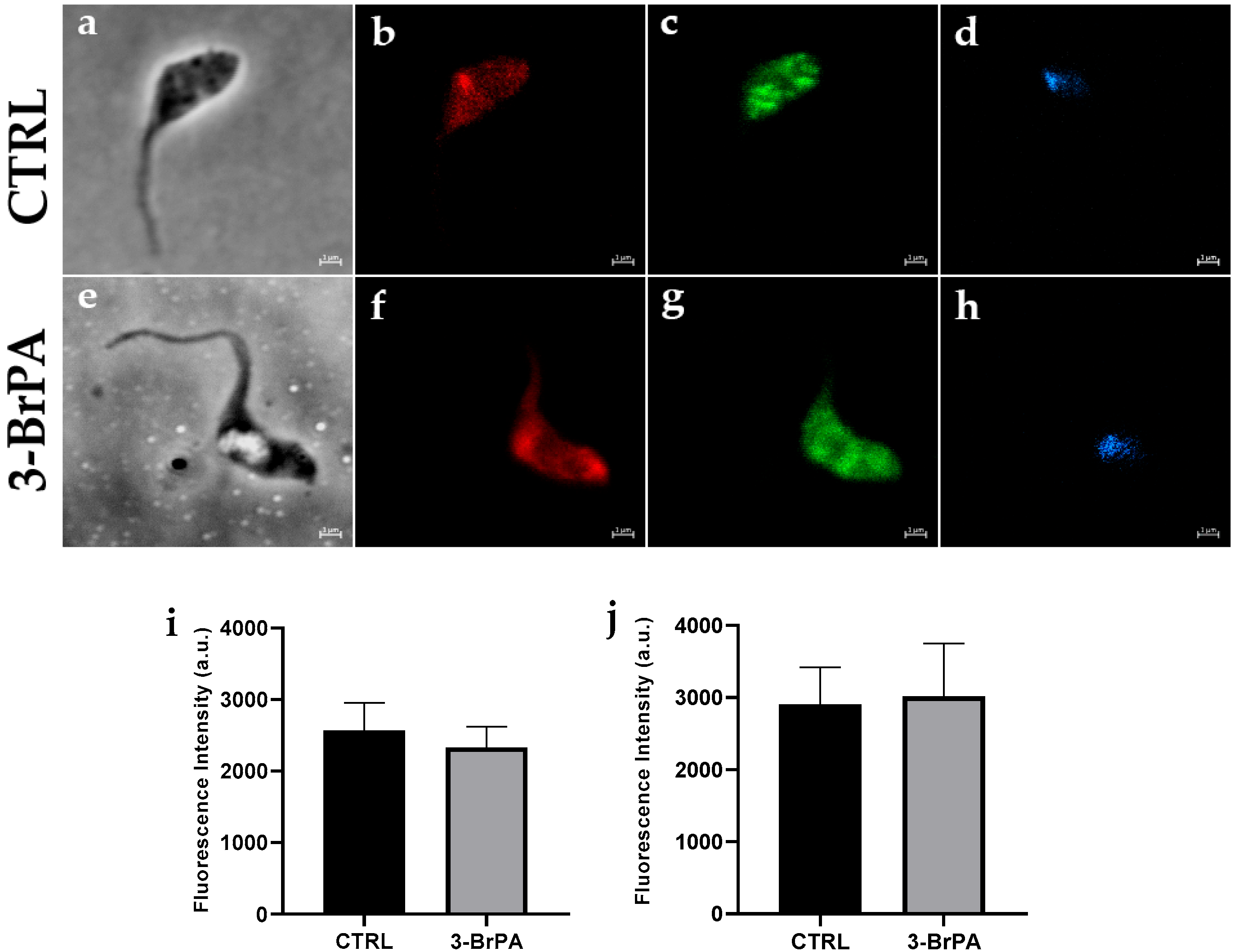
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chagas, C. Nova tripanozomiase humana. Estudos sobre a morfolojía e o ciclo evolutivo de Schizotrypanum cruzi n. gen., n. sp., ajente etiolójico de nova entidade morbida do homen. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- World Health Organization. Chagas Disease (Also Known as American Trypanosomiasis). 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 5 April 2025).
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde (2021) Doença de Chagas. Special Number. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2021/boletim_especial_chagas_14abr21_b.pdf/view (accessed on 10 June 2025).
- Torrico, F.; Alonso-Veja, C.; Suarez, E.; Rodriguez, P.; Torrico, M.C.; Dramaix, M.; Truyens, C.; Carlier, Y. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am. J. Trop. Med. Hyg. 2004, 70, 201–209. [Google Scholar] [CrossRef] [PubMed]
- U.S. Centers for Disease Control and Prevention Chagas Disease. Available online: https://www.cdc.gov/chagas/index.html (accessed on 5 April 2025).
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R.; Junqueira, A.C.; Fernandes, O.; Valente, S.A.; Miles, M.A. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 2002, 18, 171–176. [Google Scholar] [CrossRef]
- Pereira, K.S.; Schmidt, F.L.; Barbosa, R.L.; Guaraldo, A.M.; Franco, R.M.; Dias, V.L.; Passos, L.A. Transmission of chagas disease (American trypanosomiasis) by food. Adv. Food Nutr. Res. 2010, 59, 63–85. [Google Scholar]
- Baker, J.P.; Rabin, B.R. Effects of bromopyruvate on the control and catalytic properties of glutamate dehydrogenase. Eur. J. Biochem. 1969, 11, 154–159. [Google Scholar] [CrossRef]
- Fonda, M.L.; DeGrella, R.F. Inactivation of glutamate decarboxylase by bromopyruvate. Biochem. Biophys. Res. Commun. 1974, 56, 451–458. [Google Scholar] [CrossRef]
- Geschwind, J.F.; Ko, Y.H.; Torbenson, M.S.; Magee, C.; Pedersen, P.L. Novel therapy for liver cancer: Direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002, 62, 3909–3913. [Google Scholar]
- Chen, Z.; Zhang, H.; Lu, W.; Huang, P. Role of mitochondria-associated hexoquinase II in cancer cell death induced by 3-bromopyruvate. Biochim. Biophys. Acta 2009, 1787, 553–560. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 2006, 25, 4777–4786. [Google Scholar] [CrossRef]
- Dell’Antone, P. Targets of 3-bromopyruvate, a new, energy depleting, anticancer agent. Med. Chem. 2009, 5, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy-Kanniappan, S.; Geschwind, J.F.; Kunjithapatham, R.; Buijs, M.; Vossen, J.A.; Tchernyshyov, I.; Cole, R.N.; Syed, L.H.; Rao, P.P.; Ota, S.; et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009, 29, 4909–4918. [Google Scholar] [PubMed]
- Pereira da Silva, A.P.; El-Bacha, T.; Kyaw, N.; dos Santos, R.S.; da-Silva, W.S.; Almeida, F.C.; Da Poian, A.T.; Galina, A. Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem. J. 2009, 417, 717–726. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Dick, C.F.; Meyer-Fernandes, J.R. The Role of Inorganic Phosphate Transporters in Highly Proliferative Cells: From Protozoan Parasites to Cancer Cells. Membranes 2022, 13, 42. [Google Scholar] [CrossRef]
- Lis, P.; Zarzycki, M.; Ko, Y.H.; Casal, M.; Pedersen, P.L.; Goffeau, A.; Ułaszewski, S. Transport and cytotoxicity of the anticancer drug 3-bromopyruvate in the yeast Saccharomyces cerevisiae. J. Bioenerg. Biomembr. 2012, 44, 155–161. [Google Scholar] [CrossRef]
- Dyląg, M.; Lis, P.; Niedźwiecka, K.; Ko, Y.H.; Pedersen, P.L.; Goffeau, A.; Ułaszewski, S. 3-Bromopyruvate: A novel antifungal agent against the human pathogen Cryptococcus neoformans. Biochem. Biophys. Res. Commun. 2013, 434, 322–327. [Google Scholar] [CrossRef]
- de Lima, L.P.; Seabra, S.H.; Carneiro, H.; Barbosa, H.S. Effect of 3-bromopyruvate and atovaquone on infection during in vitro interaction of Toxoplasma gondii and LLC-MK2 cells. Antimicrob. Agents Chemother. 2015, 59, 5239–5249. [Google Scholar] [CrossRef]
- Barnard, J.P.; Reynafarje, B.; Pedersen, P.L. Glucose catabolism in African trypanosomes. Evidence that the terminal step is catalyzed by a pyruvate transporter capable of facilitating uptake of toxic analogs. J. Biol. Chem. 1993, 268, 3654–3661. [Google Scholar] [CrossRef]
- Vanderheyden, N.; Wong, J.; Docampo, R. A pyruvate-proton symport and an H+-ATPase regulate the intracellular pH of Trypanosoma brucei at different stages of its life cycle. Biochem. J. 2000, 346, 53–62. [Google Scholar] [CrossRef]
- Martínez-Flórez, A.; Galizzi, M.; Izquierdo, L.; Bustamante, J.M.; Rodriguez, A.; Rodriguez, F.; Rodríguez-Cortés, A.; Alberola, J. Repurposing bioenergetic modulators against protozoan parasites responsible for tropical diseases. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 17–27. [Google Scholar] [CrossRef]
- Gomes, M.T.; Paes-Vieira, L.; Gomes-Vieira, A.L.; Cosentino-Gomes, D.; da Silva, A.P.P.; Giarola, N.L.L.; Da Silva, D.; Sola-Penna, M.; Galina, A.; Meyer-Fernandes, J.R. 3-Bromopyruvate: A new strategy for inhibition of glycolytic enzymes in Leishmania amazonensis. Exp. Parasitol. 2021, 229, 108154. [Google Scholar] [CrossRef]
- Buijs, M.; Vossen, J.A.; Geschwind, J.F.; Ishimori, T.; Engles, J.M.; Acha-Ngwodo, O.; Wahl, R.L.; Vali, M. Specificity of the anti-glycolytic activity of 3-bromopyruvate confirmed by FDG uptake in a rat model of breast cancer. Investig. New Drugs 2009, 27, 120–123. [Google Scholar] [CrossRef]
- Wilson, R.; Spier, R.E. Biochemistry of hybridoma technology. Dev. Biol. Stand. 1987, 66, 161–167. [Google Scholar]
- Saraiva, F.M.S.; Cosentino-Gomes, D.; Inacio, J.D.F.; Almeida-Amaral, E.E.; Louzada-Neto, O.; Rossini, A.; Nogueira, N.P.; Meyer-Fernandes, J.R.; Paes, M.C. Hypoxia Effects on Trypanosoma cruzi Epimastigotes Proliferation, Differentiation, and Energy Metabolism. Pathogens 2022, 11, 897. [Google Scholar] [CrossRef]
- Asady, B.; Dick, C.F.; Ehrenman, K.; Sahu, T.; Romano, J.D.; Coppens, I. A single Na+-Pi cotransporter in Toxoplasma plays key roles in phosphate import and control of parasite osmoregulation. PLoS Pathog. 2020, 16, e1009067. [Google Scholar] [CrossRef]
- Papadaki, A.; Boleti, H. Measurement of Acid Ecto-phosphatase Activity in Live Leishmania donovani Parasites. Bio Protoc. 2019, 9, e3384. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Chetri, P.B.; Shukla, R.; Tripathi, T. Identification and characterization of glyceraldehyde 3-phosphate dehydrogenase from Fasciola gigantica. Parasitol. Res. 2019, 118, 861–872. [Google Scholar] [CrossRef]
- Opperdoes, F.R.; Borst, P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: The glycosome. FEBS Lett. 1977, 80, 360–364. [Google Scholar] [CrossRef]
- Zechner, C.; Rhee, E.P. Phosphate sensing in health and disease. Curr. Opin. Nephrol. Hypertens. 2024, 33, 361–367. [Google Scholar] [CrossRef]
- Zuma, A.A.; Dos Santos Barrias, E.; de Souza, W. Basic Biology of Trypanosoma cruzi. Curr. Pharm. Des. 2021, 27, 1671–1732. [Google Scholar] [CrossRef]
- Silber, A.M.; Tonelli, R.R.; Lopes, C.G.; Cunha-e-Silva, N.; Torrecilhas, A.C.T.; Schumacher, R.I.; Colli, W.; Alves, M.J.M. Glucose uptake in the mammalian stages of Trypanosoma cruzi. Mol. Biochem. Parasitol. 2009, 168, 102–108. [Google Scholar] [CrossRef]
- Silva Dias Vieira, C.; Pinheiro Aguiar, R.; de Almeida Nogueira, N.P.; Costa Dos Santos Junior, G.; Paes, M.C. Glucose metabolism sustains heme-induced Trypanosoma cruzi epimastigote growth in vitro. PLoS Negl. Trop. Dis. 2023, 17, e0011725. [Google Scholar] [CrossRef]
- Ko, Y.H.; Smith, B.L.; Wang, Y.; Pomper, M.G.; Rini, D.A.; Torbenson, M.S.; Hullihen, J.; Pedersen, P.L. Advanced cancers: Eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem. Biophys. Res. Commun. 2004, 324, 269–275. [Google Scholar] [CrossRef]
- Wu, P.; Ma, L.; Hou, X.; Wang, M.; Wu, Y.; Liu, F.; Deng, X.W. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 2003, 132, 1260–1271. [Google Scholar] [CrossRef]
- Segal, H.L.; Boyer, P.D. The role of sulfhydryl groups in the activity of d-glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 1953, 204, 265–281. [Google Scholar] [CrossRef]
- Shoshan, M.C. 3-Bromopyruvate: Targets and outcomes. J. Bioenerg. Biomembr. 2012, 44, 7–15. [Google Scholar] [CrossRef]
- Gahura, O.; Hierro-Yap, C.; Zíková, A. Redesigned and reversed: Architectural and functional oddities of the trypanosomal ATP synthase. Parasitology 2021, 148, 1151–1160. [Google Scholar] [CrossRef]
- Stefano, G.B.; Kream, R.M. Glycolytic Coupling to Mitochondrial Energy Production Ensures Survival in an Oxygen Rich Environment. Med. Sci. Monit. 2016, 22, 2571–2575. [Google Scholar] [CrossRef]
- Quiñones, W.; Acosta, H.; Gonçalves, C.S.; Motta, M.C.M.; Gualdrón-López, M.; Michels, P.A.M. Structure, Properties, and Function of Glycosomes in Trypanosoma cruzi. Front. Cell Infect. Microbiol. 2020, 10, 25. [Google Scholar] [CrossRef]
- Bakker, B.M.; Mensonides, F.I.; Teusink, B.; van Hoek, P.; Michels, P.A.; Westerhoff, H.V. Compartmentation protects trypanosomes from the dangerous design of glycolysis. Proc. Natl. Acad. Sci. USA 2000, 97, 2087–2092. [Google Scholar] [CrossRef]
- Maugeri, D.A.; Cannata, J.J.; Cazzulo, J.J. Glucose metabolism in Trypanosoma cruzi. Essays Biochem. 2011, 51, 15–30. [Google Scholar]
- Souza, R.O.O.; Damasceno, F.S.; Marsiccobetre, S.; Biran, M.; Murata, G.; Curi, R.; Bringaud, F.; Silber, A.M. Fatty acid oxidation participates in resistance to nutrient-depleted environments in the insect stages of Trypanosoma cruzi. PLoS Pathog. 2021, 7, e1009495. [Google Scholar] [CrossRef]
- Lipiński, O.; Sonani, R.R.; Dubin, G. Crystal structure of glycerol kinase from Trypanosoma cruzi, a potential molecular target in chagas disease. Acta Crystallogr. D Struct. Biol. 2024, 80, 629–638. [Google Scholar] [CrossRef]
- Alencar, M.B.; Marsiccobetre, S.; Mengarda, A.C.; Ballari, M.S.; Silber, A.M. Energy metabolism in Trypanosoma cruzi: The validated and putative bioenergetic and carbon sources. mBio 2025, 20, e02215–e02224. [Google Scholar] [CrossRef]
- Sylvester, D.; Krassner, S.M. Proline metabolism in Trypanosoma cruzi epimastigotes. Comp. Biochem. Physiol. 1976, 55, 443–447. [Google Scholar] [CrossRef]
- Nolan, D.P.; Voorheis, H.P. The mitochondrion in bloodstream forms of Trypanosoma brucei is energized by the electrogenic pumping of protons catalysed by the F1F0-ATPase. Eur. J. Biochem. 1992, 209, 207–216. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, R.O.; Barreto-Campos, D.; Barbosa-de-Barros, J.; Frechiani, G.; Carvalho-Kelly, L.F.; Carvalho-de-Araújo, A.D.; Meyer-Fernandes, J.R.; Dick, C.F. 3-Bromopyruvate Impairs Mitochondrial Function in Trypanosoma cruzi. Pathogens 2025, 14, 631. https://doi.org/10.3390/pathogens14070631
da Costa RO, Barreto-Campos D, Barbosa-de-Barros J, Frechiani G, Carvalho-Kelly LF, Carvalho-de-Araújo AD, Meyer-Fernandes JR, Dick CF. 3-Bromopyruvate Impairs Mitochondrial Function in Trypanosoma cruzi. Pathogens. 2025; 14(7):631. https://doi.org/10.3390/pathogens14070631
Chicago/Turabian Styleda Costa, Rafaella Oliveira, Davi Barreto-Campos, Juliana Barbosa-de-Barros, Giovanna Frechiani, Luiz Fernando Carvalho-Kelly, Ayra Diandra Carvalho-de-Araújo, José Roberto Meyer-Fernandes, and Claudia Fernanda Dick. 2025. "3-Bromopyruvate Impairs Mitochondrial Function in Trypanosoma cruzi" Pathogens 14, no. 7: 631. https://doi.org/10.3390/pathogens14070631
APA Styleda Costa, R. O., Barreto-Campos, D., Barbosa-de-Barros, J., Frechiani, G., Carvalho-Kelly, L. F., Carvalho-de-Araújo, A. D., Meyer-Fernandes, J. R., & Dick, C. F. (2025). 3-Bromopyruvate Impairs Mitochondrial Function in Trypanosoma cruzi. Pathogens, 14(7), 631. https://doi.org/10.3390/pathogens14070631





