A Systematic Review on the Occurrence of Babesia spp. and Anaplasma spp. in Ticks and Wild Boar from Europe—A 15-Year Retrospective Study
Abstract
1. Introduction
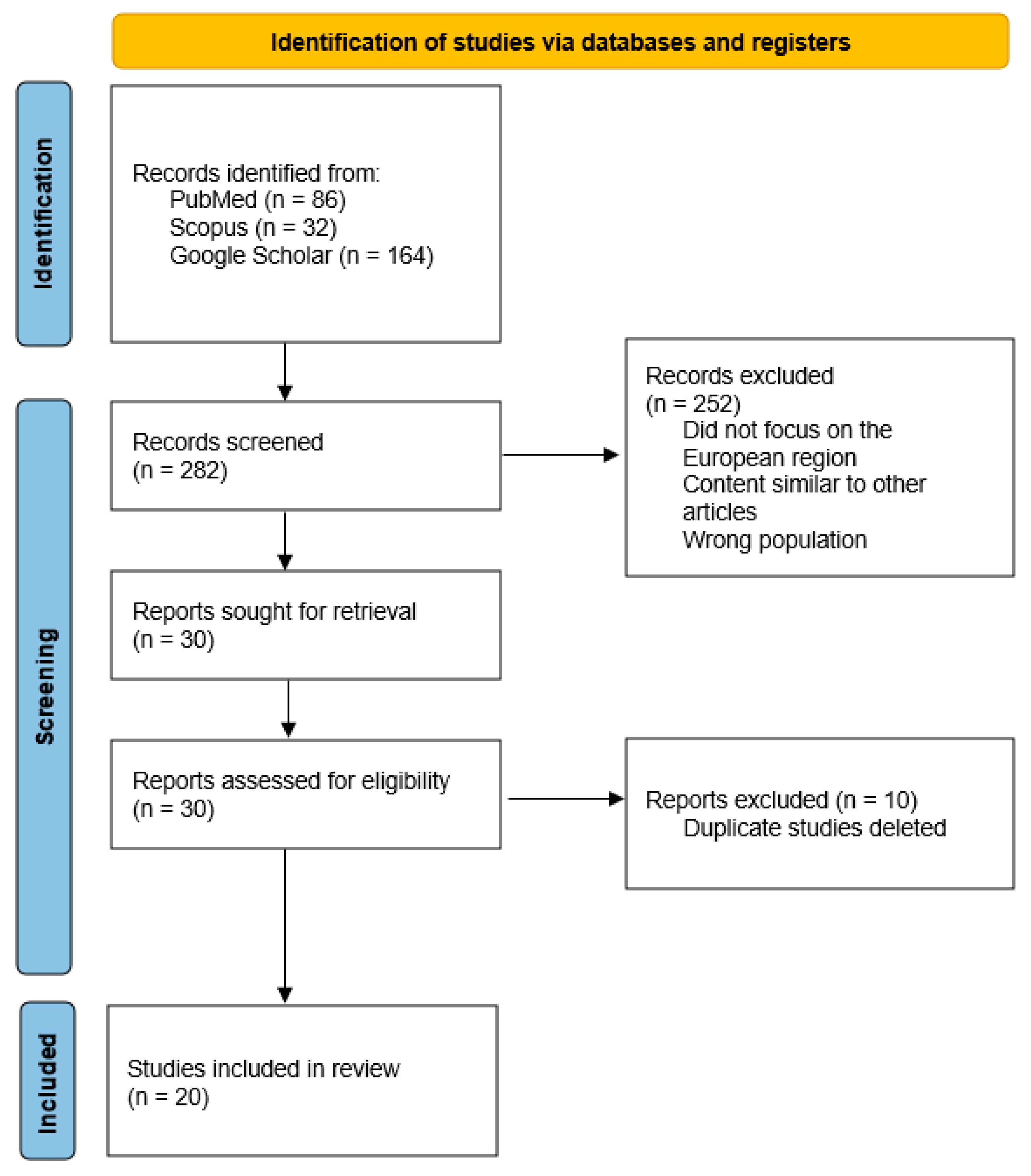
2. Materials and Methods
- Were conducted in Europe;
- Analyzed wild boars and/or their associated ticks;
- Reported the prevalence of Babesia spp. and/or Anaplasma spp. infections.
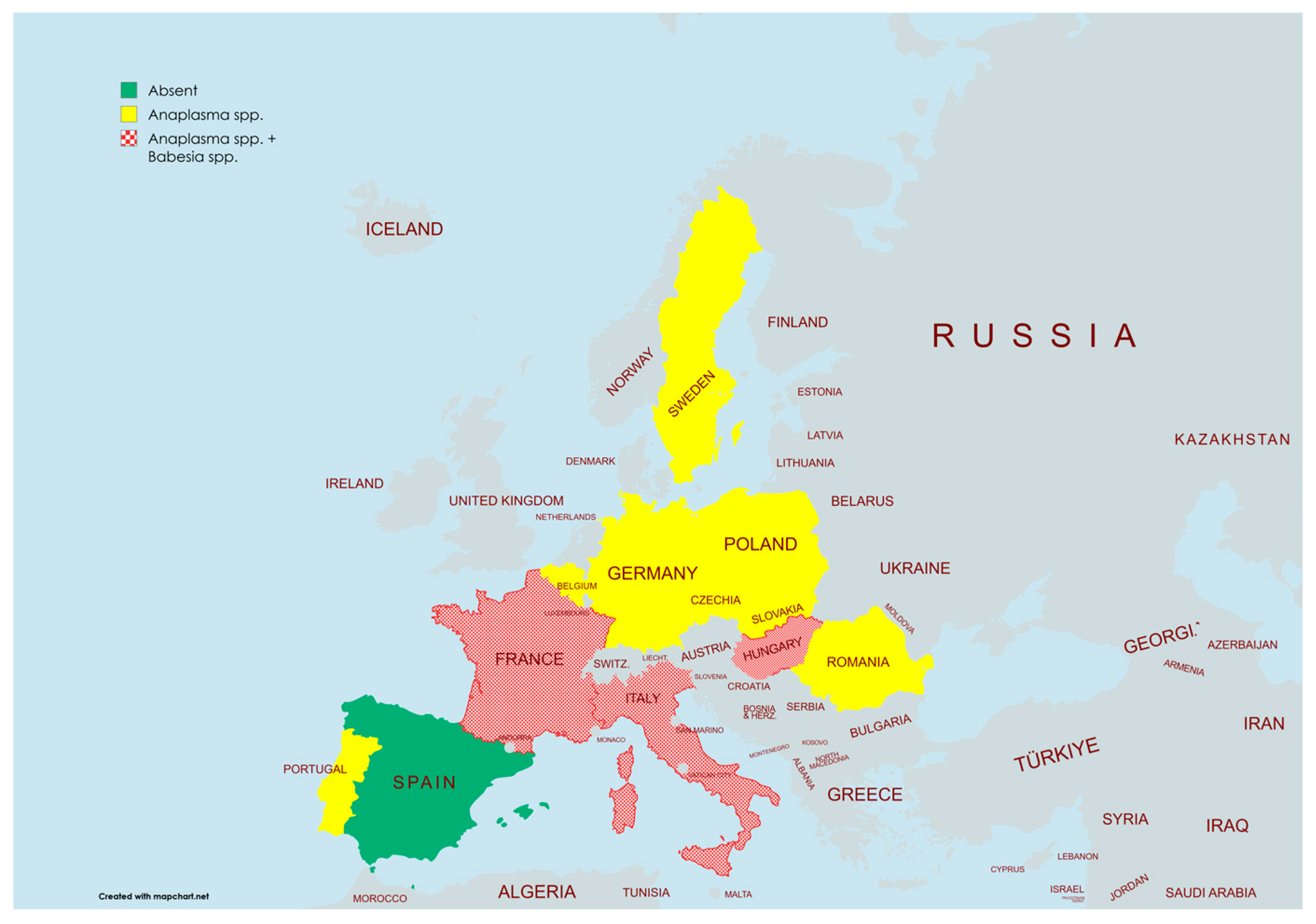
3. Results and Discussion
- Green (Absent): Countries where no Anaplasma spp. or Babesia spp. have been detected (e.g., Spain).
- Yellow (Anaplasma spp.): Countries where Anaplasma spp. has been detected but not Babesia spp. (e.g., Poland, Germany, Romania).
- Patterned Red (Anaplasma spp. + Babesia spp.): Countries where both Anaplasma spp. and Babesia spp. have been detected (e.g., France, Italy, Hungary).
General Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Stuen, S.; Scharf, W.; Schauer, S.; Freyburger, F.; Bergström, K.; von Loewenich, F.D. Experimental Infection in Lambs with a Red Deer (Cervus elaphus) Isolate of Anaplasma phagocytophilum. J. Wildl. Dis. 2010, 46, 803–809. [Google Scholar] [CrossRef]
- Ballari, S.A.; Barrios-García, M.N. A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges. Mammal Rev. 2014, 44, 124–134. [Google Scholar] [CrossRef]
- Massei, G.; Genov, P.V. The environmental impact of wild boar. Galemys 2004, 16, 135–145. [Google Scholar] [CrossRef]
- Pittiglio, C.; Khomenko, S.; Beltran-Alcrudo, D. Wild boar mapping using population-density statistics: From polygons to high resolution raster maps. PLoS ONE 2018, 13, e0193295. [Google Scholar] [CrossRef]
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and Their Management in the 21st Century; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Lewis, J.S.; Farnsworth, M.L.; Burdett, C.L.; Theobald, D.M.; Gray, M.; Miller, R.S. Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci. Rep. 2017, 7, 44152. [Google Scholar] [CrossRef] [PubMed]
- Sales, L.P.; Ribeiro, B.R.; Hayward, M.W.; Paglia, A.; Passamani, M.; Loyola, R. Niche conservatism and the invasive potential of the wild boar. J. Anim. Ecol. 2017, 86, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.L.; Burdett, C.L.; Farnsworth, M.L.; Lutman, M.W.; Theobald, D.M.; Riggs, P.D.; Grear, D.A.; Miller, R.S. Modeling and mapping the probability of occurrence of invasive wild pigs across the contiguous United States. PLoS ONE 2015, 10, e0133771. [Google Scholar] [CrossRef]
- Snow, N.P.; Jarzyna, M.A.; VerCauteren, K.C. Interpreting and predicting the spread of invasive wild pigs. J. Appl. Ecol. 2017, 54, 2022–2032. [Google Scholar] [CrossRef]
- Morelle, K.; Fattebert, J.; Mengal, C.; Lejeune, P. Invading or recolonizing? Patterns and drivers of wild boar population expansion into Belgian agroecosystems. Agric. Ecosyst. Environ. 2016, 222, 267–275. [Google Scholar] [CrossRef]
- Markov, N.; Pankova, N.; Morelle, K. Where winter rules: Modeling wild boar distribution in its north-eastern range. Sci. Total Environ. 2019, 687, 1055–1064. [Google Scholar] [CrossRef]
- Bieber, C.; Ruf, T. Population dynamics in wild boar Sus scrofa: Ecology, elasticity of growth rate and implications for the management of pulsed resource consumers. J. Appl. Ecol. 2005, 42, 1203–1213. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Jędrzejewska, B.; Okarma, H.; Ruprecht, A.L. Wolf predation and snow cover as mortality factors in the ungulate community of the Białowieża National Park, Poland. Oecologia 1992, 90, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Hipólito, D.; Teixeira, D.; Fonseca, C.; Tinoco, T.R. Hunting bag statistics of wild mammals in Portugal (1989–2022): On the need to improve data report and compilation. Eur. J. Wildl. Res. 2024, 70, 96. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, C.; Fernández-López, J.; Vicente, J.; Blanco-Aguiar, J.A.; Acevedo, P. Revisiting wild boar spatial models based on hunting yields to assess their predictive performance on interpolation and extrapolation areas. Ecol. Model. 2022, 471, 110041. [Google Scholar] [CrossRef]
- Bergqvist, G.; Kindberg, J.; Elmhagen, B. From virtually extinct to superabundant in 35 years: Establishment, population growth and shifts in management focus of the Swedish wild boar (Sus scrofa) population. BMC Zool. 2024, 9, 14. [Google Scholar] [CrossRef]
- Kopij, G. Population density of the wild boar (Sus scrofa) in south-western Poland in 1981–2020. Theriologia Ukrainica 2022, 24, 171–183. [Google Scholar] [CrossRef]
- Yin, H.; Luo, J.; Liu, Z.; Yang, J.; Mu, Y.; Zhang, P.; Guan, G. Babesiosis in domestic animals in China: Epidemiology and diagnosis. Transbound. Emerg. Dis. 2020, 67, 1294–1307. [Google Scholar]
- Hikosaka, K.; Nakamura, Y.; Tateno, M.; Matsuu, A.; Asada, M. A new species of Babesia infective to domestic pigs. J. Vet. Med. Sci. 2015, 77, 1655–1657. [Google Scholar]
- René-Martellet, M.; Lebert, I.; Chêne, J.; Massot, R.; Leon, M.; Leal, A.; Badavelli, S.; Chalvet-Monfray, K.; Ducrot, C.; Abrial, D.; et al. Diagnosis and incidence risk of clinical canine monocytic ehrlichiosis under field conditions in Southern Europe. Parasit. Vectors 2015, 8, 3. [Google Scholar] [CrossRef]
- de Waal, D.T. Porcine babesiosis. In Infectious Diseases of Livestock; Coetzer, J.A.W., Thomson, G.E., Maclachlan, N.J., Penrith, M.L., Eds.; Anipedia: Pretoria, South Africa, 2019. [Google Scholar]
- Dumitrache, M.O.; Matei, I.A.; Ionică, A.M.; Kalmár, Z.; Sándor, A.D.; Mărcuțan, I.D.; Gherman, C.M. Molecular detection and characterization of Anaplasma species in wild boars (Sus scrofa) from North-Eastern Romania. Parasit Vectors 2006, 11, 1–9. [Google Scholar]
- Galon, N.; Kohn, B.; Burridge, M.J. Anaplasma phagocytophilum and Anaplasma marginale: Comparison of nucleotide sequences for phylogenetic studies. BMC Microbiol. 2018, 18, 1–10. [Google Scholar]
- Mărcuţan, I.D.; Mihalca, A.D.; D’Amico, G.; Sándor, A.D.; Modrý, D.; Gherman, C.M. Molecular Detection of Anaplasma phagocytophilum in Wild Boars (Sus scrofa) from Romania. Vet. Parasitol. 2016, 228, 98–101. [Google Scholar]
- Pilloux, L.; Baumgartner, A.; Jaton, K.; Lienhard, R.; Ackermann-Gäumann, R.; Beuret, C.; Greub, G. Prevalence of Anaplasma phagocytophilum and Coxiella burnetii in Ixodes ricinus Ticks in Switzerland: An Underestimated Epidemiologic Risk. New Microbes New Infect. 2019, 27, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Scharf, W.; Schauer, S.; Freyburger, F.; Petrovec, M.; Schaarschmidt-Kiener, D.; Liebisch, G.; Runge, M.; Ganter, M.; Kehl, A.; Dumler, J.S.; et al. Distinct Host Species Correlate with Anaplasma phagocytophilum ankA Gene Clusters. J. Clin. Microbiol. 2011, 49, 790–796. [Google Scholar] [CrossRef]
- Rikihisa, Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: Subversive Manipulators of Host Cells. Nat. Rev. Microbiol. 2010, 8, 328–339. [Google Scholar] [CrossRef]
- Popov, V.L.; Han, V.C.; Chen, S.M.; Dumler, J.S.; Feng, H.M.; Andreadis, T.G.; Tesh, R.B.; Walker, D.H. Ultrastructural Differentiation of the Genogroups in the Genus Ehrlichia. J. Med. Microbiol. 1998, 47, 235–251. [Google Scholar] [CrossRef]
- Troese, M.J.; Kahlon, A.; Ragland, S.A.; Ottens, A.K.; Ojogun, N.; Nelson, K.T.; Walker, N.J.; Borjesson, D.L.; Carlyon, J.A. Proteomic Analysis of Anaplasma phagocytophilum During Infection of Human Myeloid Cells Identifies a Protein that is Pronouncedly Upregulated on the Infectious Dense-Cored Cell. Infect. Immun. 2011, 79, 4696–4707. [Google Scholar] [CrossRef]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Mal Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- de la Fuente, J.; Contreras, M. Tick vaccines: Current status and future directions. Expert Rev. Vaccines 2015, 14, 1367–1376. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Gray, J.S.; Kahl, O.; Lane, R.S.; Nijhof, A.M. Research on the ecology of ticks and tick-borne pathogens—methodological principles and caveats. Front. Cell Infect Microbiol. 2013, 3, 29. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013, 6, 1. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Clin. Res. Ed. 2021, 372, n71. [Google Scholar]
- MapChart. Available online: https://www.mapchart.net/europe.html (accessed on 16 March 2025).
- Defaye, B.; Moutailler, S.; Pietri, C.; Galon, C.; Grech-Angelini, S.; Pasqualini, V.; Quilichini, Y. Molecular Detection of Zoonotic and Non-Zoonotic Pathogens from Wild Boars and Their Ticks in the Corsican Wetlands. Pathogens 2021, 10, 1643. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Szekeres, S.; Horváth, G.; Takács, N.; Bekő, K.; Kontschán, J.; Gyuranecz, M.; Tóth, B.; Sándor, A.D.; Juhász, A.; et al. Diversity of Tick Species and Associated Pathogens on Peri-Urban Wild Boars—First Report of the Zoonotic Babesia cf. crassa from Hungary. Ticks Tick Borne Dis. 2022, 13, 101936. [Google Scholar] [CrossRef] [PubMed]
- Zanet, S.; Trisciuoglio, A.; Bottero, E.; de Mera, I.G.; Gortazar, C.; Carpignano, M.G.; Ferroglio, E. Piroplasmosis in Wildlife: Babesia and Theileria Affecting Free-Ranging Ungulates and Carnivores in the Italian Alps. Parasit. Vectors 2014, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Zobba, R.; Nuvoli, A.M.; Sotgiu, F.; Lecis, R.; Spezzigu, A.; Dore, G.M.; Masia, M.A.; Cacciotto, C.; Parpaglia, M.L.; Dessì, D.; et al. Molecular Epizootiology and Diagnosis of Porcine Babesiosis in Sardinia, Italy. Vector Borne Zoonotic Dis. 2014, 14, 716–723. [Google Scholar] [CrossRef]
- Sgroi, G.; D’Alessio, N.; Auriemma, C.; Salant, H.; Gallo, A.; Riccardi, M.G.; Alfano, F.; Rea, S.; Scarcelli, S.; Ottaviano, M.; et al. First Molecular Detection of Babesia vulpes and Babesia capreoli in Wild Boars from Southern Italy. Front. Vet. Sci. 2023, 10, 1201476. [Google Scholar] [CrossRef]
- Matei, I.A.; Kalmár, Z.; Balea, A.; Mihaiu, M.; Sándor, A.D.; Cocian, A.; Crăciun, S.; Bouari, C.; Briciu, V.T.; Fiț, N. The Role of Wild Boars in the Circulation of Tick-Borne Pathogens: The First Evidence of Rickettsia monacensis Presence. Animals 2023, 13, 1743. [Google Scholar] [CrossRef]
- Díaz-Cao, J.M.; Adaszek, Ł.; Dzięgiel, B.; Paniagua, J.; Caballero-Gómez, J.; Winiarczyk, S.; Winiarczyk, D.; Cano-Terriza, D.; García-Bocanegra, I. Prevalence of Selected Tick-Borne Pathogens in Wild Ungulates and Ticks in Southern Spain. Transbound. Emerg. Dis. 2022, 69, 1084–1094. [Google Scholar] [CrossRef]
- Fabri, N.D.; Sprong, H.; Hofmeester, T.R.; Heesterbeek, H.; Donnars, B.F.; Widemo, F.; Ecke, F.; Cromsigt, J.P.G.M. Wild ungulate species differ in their contribution to the transmission of Ixodes ricinus-borne pathogens. Parasit Vectors 2021, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.E.; Andersson, M.O. Babesia species in questing Ixodes ricinus, Sweden. Ticks Tick Borne Dis. 2016, 7, 10–12. [Google Scholar] [CrossRef]
- Nahayo, A.; Bardiau, M.; Volpe, R.; Pirson, J.; Paternostre, J.; Fett, T.; Linden, A. Molecular Evidence of Anaplasma phagocytophilum in Wild Boar (Sus scrofa) in Belgium. BMC Vet. Res. 2014, 10, 80. [Google Scholar] [CrossRef]
- Hrazdilová, K.; Lesiczka, P.M.; Bardoň, J.; Vyroubalová, Š.; Šimek, B.; Zurek, L.; Modrý, D. Wild Boar as a Potential Reservoir of Zoonotic Tick-Borne Pathogens. Ticks Tick Borne Dis. 2021, 12, 101558. [Google Scholar] [CrossRef]
- Dugat, T.; Haciane, D.; Durand, B.; Lagrée, A.C.; Haddad, N.; Boulouis, H.J. Identification of a Potential Marker of Anaplasma phagocytophilum Associated with Cattle Abortion. Transbound. Emerg. Dis. 2017, 64, e1–e3. [Google Scholar] [CrossRef]
- Grech-Angelini, S.; Stachurski, F.; Vayssier-Taussat, M.; Devillers, E.; Casabianca, F.; Lancelot, R.; Uilenberg, G.; Moutailler, S. Tick-Borne Pathogens in Ticks (Acari: Ixodidae) Collected from Various Domestic and Wild Hosts in Corsica (France), a Mediterranean Island Environment. Transbound. Emerg. Dis. 2019, 67, 745–757. [Google Scholar] [CrossRef]
- Silaghi, C.; Pfister, K.; Overzier, E. Molecular Investigation for Bacterial and Protozoan Tick-Borne Pathogens in Wild Boars (Sus scrofa) from Southern Germany. Vector Borne Zoonotic Dis. 2014, 14, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Bertelloni, F.; Cecconi, G.; Sgorbini, M.; Cerri, D. Zoonotic Tick-Borne Bacteria among Wild Boars (Sus scrofa) in Central Italy. Asian Pac. J. Trop. Med. Press 2017, 7, 141–143. [Google Scholar] [CrossRef]
- Myczka, A.W.; Steiner-Bogdaszewska, Ż.; Filip-Hutsch, K.; Oloś, G.; Czopowicz, M.; Laskowski, Z. Detection of Anaplasma phagocytophilum in Wild and Farmed Cervids in Poland. Pathogens 2021, 10, 1190. [Google Scholar] [CrossRef]
- Michalski, M.M.; Kubiak, K.; Szczotko, M.; Dmitryjuk, M. Tick-Borne Pathogens in Ticks Collected from Wild Ungulates in North-Eastern Poland. Pathogens 2021, 10, 587. [Google Scholar] [CrossRef]
- Pereira, A.; Parreira, R.; Nunes, M.; Casadinho, A.; Vieira, M.L.; Campino, L.; Maia, C. Molecular Detection of Tick-Borne Bacteria and Protozoa in Cervids and Wild Boars from Portugal. Parasit. Vectors 2016, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Cadar, D.; Krupaci, F.A.; Bordeanu, A.D.; Spînu, M. Prevalence of Anaplasma phagocytophilum Infection in European Wild Boar (Sus scrofa) Populations from Transylvania, Romania. Epidemiol. Infect. 2014, 142, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Dreghiciu, I.C.; Imre, M.; Oprescu, I.; Florea, T.; Ghilean, B.M.; Sîrbu, B.A.M.; Iorgoni, V.; Badea, C.; Giubega, S.; Mederle, N.; et al. Molecular Detection of Anaplasma phagocytophilum in Wild Boar (Sus scrofa) from Hunedoara and Timiș Counties—Preliminary Study. Rev. Rom. Med. Vet. 2023, 33, 59–62. [Google Scholar]
- Kazimírová, M.; Hamšíková, Z.; Špitalská, E.; Minichová, L.; Mahríková, L.; Caban, R.; Sprong, H.; Fonville, M.; Schnittger, L.; Kocianová, E. Diverse Tick-Borne Microorganisms Identified in Free-Living Ungulates in Slovakia. Parasit. Vectors 2018, 11, 495. [Google Scholar] [CrossRef]
- Polin, H.; Hufnagl, P.; Hauschmid, R.; Gruber, F.; Guther, L. Molecular Evidence of Anaplasma phagocytophilum in Ixodes ricinus Ticks and Wild Animals in Austria. J. Clin. Microbiol. 2004, 42, 2285–2286. [Google Scholar] [CrossRef]
- Portillo, A.; Perez-Martinez, L.; Santibanez, S.; Santibanez, P.; Palomar, A.M.; Oteo, J.A. Anaplasma spp. in Wild Mammals and Ixodes ricinus from the North of Spain. Vector Borne Zoonotic Dis. 2011, 11, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.T.; Pallas, E.; Gestal, J.J.; Guitián, F.J.; Olmeda, A.; Telford, S.R. Ixodes hexagonus is the Main Candidate as Vector of Theileria annae in Northwest Spain. Vet. Parasitol. 2003, 112, 157–163. [Google Scholar] [CrossRef]
- Obsomer, V.; Wirtgen, M.; Linden, A.; Claerebout, E.; Heyman, P.; Heylen, D.; Madder, M.; Maris, J.; Lebrun, M.; Tack, W.; et al. Spatial Disaggregation of Tick Occurrence and Ecology at a Local Scale as a Preliminary Step for Spatial Surveillance of Tick-Borne Diseases: General Framework and Health Implications in Belgium. Parasit. Vectors 2013, 6, 190. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification; Springer Nature: Cham, Switzerland, 2017; p. 368. [Google Scholar]
- Bajer, A.; Dwużnik-Szarek, D. The Specificity of Babesia-Tick Vector Interactions: Recent Advances and Pitfalls in Molecular and Field Studies. Parasit. Vectors 2021, 14, 507. [Google Scholar] [CrossRef]
- Galindo, R.C.; Ayllon, N.; Strasek, S.; Boadella, M.; Beltran-Beck, B.; Mazariegos, M. Gene Expression Profile Suggests that Pigs (Sus scrofa) are Susceptible to Anaplasma phagocytophilum but Control Infection. Parasites Vectors 2012, 5, 181. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Liu, Q.; Chen, C.; Li, J.; Long, B.; Yu, H.; Zhang, Z.; He, J.; Qu, Z.; et al. Molecular analysis of Anaplasma phagocytophilum isolated from patients with febrile diseases of unknown etiology in China. PLoS ONE 2013, 8, e57155. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Bown, K.; Horrocks, B.K.; Woldehiwet, Z.; Bennett, M. Granulocytic Ehrlichia infection in ixodid ticks and mammals in woodlands and uplands of the U.K. Med. Vet. Entomol. 1998, 12, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Liz, J.S.; Sumner, J.W.; Pfister, K.; Brossard, M. PCR detection and serological evidence of granulocytic ehrlichial infection in roe deer (Capreolus capreolus) and chamois (Rupicapra rupicapra). J. Clin. Microbiol. 2002, 40, 892–897. [Google Scholar] [CrossRef]
- Overzier, E.; Pfister, K.; Thiel, C.; Herb, I.; Mahling, M.; Silaghi, C. Anaplasma phagocytophilum in questing Ixodes ricinus Ticks: Comparison of prevalences and partial 16S rRNA gene variants in urban, pasture, and natural habitats. Appl. Environ. Microbiol. 2013, 79, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef]
- Michalik, J.; Stanczak, J.; Cieniuch, S.; Racewicz, M.; Sikora, B.; Dabert, M. Wild boars as hosts of human-pathogenic Anaplasma phagocytophilum variants. Emerg. Infect. Dis. 2012, 18, 998–1001. [Google Scholar] [CrossRef]
- Smetanová, K.; Schwarzová, K.; Kocianová, E. Detection of Anaplasma phagocytophilum, Coxiella burnetii, Rickettsia spp., and Borrelia burgdorferi s. l. in Ticks, and wild-living animals in western and middle Slovakia. Ann. N. Y. Acad. Sci. 2006, 1078, 312–315. [Google Scholar] [CrossRef]
- Hulínská, D.; Langøová, K.; Pejèoch, M.; Pavlásek, I. Detection of Anaplasma phagocytophilum in animals by real-time polymerase chain reaction. Apmis 2004, 112, 239–247. [Google Scholar] [CrossRef]
- Skarphedinsson, S.; Lyholm, B.F.; Ljungberg, M.; Sogaard, P.; Kolmos, H.J.; Nielsen, L.P. Detection and identification of Anaplasma phagocytophilum, Borrelia burgdorferi, and Rickettsia helvetica in Danish Ixodes ricinus ticks. Apmis 2007, 115, 225–230. [Google Scholar] [CrossRef]
- Christova, I.; Schouls, L.; van De Pol, I.; Park, J.; Panayotov, S.; Lefterova, V.; Kantardjiev, T.; Dumler, J.S. High prevalence of granulocytic Ehrlichiae and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Bulgaria. J. Clin. Microbiol. 2001, 39, 4172–4174. [Google Scholar] [CrossRef]
- Capelli, G.; Ravagnan, S.; Montarsi, F.; Ciocchetta, S.; Cazzin, S.; Porcellato, E.; Babiker, A.M.; Cassini, R.; Salviato, A.; Cattoli, G.; et al. Occurrence and identification of risk areas of Ixodes ricinus-borne pathogens: A cost-effectiveness analysis in north-eastern Italy. Parasit Vectors 2012, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Cinco, M.; Padovan, D.; Murgia, R.; Maroli, M.; Frusteri, L.; Heldtander, M.; Johansson, K.E.; Engvall, E.O. Coexistence of Ehrlichia phagocytophila and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Italy as determined by 16S rRNA gene sequencing. J. Clin. Microbiol. 1997, 35, 3365–3366. [Google Scholar] [CrossRef]
- Egyed, L.; Elo, P.; Sreter-Lancz, Z.; Szell, Z.; Balogh, Z.; Sreter, T. Seasonal activity and tick-borne pathogen infection rates of Ixodes ricinus ticks in Hungary. Ticks Tick Borne Dis. 2012, 3, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Wielinga, P.R.; Gaasenbeek, C.; Fonville, M.; de Boer, A.; de Vries, A.; Dimmers, W.; Akkerhuis Op Jagers, G.; Schouls, L.M.; Borgsteede, F.; van der Giessen, J.W. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in The Netherlands. Appl. Environ. Microbiol. 2006, 72, 7594–7601. [Google Scholar] [CrossRef] [PubMed]
- Reye, A.L.; Hübschen, J.M.; Sausy, A.; Müller, C.P. Prevalence and seasonality of tick-borne pathogens in questing Ixodes ricinus ticks from Luxembourg. Appl. Environ. Microbiol. 2010, 76, 2923–2931. [Google Scholar] [CrossRef]
- Wicki, R.; Sauter, P.; Mettler, C.; Natsch, A.; Enzler, T.; Pusterla, N.; Kuhnert, P.; Egli, G.; Bernasconi, M.; Lienhard, R.; et al. Swiss Army Survey in Switzerland to determine the prevalence of Francisella tularensis, members of the Ehrlichia phagocytophila genogroup, Borrelia burgdorferi sensu lato, and tick-borne encephalitis virus in ticks. Eur. J. Clin. 2000, 19, 427–432. [Google Scholar] [CrossRef]
- Burri, C.; Dupasquier, C.; Bastic, V.; Gern, L. Pathogens of emerging tick-borne diseases, Anaplasma phagocytophilum, Rickettsia spp., and Babesia spp., in Ixodes ticks collected from rodents at four sites in Switzerland (Canton of Bern). Vector Borne Zoonotic Dis. 2011, 11, 939–944. [Google Scholar] [CrossRef]
- Leutenegger, C.M.; Pusterla, N.; Mislin, C.N.; Weber, R.; Lutz, H. Molecular evidence of coinfection of ticks with Borrelia burgdorferi sensu lato and the human granulocytic ehrlichiosis agent in Switzerland. J. Clin. Microbiol. 1999, 37, 3390–3391. [Google Scholar] [CrossRef]


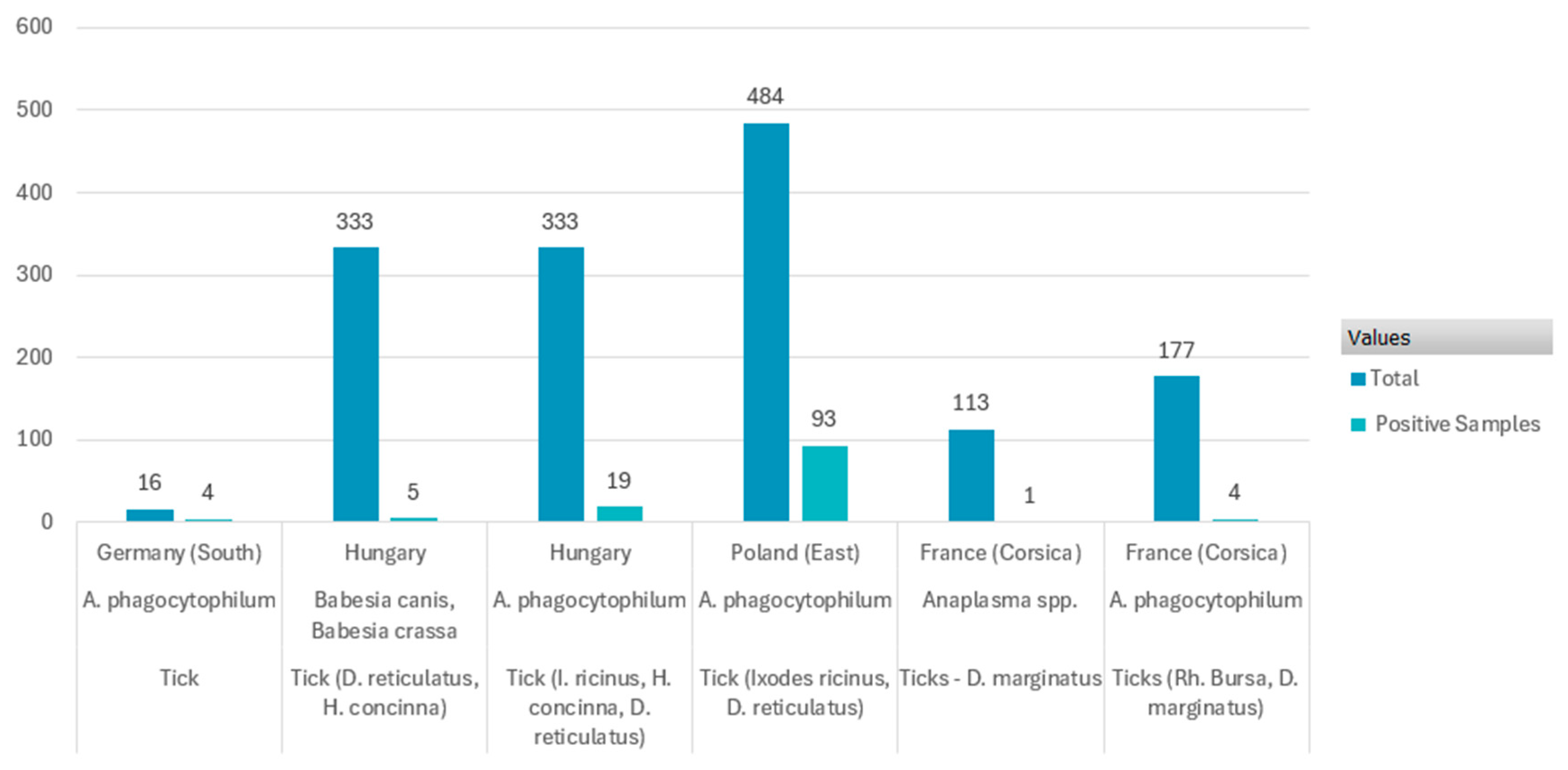

| Years of Study | Country | Diag. Method | Host/Sample | Species | No. of Samples | Positive | Prevalence | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2018–2020 | France (Corsica) | Blood | Babesia spp. | 158 | 3 | 2% | [37] | |
| 2020 | Hungary | Ticks (D. reticulatus, H. concinna) | B. canis, B. crassa | 333 | 5 | 1.50% | [38] | |
| 2008–2012 | Italy (North) | Spleen | B. bigemina | 257 | 12 | 4.67% | [39] | |
| 2010–2013 | Italy (Sardinia) | Blood | Babesia spp. | 52 | 0 | 0% | [40] | |
| 2016–2022 | Italy (South) | S. | Spleen | B. vulpes, B. capreoli | 243 | 15 | 6.20% | [41] |
| 2019–2021 | Romania | S. | Blood | Babesia spp. | 203 | 0 | 0% | [42] |
| 2009–2015 | Spain | Spleen | Babesia spp. | 269 | 0 | 0% | [43] | |
| 2018–2019 | Sweden | Spleen | Babesia spp. | 34 | 0 | 0% | [44] | |
| 2013–2014 | Sweden | Ticks (I. ricinus) | B. microti, | 519 | 17 | 3.6% | [45] | |
| B. divergens, | 1 | 0.2% | ||||||
| B. venatorum | 5 | 1% |
| Years of Study | Country | Diag. Method | Host/Sample | Species | No. of Samples | Positive | Prevalence | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2011 | Belgium | Spleen | A. phag. | 513 | 5 | 0.97% | [46] | |
| 2018–2019 | Czech Rep. | S. | Blood | A. phag. | 550 | 28 | 5.10% | [47] |
| 2009–2015 | France (Central) | Spleen | A. phag. | 29 | 13 | 44.80% | [48] | |
| 2014–2015 | France (Corsica) | Tick (Rhipicephalus bursa, D. marginatus) | A. phag. | 177 | 4 | 2% | [49] | |
| 2018–2020 | France (Corsica) | Tick (D. marginatus) | Anaplasma spp. | 113 | 1 | 0.88% | [37] | |
| 2010–2013 | Germany (South) | S. | Spleen | A. phag. | 24 | 3 | 12.50% | [50] |
| 2010–2013 | Germany (South) | S. | Tick | A. phag. | 16 | 4 | 25% | [50] |
| 2020 | Hungary | Tick (I. ricinus, H. concinna, D. reticulatus) | A. phag. | 333 | 19 | 5.70% | [38] | |
| 2013–2015 | Italy (Central) | Spleen | A. phag. | 100 | 1 | 1% | [51] | |
| 2017–2019 | Poland | S. | Spleen/liver | A. phag. | 59 | 12 | 20.34% | [52] |
| 2018–2020 | Poland (East) | Tick (I. ricinus, D. reticulatus) | A. phag. | 484 | 93 | 19.20% | [53] | |
| 2013–2015 | Portugal | S. | Blood | A. platys | 65 | 2 | 3.10% | [54] |
| 2019–2020 | Romania | S. | Blood | A. phag. | 203 | 6 | 2.96% | [42] |
| 2007–2012 | Romania (Central) | Spleen/liver/kidney | A. phag. | 870 | 39 | 4.48% | [55] | |
| 2023 | Romania (West) | Blood | A. phag. | 29 | 1 | 3.44% | [56] | |
| 2011–2014 | Slovakia | Spleen/blood | A. phag. | 39 | 11 | 28.20% | [57] | |
| 2009–2015 | Spain | Spleen | Anaplasma spp. | 269 | 0 | 0% | [43] | |
| 2018–2019 | Sweden | Spleen | Anaplasma spp. | 34 | 24 | 70.60% | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreghiciu, I.C.; Hoffman, D.; Florea, T.; Oprescu, I.; Dumitru, S.; Imre, M.; Iorgoni, V.; Plesko, A.; Morariu, S.; Ilie, M.S. A Systematic Review on the Occurrence of Babesia spp. and Anaplasma spp. in Ticks and Wild Boar from Europe—A 15-Year Retrospective Study. Pathogens 2025, 14, 612. https://doi.org/10.3390/pathogens14070612
Dreghiciu IC, Hoffman D, Florea T, Oprescu I, Dumitru S, Imre M, Iorgoni V, Plesko A, Morariu S, Ilie MS. A Systematic Review on the Occurrence of Babesia spp. and Anaplasma spp. in Ticks and Wild Boar from Europe—A 15-Year Retrospective Study. Pathogens. 2025; 14(7):612. https://doi.org/10.3390/pathogens14070612
Chicago/Turabian StyleDreghiciu, Ioan Cristian, Diana Hoffman, Tiana Florea, Ion Oprescu, Simona Dumitru, Mirela Imre, Vlad Iorgoni, Anamaria Plesko, Sorin Morariu, and Marius Stelian Ilie. 2025. "A Systematic Review on the Occurrence of Babesia spp. and Anaplasma spp. in Ticks and Wild Boar from Europe—A 15-Year Retrospective Study" Pathogens 14, no. 7: 612. https://doi.org/10.3390/pathogens14070612
APA StyleDreghiciu, I. C., Hoffman, D., Florea, T., Oprescu, I., Dumitru, S., Imre, M., Iorgoni, V., Plesko, A., Morariu, S., & Ilie, M. S. (2025). A Systematic Review on the Occurrence of Babesia spp. and Anaplasma spp. in Ticks and Wild Boar from Europe—A 15-Year Retrospective Study. Pathogens, 14(7), 612. https://doi.org/10.3390/pathogens14070612











