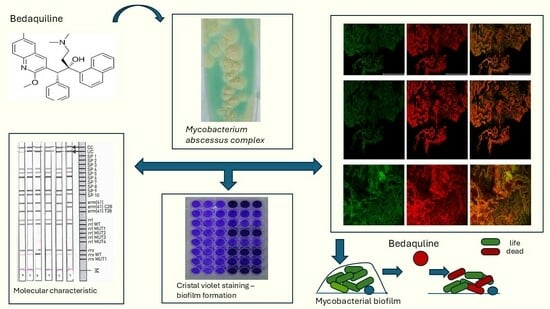
Evaluation of the In Vitro Activity of Bedaquiline, Delamanid, and Clofazimine Against Mycobacterium abscessus Complex and Their Antibiofilm Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Mycobacterial Strains
2.2. Molecular Identification of Subspecies Within the M. abscesuss Complex and Molecular Mechanisms of Resistance Assessed by the DNA Strip Method
2.3. Morphology of the Colony of Mycobacterial Strains
2.4. Phenotypic Drug Susceptibility Testing by Routinely Used Method
2.5. Susceptibility Testing to Bedaquiline, Delamanid and Clofazimine by the Broth Microdilution Method
2.6. Biofilm-Formation Assay by Crystal Violet Staining
2.7. Evaluation of the Antibiofilm Activity of Bedaquiline
2.8. Bacterial Cell-Viability Assessment and Microscopy
2.9. Statistical Analysis
3. Results
3.1. Molecular Characterisation of Tested M. abscessus Isolates
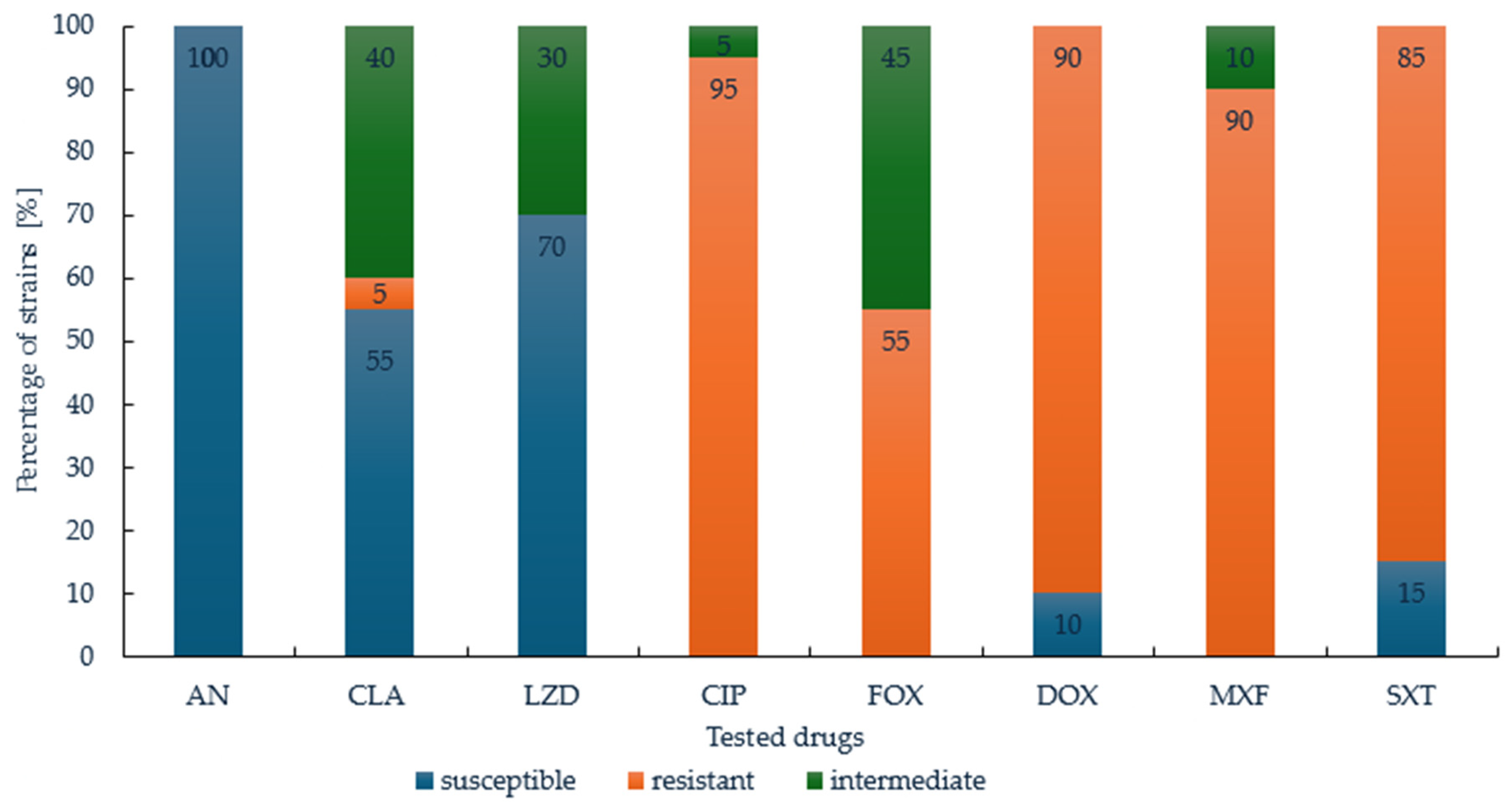
3.2. Susceptibility of M. abscessus to Therapeutics Routinely Used in RGM Treatment
3.3. Potential of Bedaquiline, Delamanid, and Clofazimine Against M. abscessus
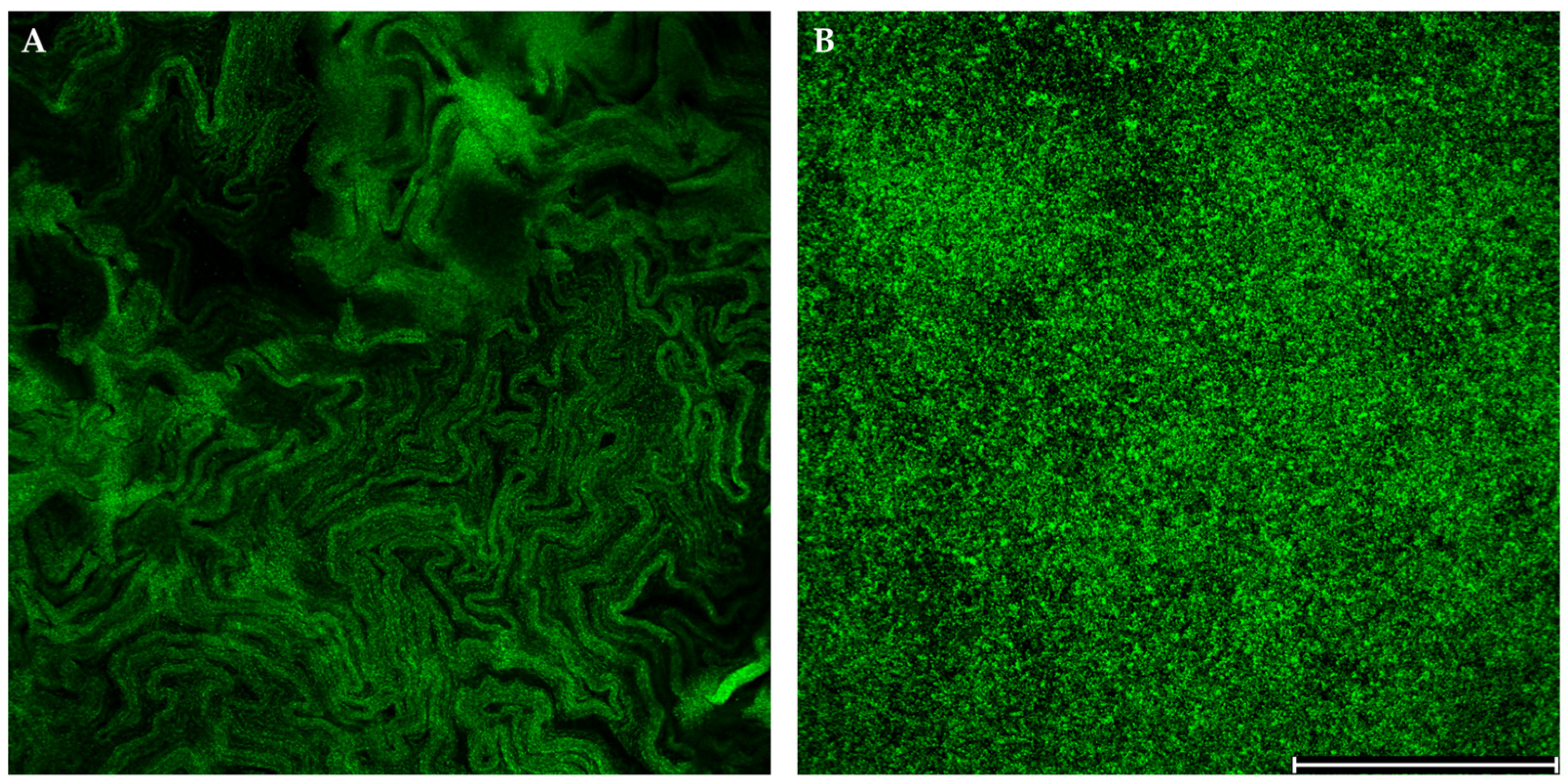
3.4. Differentiation of Morphotypes and Biofilm Formation
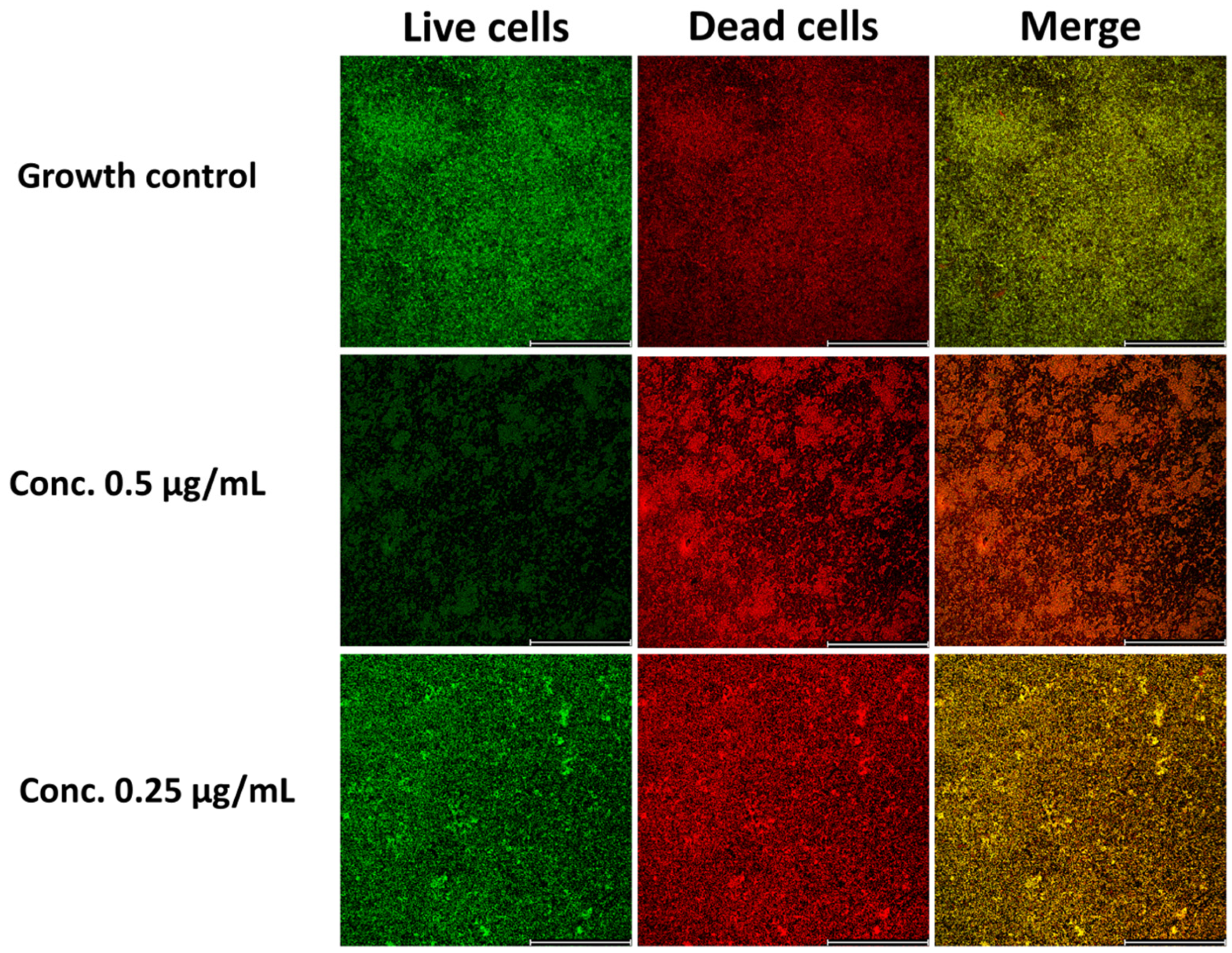
3.5. Bedaquilline as a Good Antibiofilm Agent
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDQ | Bedaquiline |
| CFU | Colony-Forming Units |
| CLO | Clofazimine |
| DEL | Delamanid |
| CV | Crystal Violet |
| LJ | Löwenstein–Jensen medium |
| MABc | Mycobacterium abscessus complex |
| MBIC | Minimal Biofilm Inhibitory Concentration |
| MIC | Minimal Inhibitory Concentration |
| NTM | Non-Tuberculous Mycobacteria |
| OD | Optical Density |
| PI | Propidium Iodide |
| DST | Drug Susceptibility Testing |
Appendix A
| Antimicrobial Agent | MIC (µg/mL) | ||
|---|---|---|---|
| S | I | R | |
| Amikacin (IV) | ≤16 | 32 | ≥64 |
| Cefoxitin | ≤16 | 32–64 | ≥128 |
| Ciprofloxacin | ≤1 | 2 | ≥4 |
| Clarithromycin | ≤2 | 4 | ≥8 |
| Imipenem | ≤4 | 8–16 | ≥32 |
| Linezolid | ≤8 | 16 | ≥32 |
| Meropenem | ≤4 | 8–16 | ≥32 |
| Moxifloxacin | ≤1 | 2 | ≥4 |
| Trimetoprim-sulfametoxazol | ≤2/38 | - | ≥4/76 |
| Tigecycline | - | - | - |
| Tobramycin | ≤2 | 4 | ≥8 |
References
- Victoria, L.; Gupta, A.; Gómez, J.L.; Robledo, J. Mycobacterium abscessus complex: A Review of Recent Developments in an Emerging Pathogen. Front. Cell. Infect. Microbiol. 2021, 11, 659997. [Google Scholar] [CrossRef] [PubMed]
- Abdela al, H.F.M.; Chan, E.D.; Young, L.; Baldwin, S.L.; Coler, R.N. Mycobacterium abscessus: It’s Complex. Microorganisms 2022, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Shaw, P.A.; Strickland, D.; Jackson, L.A.; Raebel, M.A.; Blosky, M.A.; Montes De Oca, R.; Shea, Y.R.; Seitz, A.E.; Holland, S.M.; et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am. J. Respir. Crit. Care Med. 2010, 182, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Snell, G.; Reed, A.; Stern, M.; Hadjiliadis, D. The evolution of lung transplantation for cystic fibrosis: A 2017 update. J. Cyst. Fibros. 2017, 16, 553–564. [Google Scholar] [CrossRef]
- Wetzstein, N.; Diricks, M.; Kohl, T.A.; Wichelhaus, T.A.; Andres, S.; Paulowski, L.; Schwarz, C.; Lewin, A.; Kehrmann, J.; Kahl, B.C.; et al. Molecular Epidemiology of Mycobacterium abscessus Isolates Recovered from German Cystic Fibrosis Patients. Microbiol. Spectr. 2022, 10, 0171422. [Google Scholar] [CrossRef]
- Faria, S.; Joao, I.; Jordao, L. General Overview on Nontuberculous Mycobacteria, Biofilms, and Human Infection. J. Pathog. 2015, 2015, 809014. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Heo, B.E.; Jeon, S.; Ash, A.; Lee, H.; Moon, C.; Jang, J. Exploring antibiotic resistance mechanisms in Mycobacterium abscessus for enhanced therapeutic approaches. Front. Microbiol. 2024, 15, 1331508. [Google Scholar] [CrossRef]
- Dokic, A.; Peterson, E.; Arrieta-Ortiz, M.L.; Pan, M.; Di Maio, A.; Baliga, N.; Bhatt, A. Mycobacterium abscessus biofilms produce an extracellular matrix and have a distinct mycolic acid profile. Cell Surf. 2021, 7, 100051. [Google Scholar] [CrossRef]
- Gloag, E.S.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Mycobacterium abscessus biofilms have viscoelastic properties which may contribute to their recalcitrance in chronic pulmonary infections. Sci. Rep. 2021, 11, 5020. [Google Scholar] [CrossRef]
- Luthra, S.; Rominski, A.; Sander, P. The Role of Antibiotic-Target-Modifying and Antibiotic-Modifying Enzymes in Mycobacterium abscessus Drug Resistance. Front. Microbiol. 2018, 9, 2179. [Google Scholar] [CrossRef]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Mulyukin, A.L.; Recchia, D.; Kostrikina, N.A.; Artyukhina, M.V.; Martini, B.A.; Stamilla, A.; Degiacomi, G.; Salina, E.G. Distinct Effects of Moxifloxacin and Bedaquiline on Growing and ‘Non-Culturable’ Mycobacterium abscessus. Microorganisms 2023, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614, Erratum in Microorganisms 2024, 12, 1961. [Google Scholar] [CrossRef] [PubMed]
- Born, S.E.M.; Reichlen, M.J.; Bartek, I.L.; Benoit, J.B.; Frank, D.N.; Voskuil, M.I. Population heterogeneity in Mycobacterium smegmatis and Mycobacterium abscessus. Microbiology 2023, 169, 001402. [Google Scholar] [CrossRef]
- López-Roa, P.; Esteban, J.; Muñoz-Egea, M.C. Updated Review on the Mechanisms of Pathogenicity in Mycobacterium abscessus, a Rapidly Growing Emerging Pathogen. Microorganisms 2022, 11, 90. [Google Scholar] [CrossRef]
- Kwak, N.; Dalcolmo, M.P.; Daley, C.L.; Eather, G.; Gayoso, R.; Hasegawa, N.; Jhun, B.W.; Koh, W.-J.; Namkoong, H.; Park, J.; et al. Mycobacterium abscessus pulmonary disease: Individual patient data meta-analysis. Eur. Respir. J. 2019, 54, 1801991. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, Approved Standard, 3rd ed.; CLSI document, M24; Woods, G.L., Ed.; Clinical and Laboratory Standards Institute: Wayne PA, USA, 2018. [Google Scholar]
- CLSI. Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, 3rd ed.; Suplement Edition M62; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Dupont, C.; Viljoen, A.; Thomas, S.; Roquet-Banères, F.; Herrmann, J.-L.; Pethe, K.; Kremer, L. Bedaquiline Inhibits the ATP Synthase in Mycobacterium abscessus and Is Effective in Infected Zebrafish. Antimicrob. Agents Chemother. 2017, 61, 01225–17. [Google Scholar] [CrossRef]
- Ahmad Khosravi, N.; Sirous, M.; Khosravi, A.D.; Saki, M. A Narrative Review of Bedaquiline and Delamanid: New Arsenals Against Multidrug-Resistant and Extensively Drug-Resistant Mycobacterium tuberculosis. J. Clin. Lab. Anal. 2024, 38, 25091. [Google Scholar] [CrossRef]
- Motta, I.; Boeree, M.; Chesov, D.; Dheda, K.; Günther, G.; Horsburgh, C.R.; Kherabi, Y.; Lange, C.; Lienhardt, C.; McIlleron, H.M.; et al. Recent advances in the treatment of tuberculosis. Clin. Microbiol. Infect. 2024, 30, 1107–1114. [Google Scholar] [CrossRef]
- Wang, M.; Men, P.; Zhang, W.; Wu, J.; Gu, Y.; Wang, F.; Huang, H.; Yu, X.; Duan, H. Bedaquiline susceptibility testing of Mycobacterium abscessus complex and Mycobacterium avium complex: A meta-analysis study. J. Glob. Antimicrob. Resist. 2024, 7, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ye, M.; Guo, Q.; Zhang, Z.; Yang, S.; Ma, W.; Yu, F.; Chu, H. Determination of MIC Distribution and Mechanisms of Decreased Susceptibility to Bedaquiline among Clinical Isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2018, 62, 00175–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jhun, B.W.; Moon, S.M.; Kim, S.-Y.; Jeon, K.; Kwon, O.J.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. In Vitro Activity of Bedaquiline and Delamanid against Nontuberculous Mycobacteria, Including Macrolide-Resistant Clinical Isolates. Antimicrob. Agents Chemother. 2019, 63, 00665–19. [Google Scholar] [CrossRef]
- Meir, M.; Barkan, D. Alternative and Experimental Therapies of Mycobacterium abscessus Infections. Int. J. Mol. Sci. 2020, 21, 6793. [Google Scholar] [CrossRef]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.; Neefs, J.M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.S.; Lakshmanan, M. Delamanid: A new armor in combating drug-resistant tuberculosis. J. Pharmacol. Pharmacother. 2014, 5, 222–224. [Google Scholar] [CrossRef]
- Venkatesan, K.; Mathur, A.; Girdhar, B.K.; Bharadwaj, V.P. The effect of clofazimine on the pharmacokinetics of rifampicin and dapsone in leprosy. J. Antimicrob. Chemother. 1986, 18, 715–718. [Google Scholar] [CrossRef]
- Oommen, S.T.; Natu, M.V.; Mahajan, M.K.; Kadyan, R.S. Lymphangiographic evaluation of patients with clinical lepromatous leprosy on clofazimine. Int. J. Lepr. Other Mycobact. Dis. 1994, 62, 32–36. [Google Scholar]
- Mungroo, M.R.; Khan, N.A.; Siddiqui, R. Mycobacterium leprae: Pathogenesis, diagnosis, and treatment options. Microb. Pathog. 2020, 149, 104475. [Google Scholar] [CrossRef]
- Stadler, J.A.M.; Maartens, G.; Meintjes, G.; Wasserman, S. Clofazimine for the treatment of tuberculosis. Front. Pharmacol. 2023, 14, 1100488. [Google Scholar] [CrossRef]
- Riccardi, N.; Giacomelli, A.; Canetti, D.; Comelli, A.; Intini, E.; Gaiera, G.; Diaw, M.M.; Udwadia, Z.; Besozzi, G.; Codecasa, L.; et al. Clofazimine: An old drug for never-ending diseases. Clofazimine: An old drug for never-ending diseases. Future Microbiol. 2020, 15, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Alam, S.; Liu, Z.; Khatun, M.S.; Yusuf, B.; Hameed, H.M.A.; Tian, X.; Chhotaray, C.; Basnet, R.; Abraha, H.; et al. Molecular mechanisms of resistance and treatment efficacy of clofazimine and bedaquiline against Mycobacterium tuberculosis. Front. Med. 2024, 10, 1304857. [Google Scholar] [CrossRef] [PubMed]
- Hajikhani, B.; Nasiri, M.J.; Hosseini, S.S.; Khalili, F.; Karimi-Yazdi, M.; Hematian, A.; Nojookambari, N.Y.; Goudarzi, M.; Dadashi, M.; Mirsaeidi, M. Clofazimine susceptibility testing of Mycobacterium avium complex and Mycobacterium abscessus: A meta-analysis study. J. Glob. Antimicrob. Resist. 2021, 26, 188–193. [Google Scholar] [CrossRef]
- Schulthess, B.; Akdoğan Kittana, F.N.; Hömke, R.; Sander, P. In Vitro Bedaquiline and Clofazimine Susceptibility Testing in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2022, 66, 0234621. [Google Scholar] [CrossRef]
- Omar, S.; Whitfield, M.G.; Nolan, M.B.; Ngom, J.T.; Ismail, N.; Warren, R.M.; Klopper, M. Bedaquiline for treatment of non-tuberculous mycobacteria (NTM): A systematic review and meta-analysis. J. Antimicrob. Chemother. 2024, 79, 211–240. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium avium and Mycobacterium abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- GenoType NTM-DR VER 1.0 Molecular Genetic Assay for Detection of Resistance to Macrolides and Aminoglycosides in various Nontuberculous Mycobacterial Species (NTM) from Cultured Material. GenoType NTM-DR VER 1.0 Instructions for Use (IFU-297-03), 2019, 4–9. Available online: https://www.hain-lifescience.de/en/instructions-for-use.html (accessed on 15 July 2014).
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Parrish, N.M.; Ko, C.G.; Dick, J.D.; Jones, P.B.; Ellingson, J.L. Growth, Congo Red agar colony morphotypes and antibiotic susceptibility testing of Mycobacterium avium subspecies paratuberculosis. Clin. Med. Res. 2024, 2, 107–114. [Google Scholar] [CrossRef][Green Version]
- Invitrogen LIVE/DEAD® BacLight Bacterial Viability Kits. 2004. Available online: https://www.thermofisher.cn/order/catalog/product/L7012 (accessed on 15 July 2014).
- Griffith, D.E.; Daley, C.L. Treatment of Mycobacterium abscessus. Pulmonary Disease. Chest 2022, 161, 64–75. [Google Scholar] [CrossRef]
- Hedin, W.; Fröberg, G.; Fredman, K.; Chryssanthou, E.; Selmeryd, I.; Gillman, A.; Orsini, L.; Runold, M.; Jönsson, B.; Schön, T.; et al. A Rough Colony Morphology of Mycobacterium abscessus Is Associated With Cavitary Pulmonary Disease and Poor Clinical Outcome. J. Infect. Dis. 2023, 227, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.A.; Brown-Elliott, B.A.; Wallace, R.J., Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob. Agents Chemother. 2009, 53, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yoo, H.K.; Kim, S.H.; Koh, W.J.; Kim, C.K.; Park, Y.K.; Kim, H.J. Detection and assessment of clarithromycin inducible resistant strains among Korean Mycobacterium abscessus clinical strains: PCR methods. J. Clin. Lab. Anal. 2014, 28, 409–414, Erratum in J. Clin. Lab. Anal. 2016, 30, 356. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.; de Waard, J.H.; Araque, M. Molecular mechanisms of clarithromycin resistance in Mycobacterium abscessus complex clinical isolates from Venezuela. J. Glob. Antimicrob. Resist. 2015, 3, 205–209. [Google Scholar] [CrossRef]
- Carneiro, M.D.S.; Nunes, L.S.; David, S.M.M.; Barth, A.L. Lack of association between rrl and erm(41) mutations and clarithromycin resistance in Mycobacterium abscessus complex. Mem. Inst. Oswaldo Cruz 2017, 112, 775–778. [Google Scholar] [CrossRef]
- Ananta, P.; Kham-Ngam, I.; Chetchotisakd, P.; Chaimanee, P.; Reechaipichitkul, W.; Namwat, W.; Lulitanond, V.; Faksri, K. Analysis of drug-susceptibility patterns and gene sequences associated with clarithromycin and amikacin resistance in serial Mycobacterium abscessus isolates from clinical specimens from Northeast Thailand. PLoS ONE 2018, 13, 0208053. [Google Scholar] [CrossRef]
- Nie, W.; Duan, H.; Huang, H.; Lu, Y.; Chu, N. Species Identification and Clarithromycin Susceptibility Testing of 278 Clinical Nontuberculosis Mycobacteria Isolates. Biomed. Res. Int. 2015, 2015, 506598. [Google Scholar] [CrossRef]
- Pang, Y.; Zheng, H.; Tan, Y.; Song, Y.; Zhao, Y. In Vitro Activity of Bedaquiline against Nontuberculous Mycobacteria in China. Antimicrob. Agents Chemother. 2017, 1, 02627-16. [Google Scholar] [CrossRef]
- Le Moigne, V.; Raynaud, C.; Moreau, F.; Dupont, C.; Nigou, J.; Neyrolles, O.; Kremer, L.; Herrmann, J.-L. Efficacy of Bedaquiline, Alone or in Combination with Imipenem, against Mycobacterium abscessus in C3HeB/FeJ Mice. Antimicrob. Agents Chemother. 2020, 64, 00114–00120. [Google Scholar] [CrossRef]
- Ruth, M.M.; Sangen, J.J.N.; Remmers, K.; Pennings, L.J.; Svensson, E.; E Aarnoutse, R.; Zweijpfenning, S.M.H.; Hoefsloot, W.; Kuipers, S.; Magis-Escurra, C.; et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J. Antimicrob. Chemother. 2019, 74, 935–943. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef] [PubMed]
- Pfaeffle, H.O.I.; Alameer, R.M.; Marshall, M.H.; Houpt, E.R.; Albon, D.P.; Heysell, S.K. Clofazimine for treatment of multidrug-resistant non-tuberculous mycobacteria. Pulm. Pharmacol. Ther. 2021, 70, 102058. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Fujiwara, K.; Furuuchi, K.; Ito, M.; Hanada, K.; Kodama, T.; Aono, A.; Mitarai, S.; Yoshiyama, T.; Kurashima, A.; et al. Clofazimine serum concentration and safety/efficacy in nontuberculous mycobacterial pulmonary disease treatment. Respir. Med. 2024, 231, 107718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, Y.; Islam, M.M.; Cao, Y.; Lu, X.; Zeng, S.; Hameed, H.M.A.; Zhou, P.; Cai, X.; Wang, S.; et al. Assessment of Clofazimine and TB47 Combination Activity against Mycobacterium abscessus Using a Bioluminescent Approach. Antimicrob. Agents Chemother. 2020, 64, e02627-16. [Google Scholar] [CrossRef]
- Howard, S.T.; Rhoades, E.; Recht, J.; Pang, X.; Alsup, A.; Kolter, R.; Lyons, C.R.; Byrd, T.F. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 2006, 152, 1581–1590. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Boudehen, Y.M.; Kremer, L. Characterization of Mycobacterium abscessus colony-biofilms based on bi-dimensional images. Antimicrob. Agents Chemother. 2023, 67, 0040223. [Google Scholar] [CrossRef]
- Griffith, D.E.; Girard, W.M.; Wallace, R.J., Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am. Rev. Respir. Dis. 1993, 147, 1271–1278. [Google Scholar] [CrossRef]
- Pawlik, A.; Garnier, G.; Orgeur, M.; Tong, P.; Lohan, A.; Le Chevalier, F.; Sapriel, G.; Roux, A.; Conlon, K.; Honoré, N.; et al. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. Mol. Microbiol. 2013, 90, 612–629. [Google Scholar] [CrossRef]
- Nandanwar, N.; Gibson, J.E.; Neely, M.N. Growth medium and nitric oxide alter Mycobacterium abscessus morphotype and virulence. Microbiol. Res. 2021, 253, 126887. [Google Scholar] [CrossRef]
- Ferrell, K.C.; Johansen, M.D.; Triccas, J.A.; Counoupas, C. Virulence Mechanisms of Mycobacterium abscessus: Current Knowledge and Implications for Vaccine Design. Front. Microbiol. 2022, 13, 842017. [Google Scholar] [CrossRef]
- Rüger, K.; Hampel, A.; Billig, S.; Rücker, N.; Suerbaum, S.; Bange, F.C. Characterization of rough and smooth morphotypes of Mycobacterium abscessus isolates from clinical specimens. J. Clin. Microbiol. 2014, 52, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ye, M.; Zhao, L.; Guo, Q.; Chen, J.; Xu, B.; Zhan, M.; Zhang, Y.; Zhang, Z.; Chu, H. Glycopeptidolipid Genotype Correlates With the Severity of Mycobacterium abscessus Lung Disease. J. Infect. Dis. 2020, 221 (Suppl. S2), S257–S262. [Google Scholar] [CrossRef] [PubMed]
- Conyers, L.E.; Saunders, B.M. Treatment for non-tuberculous mycobacteria: Challenges and prospects. Front. Microbiol. 2024, 15, 1394220. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; García-Coca, M. Mycobacterium Biofilms. Front. Microbiol. 2018, 8, 2651. [Google Scholar] [CrossRef]
- Kothavade, R.J.; Dhurat, R.S.; Mishra, S.N.; Kothavade, U.R. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 161–188. [Google Scholar] [CrossRef]
- Parmar, S.; Tocheva, E.I. The cell envelope of Mycobacterium abscessus and its role in pathogenesis. PLoS Pathog. 2023, 19, 1011318. [Google Scholar] [CrossRef]
- Lee, M.R.; Sheng, W.H.; Hung, C.C.; Yu, C.J.; Lee, L.N.; Hsueh, P.R. Mycobacterium abscessus Complex Infections in Humans. Emerg. Infect. Dis. 2015, 21, 1638–1646. [Google Scholar] [CrossRef]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Shi, L.; Günther, S.; Hübschmann, T.; Wick, L.Y.; Harms, H.; Müller, S. Limits of propidium iodide as a cell viability indicator for environmental bacteria. Cytometry A 2007, 71, 592–598. [Google Scholar] [CrossRef]
- Clary, G.; Sasindran, S.J.; Nesbitt, N.; Mason, L.; Cole, S.; Azad, A.K.; McCoy, K.; Schlesinger, L.S.; Honda, J.R. Mycobacterium abscessus smooth and rough morphotypes form antimicrobial-tolerant biofilm phenotypes but are killed by acetic acid. Antimicrob. Agents Chemother. 2018, 62, e01782-17. [Google Scholar] [CrossRef]
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. A laboratory perspective on Mycobacterium abscessus biofilm research. Front. Microbiol. 2024, 15, 1392606. [Google Scholar]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, 2437. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995, Erratum in Front. Cell. Infect. Microbiol. 2025, 15, 1564010. [Google Scholar] [CrossRef] [PubMed]
- Meliefste, H.M.; Mudde, S.E.; Ammerman, N.C.; de Steenwinkel, J.E.M.; Bax, H.I. A laboratory perspective on Mycobacterium abscessus biofilm culture, characterization, and drug activity testing. Front. Microbiol. 2024, 15, 1392606. [Google Scholar] [CrossRef]

| No. | Species | Macrolide Resistance | Aminoglycoside Resistance | ||||
|---|---|---|---|---|---|---|---|
| Clarithromycin MIC (µg/mL) | Detected Mutation | Amikacin MIC (µg/mL) | Detected Mutation | ||||
| 1 | M. abscessus subsp. abscessus | 0.12 | S | - | 16 | S | - |
| 2 | M. abscessus subsp. abscessus | 8 | R | erm(41) T28 | 16 | S | - |
| 3 | M. abscessus subsp. abscessus | 8 | R | erm(41) T28 | 16 | S | - |
| 4 | M. abscessus subsp. abscessus | 16 | R | erm(41) T28 | 16 | S | - |
| 5 | M. abscessus subsp. abscessus | 16 | R | erm(41) T28 | 16 | S | - |
| 6 | M. abscessus subsp. abscessus | 8 | R | erm(41) T28 | 16 | S | - |
| 7 | M. abscessus subsp. abscessus | 16 | R | erm(41) T28 | 16 | S | - |
| 8 | M. abscessus subs massiliense | 0.125 | S | - | 16 | S | - |
| 9 | M. abscessus subsp. abscessus | 16 | R | erm(41) T28, rrl | 16 | S | rrs—T1408G |
| 10 | M. abscessus subs massiliense | 0.06 | S | - | 4 | S | - |
| 11 | M. abscessus subs massiliense | 0.06 | S | - | 16 | S | - |
| 12 | M. abscessus subsp. bolletii | 8 | R | erm(41) T28 | 16 | S | - |
| 13 | M. abscessus subsp. abscessus | 0.25 | S | erm(41) T28 | 16 | S | - |
| 14 | M. abscessus subsp. abscessus | 16 | R | erm(41) T28 | 16 | S | - |
| 15 | M. abscessus subsp. abscessus | 0.5 | S | erm(41) T28 | 2 | S | - |
| 16 | M. abscessus subs massiliense | 0.12 | S | - | 4 | S | - |
| 17 | M. abscessus subs massiliense | ≤0.06 | S | - | ≤1 | S | - |
| 18 | M. abscessus subs abscessus | 0.06 | S | erm(41) T28 | 16 | S | - |
| 19 | M. abscessus subsp. massiliense | 0.06 | S | - | 16 | S | - |
| 20 | M. abscessus subsp. abscessus | 0.5 | S | erm(41) T28 | 2 | S | - |
| Antimicrobial Agent | MIC (μg/mL) | ||
|---|---|---|---|
| MIC50 | MIC90 | Range | |
| AN | 16 | 16 | 1–16 |
| FOX | 128 | 128 | 32–128 |
| CIP | 4 | 4 | 2–4 |
| DOX | 16 | 16 | 1–16 |
| LZD | 2 | 16 | 1–16 |
| MXF | 4 | 8 | 2–8 |
| SXT | 4/76 | 8/152 | 4/76–8/152 |
| TGC | 0.5 | 1 | 0.25–2 |
| CLA | 0.06 | 4 | 0.5–16 |
| MIC (μg/mL) | |||
|---|---|---|---|
| Antimicrobial Agent | MIC50 | MIC90 | Range |
| BDQ | 0.5 | 0.5 | 0.125–1 |
| DEL | >16 | >16 | >16 |
| CLO | >8 | >8 | >8 |
| Strains No. | Species | Morphotype 7H10 Agar | Crystal Violet Staining Method | ||
|---|---|---|---|---|---|
| OD595 | OD595/OD595control Ratio * | Characterization | |||
| 1 | M. abscessus subsp. abscessus | Rough | 2.45 | 1.99 | weak biofilm |
| 2 | M. abscessus subsp. abscessus | Rough | 4.89 | 3.74 | moderate biofilm |
| 3 | M. abscessus subsp. abscessus | Rough | 2.35 | 1.76 | weak biofilm |
| 4 | M. abscessus subsp. abscessus | Smooth | 4.69 | 3.59 | moderate biofilm |
| 5 | M. abscessus subsp. abscessus | Rough | 3.78 | 3.15 | moderate biofilm |
| 6 | M. abscessus subsp. abscessus | Mixed | 5.98 | 4.63 | strong biofilm |
| 7 | M. abscessus subsp. abscessus | Mixed | 5.98 | 4.96 | strong biofilm |
| 8 | M. abscessus subs massiliense | Rough | 2.08 | 1.64 | weak biofilm |
| 9 | M. abscessus subsp. abscessus | Rough | 2.39 | 1.98 | weak biofilm |
| 10 | M. abscessus subsp. massiliense | Rough | 1.27 | 1.02 | weak biofilm |
| 11 | M. abscessus subsp. massiliense | Rough | 3.56 | 3.02 | moderate biofilm |
| 12 | M. abscessus subsp. bolletii | Smooth | 5.43 | 4.55 | strong biofilm |
| 13 | M. abscessus subsp. abscessus | Rough | 4.86 | 3.51 | moderate biofilm |
| 14 | M. abscessus subsp. abscessus | Rough | 2.81 | 1.90 | weak biofilm |
| 15 | M. abscessus subsp. abscessus | Rough | 2.25 | 1.73 | weak biofilm |
| 16 | M. abscessus subsp. massiliense | Rough | 5.48 | 4.34 | strong biofilm |
| 17 | M. abscessus subsp. massiliense | Mixed | 1.93 | 1.55 | weak biofilm |
| 18 | M. abscessus subs abscessus | Rough | 2.23 | 1.44 | weak biofilm |
| 19 | M. abscessus subsp. massiliense | Rough | 2.54 | 1.37 | weak biofilm |
| 20 | M. abscessus subsp. abscessus | Mixed | 5.98 | 4.76 | strong biofilm |
| Phenotype of Colony | Ability to Form Biofilm | No. of Strains | Comparison and p-Value * |
|---|---|---|---|
| Rough | weak biofilm | 9 | Rough vs. Weak: p = 0.141 (ns a) |
| moderate biofilm | 4 | ||
| strong biofilm | 1 | ||
| Smooth | moderate biofilm | 1 | Smooth vs. Strong: p = 0.447 (ns a) |
| strong biofilm | 1 | ||
| Mixed | weak biofilm | 1 | Mixed vs. Strong: p = 0.032 (s b) |
| strong biofilm | 3 |
| Tested Strains | Bedaquiline Activity | ||
|---|---|---|---|
| MIC (µg/mL) | MBIC (µg/mL) | MBIC/MIC Ratio | |
| 1 | 0.5 | 2 | 4 |
| 2 | 0.5 | 1 | 2 |
| 3 | 1 | 1 | 1 |
| 4 | 0.5 | 1 | 2 |
| 5 | 0.5 | 0.5 | 1 |
| 6 | 0.5 | 1 | 2 |
| 7 | 0.5 | 2 | 4 |
| 8 | 0.5 | 1 | 2 |
| 9 | 0.25 | 1 | 4 |
| 10 | 0.25 | 1 | 4 |
| 11 | 0.5 | 2 | 4 |
| 12 | 0.25 | 1 | 4 |
| 13 | 0.5 | 1 | 2 |
| 14 | 0.25 | 1 | 4 |
| 15 | 0.5 | 1 | 2 |
| 16 | 0.25 | 1 | 4 |
| 17 | 0.125 | 0.25 | 2 |
| 18 | 0.5 | 2 | 4 |
| 19 | 0.5 | 2 | 4 |
| 20 | 0.25 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, K.; Wójcik, K.; Skórkowska, A.; Klesiewicz, K. Evaluation of the In Vitro Activity of Bedaquiline, Delamanid, and Clofazimine Against Mycobacterium abscessus Complex and Their Antibiofilm Potential. Pathogens 2025, 14, 582. https://doi.org/10.3390/pathogens14060582
Kania K, Wójcik K, Skórkowska A, Klesiewicz K. Evaluation of the In Vitro Activity of Bedaquiline, Delamanid, and Clofazimine Against Mycobacterium abscessus Complex and Their Antibiofilm Potential. Pathogens. 2025; 14(6):582. https://doi.org/10.3390/pathogens14060582
Chicago/Turabian StyleKania, Katarzyna, Katarzyna Wójcik, Alicja Skórkowska, and Karolina Klesiewicz. 2025. "Evaluation of the In Vitro Activity of Bedaquiline, Delamanid, and Clofazimine Against Mycobacterium abscessus Complex and Their Antibiofilm Potential" Pathogens 14, no. 6: 582. https://doi.org/10.3390/pathogens14060582
APA StyleKania, K., Wójcik, K., Skórkowska, A., & Klesiewicz, K. (2025). Evaluation of the In Vitro Activity of Bedaquiline, Delamanid, and Clofazimine Against Mycobacterium abscessus Complex and Their Antibiofilm Potential. Pathogens, 14(6), 582. https://doi.org/10.3390/pathogens14060582