Detection and Inhibition of Clostridium perfringens by Cocktail of Star Anise and Thymus Extracts in Chicken Meat Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Collection of Samples
2.2. Star Anise and Thymus Extracts Preparation
2.3. Phytochemical Analysis of Extracts
2.4. Anti-Clostridial Activity of Extracts
2.5. Identification of Minimum Inhibitory Concentrations (MICs)
2.6. Detection of C. perfringens in Chicken Samples
2.7. Experimental Application on Chicken Burger After Treatment by Mixture Extract
2.8. Sensory Evaluation of Chicken Burger
2.9. Statistical Analyses
3. Results and Discussion
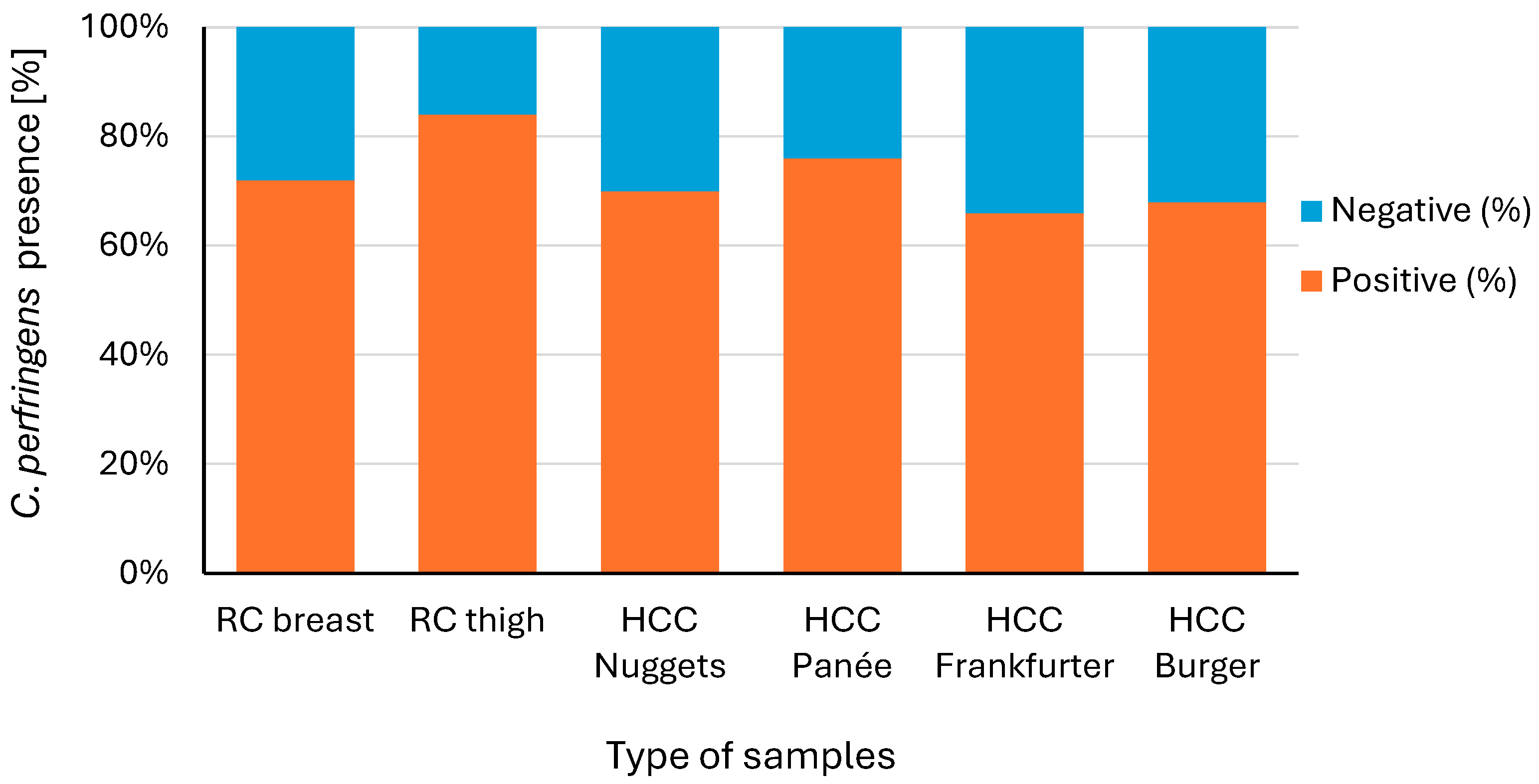
3.1. Incidence of C. perfringens in the Examined Chicken Meat Product Samples
3.2. The Use of Star Anise and Thymus Extracts as Antimicrobial Agents
3.2.1. Phytochemical Composition of Star Anise and Thymus Extracts and Their Mixture
3.2.2. Antioxidant Activity and DPPH Radical Scavenging Capacity of Extracts
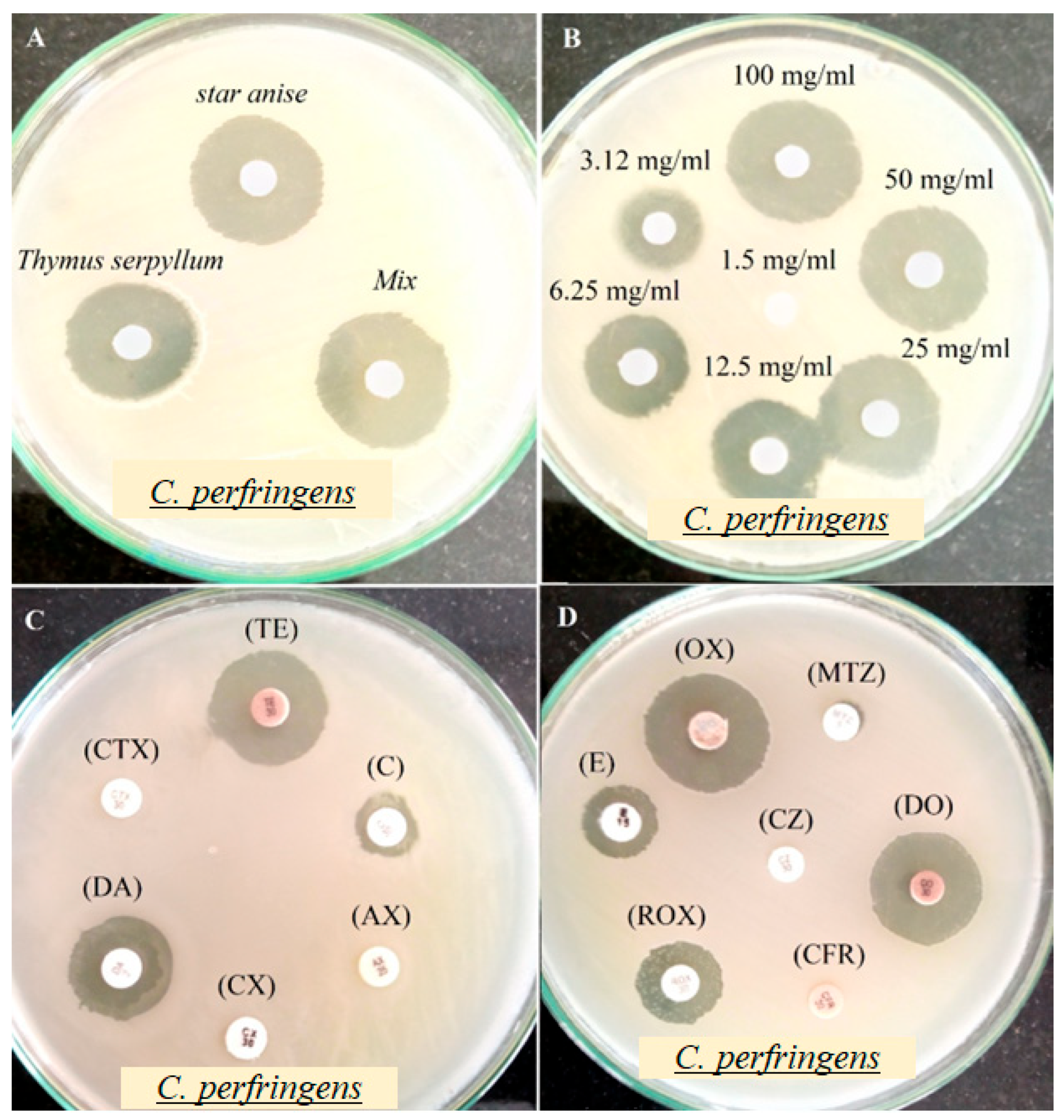
3.2.3. Influence of Extracts on C. perfringens Activity
3.3. Application of Extracts in Chicken Burger
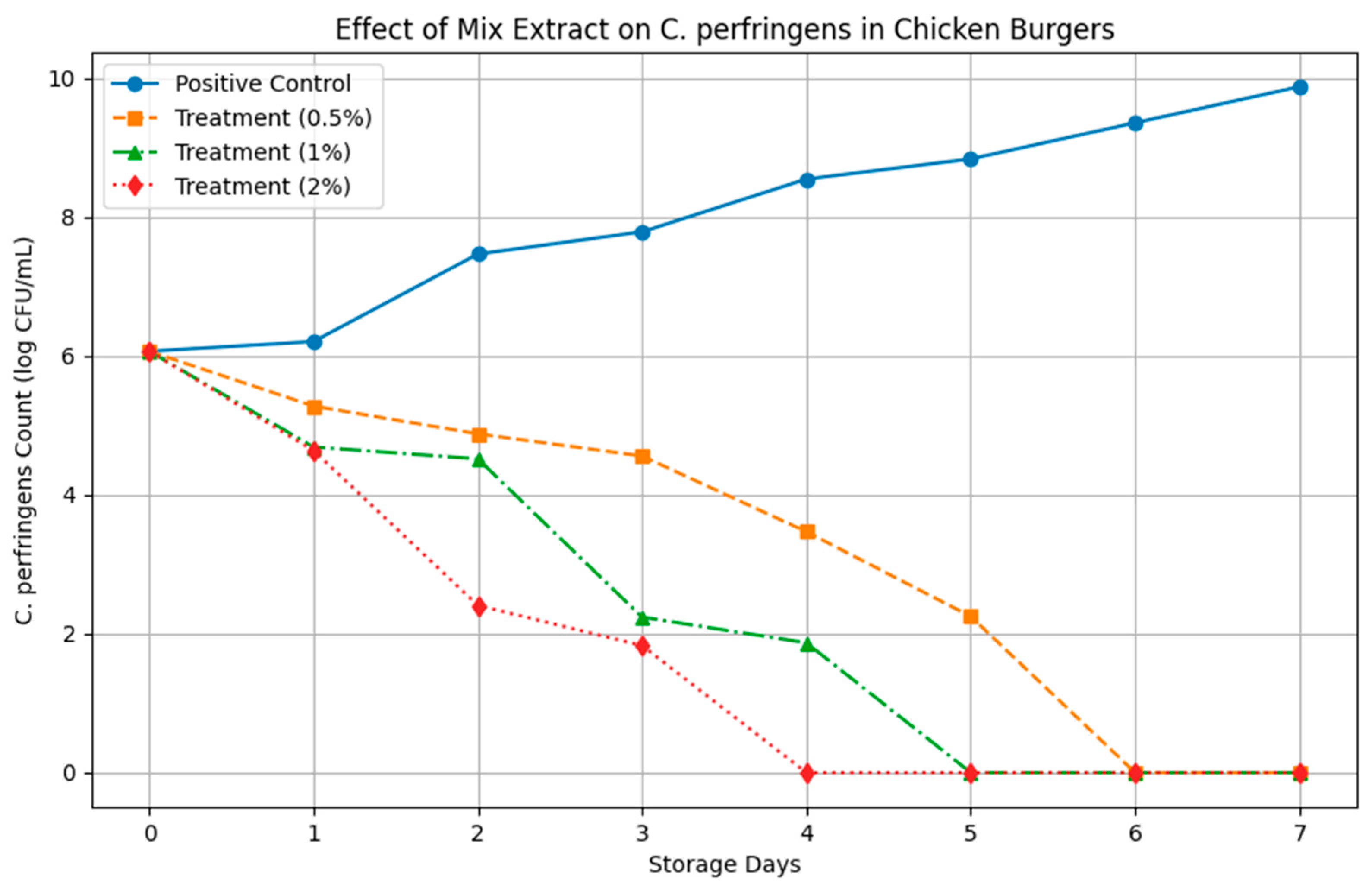
3.3.1. Influence on Development of C. perfringens During Storage
3.3.2. Sensory Evaluation of Chicken Burger Fortified with Mix Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaltout, F.; Zakaria, I.; Nabil, M. Incidence of Some Anaerobic Bacteria Isolated from Chicken Meat Products with Special Reference to Clostridium perfringens. Nutr. Food Toxicol. 2018, 2, 429–438. [Google Scholar] [CrossRef]
- Geornaras, I.; De Jesus, A.; Van Zyl, E.; Von Holy, A. Microbiological survey of a South African poultry processing plant. J. Basic Microbiol. 1995, 35, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafy, A.M.; Younis, H.A.; Osman, A.I.; Hussein, S.M.; El-Ela, A.S.A.; Mahmoud, E.A.; Elsherbiny, O.; Rashwan, A.K. Recent Advances in Detection and Control Strategies for Foodborne Bacteria in Raw and Ready-to-Eat Fruits and Vegetables. Food Front. 2025, 6, 605–629. [Google Scholar] [CrossRef]
- Guran, H.; Oksuztepe, G. Detection and typing of Clostridium perfringens from retail chicken meat parts. Lett. Appl. Microbiol. 2013, 57, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Gerges, M.; Mehany, T.; Hussein, S.M.; Eskander, M.; Tawfik, R.G.; El-Halmouch, Y.; Mansour, A.M.; Hafez, E.E.; Esatbeyoglu, T.; et al. Estimating the Prevalence of Foodborne Pathogen Campylobacter jejuni in Chicken and Its Control via Sorghum Extracts. Pathogens 2023, 12, 958. [Google Scholar] [CrossRef]
- Butler, A.J.; Thomas, M.K.; Pintar, K.D. Expert elicitation as a means to attribute 28 enteric pathogens to foodborne, waterborne, animal contact, and person-to-person transmission routes in Canada. Foodborne Pathog. Dis. 2015, 12, 335–344. [Google Scholar] [CrossRef]
- Fahmy, H.; Badr, S.; El-Khamissi, H.; Hassan, Z. Oxidative stress, biochemical and histopathological alterations induced by some synthetic food colorants on Albino rats. Arch. Agric. Sci. J. 2021, 4, 83–98. [Google Scholar] [CrossRef]
- García, S.; Araiza, M.; Gómez, M.; Heredia, N. Inhibition of growth, enterotoxin production, and spore formation of Clostridium perfringens by extracts of medicinal plants. J. Food Prot. 2002, 65, 1667–1669. [Google Scholar] [CrossRef]
- Naveena, B.; Sen, A.; Vaithiyanathan, S.; Babji, Y.; Kondaiah, N. Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci. 2008, 80, 1304–1308. [Google Scholar] [CrossRef]
- Velasco, V.; Williams, P. Improving meat quality through natural antioxidants. Chil. J. Agric. Res. 2011, 71, 313–322. [Google Scholar] [CrossRef]
- Padmashree, A.; Roopa, N.; Semwal, A.; Sharma, G.; Agathian, G.; Bawa, A.S. Star-anise (Illicium verum) and black caraway (Carum nigrum) as natural antioxidants. Food Chem. 2007, 104, 59–66. [Google Scholar] [CrossRef]
- El-Waseif, M.; Saed, B.; Fahmy, H.; Sabry, A.; Shaaban, H.; Abdelgawad, M.; Amin, A.; Farouk, A. Mayonnaise enriched with flaxseed oil: Omega-3 fatty acids content, sensory quality and stability during the storage. Foods 2022, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Naveena, B.; Vaithiyanathan, S.; Muthukumar, M.; Sen, A.; Kumar, Y.P.; Kiran, M.; Shaju, V.; Chandran, K.R. Relationship between the solubility, dosage and antioxidant capacity of carnosic acid in raw and cooked ground buffalo meat patties and chicken patties. Meat Sci. 2013, 95, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Al-Geddawi, M.; Ragab, W.; Nassar, A.; Abdelshafy, A. Antimicrobial Activity of Some Spices and Herbs Essential Oils. Assiut J. Agric. Sci. 2016, 47, 29–37. [Google Scholar] [CrossRef][Green Version]
- Ouattara, L.; Koudou, J.; Zongo, C.; Barro, N.; Savadogo, A.; Bassole, I.; Ouattara, A.; Traore, A.S. Antioxidant and antibacterial activities of three species of Lannea from Burkina Faso. J. Appl. Sci. 2011, 11, 157–162. [Google Scholar] [CrossRef]
- Abid, K.Y.; Abachi, F.T. Phytochemical Comparative Studies, Antioxidant and Antimicrobial of Artemisia and Star Anise. Pharmacogn. J. 2023, 15, 183–188. [Google Scholar] [CrossRef]
- Zeng, M.; He, Z.; Zheng, Z.; Qin, F.; Tao, G.; Zhang, S.; Gao, Y.; Chen, J. Effect of six Chinese spices on heterocyclic amine profiles in roast beef patties by ultra performance liquid chromatography-tandem mass spectrometry and principal component analysis. J. Agric. Food Chem. 2014, 62, 9908–9915. [Google Scholar] [CrossRef]
- Huang Zhan, H.Z.; Liu XiaoChang, L.X.; Jia ShiLiang, J.S.; Zhang LongTeng, Z.L.; Luo YongKang, L.Y. The effect of essential oils on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Int. J. Food Microbiol. 2018, 266, 52–59. [Google Scholar] [CrossRef]
- Asbaghian, S.; Shafaghat, A.; Zarea, K.; Kasimov, F.; Salimi, F. Comparison of volatile constituents, and antioxidant and antibacterial activities of the essential oils of Thymus caucasicus, T. kotschyanus and T. vulgaris. Nat. Prod. Commun. 2011, 6, 137–140. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Sorour, M.A.E.; El-Hamied, A.; Adel, A.; Mahmoud, A.R.; Mahmoud, E.A. Impact of liquid smoke and thyme oil on quality of chicken and turkey chilled meatballs. J. Sohag Agrisci. (JSAS) 2021, 6, 137–150. [Google Scholar] [CrossRef]
- Baharfar, R.; Azimi, R.; Mohseni, M. Antioxidant and antibacterial activity of flavonoid-, polyphenol-and anthocyanin-rich extracts from Thymus kotschyanus boiss & hohen aerial parts. J. Food Sci. Technol. 2015, 52, 6777–6783. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Li, X.; Xing, Z.; Li, F.; Shen, M.; Wang, H.; Shi, X.; Du, L. Silica/gold nanoplatform combined with a thermosensitive gel for imaging-guided interventional therapy in PDX of pancreatic cancer. Chem. Eng. J. 2020, 382, 122949. [Google Scholar] [CrossRef]
- Zhong, H.; Mu, B.; Zhang, M.; Hui, A.; Kang, Y.; Wang, A. Preparation of effective carvacrol/attapulgite hybrid antibacterial materials by mechanical milling. J. Porous Mater. 2020, 27, 843–853. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Zhang, C.; Pich, A.; Xing, L.; Shi, X. Intelligent nanogels with self-adaptive responsiveness for improved tumor drug delivery and augmented chemotherapy. Bioact. Mater. 2021, 6, 3473–3484. [Google Scholar] [CrossRef]
- Monteiro, C.R.; do Carmo, M.S.; Melo, B.O.; Alves, M.S.; Dos Santos, C.I.; Monteiro, S.G.; Bomfim, M.R.Q.; Fernandes, E.S.; Monteiro-Neto, V. In vitro antimicrobial activity and probiotic potential of Bifidobacterium and Lactobacillus against species of Clostridium. Nutrients 2019, 11, 448. [Google Scholar] [CrossRef]
- Hamad, G.M.; Taha, T.H.; El-Deeb, N.M.; Alshehri, A.M. Advanced trends in controlling Helicobacter pylori infections using functional and therapeutically supplements in baby milk. J. Food Sci. Technol. 2015, 52, 8156–8163. [Google Scholar] [CrossRef][Green Version]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Hamad, G.M.; Abu-Serie, M.M.; Ali, S.H.; Hafez, E.E. Combination probiotic supernatants reduce growth and aflatoxin production by Aspergillus spp in food contamination. Am. J. Food Sci. Technol 2018, 6, 57–67. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wong, Y.; Lee, P.; Nurdiyana, W.W. Extraction and antioxidative activity of essential oil from star anise (Illicium verum). Orient. J. Chem. 2014, 30, 1159. [Google Scholar] [CrossRef]
- Hamad, G.M.; Abdelmotilib, N.M.; Darwish, A.M.; Zeitoun, A.M. Commercial probiotic cell-free supernatants for inhibition of Clostridium perfringens poultry meat infection in Egypt. Anaerobe 2020, 62, 102181. [Google Scholar] [CrossRef]
- Duc, H.M.; Hoa, T.T.K.; Ha, C.T.T.; Van Hung, L.; Van Thang, N.; Minh Son, H.; Flory, G.A. Prevalence and Antibiotic Resistance Profile of Clostridium perfringens Isolated from Pork and Chicken Meat in Vietnam. Pathogens 2024, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Abushaala, N.M.; Soltan, O.I.; Abdel-Hameed, S.M.; Magdy, R.M.; Ahmed, E.M.H.; Elshaer, S.E.; Kamar, A.M.; Hashem, R.M.A.; Elghazaly, E.M. Prevalence and antibacterial effect of natural extracts against Vibrio parahaemolyticus and its application on Tilapia Fillets. LWT 2024, 209, 116812. [Google Scholar] [CrossRef]
- Alqurashi, R.M.; Aldossary, H.M. In vitro antioxidant and antimicrobial activity of Moringa oleifera leaf as a natural food preservative in chicken burgers. Emir. J. Food Agric. 2021, 33, 450–457. [Google Scholar] [CrossRef]
- Keeton, J.T. Effects of fat and NaCl/phosphate levels on the chemical and sensory properties of pork patties. J. Food Sci. 1983, 48, 878–881. [Google Scholar] [CrossRef]
- Mohammed, H.N. Study of some chemical, physical, sensory and bacteriology characteristics of canned chicken meat imported to Sulaymaniyah markets, Iraq. Int. J. Nutr. Metab. 2013, 5, 128–133. [Google Scholar] [CrossRef]
- Wen, Q.; McClane, B.A. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl. Environ. Microbiol. 2004, 70, 2685–2691. [Google Scholar] [CrossRef]
- Cooper, K.K.; Bueschel, D.M.; Songer, J.G. Presence of Clostridium perfringens in retail chicken livers. Anaerobe 2013, 21, 67–68. [Google Scholar] [CrossRef]
- Shaltout, F.; Zakaria, I.; Nabil, M. Detection and typing of Clostridium perfringens in some retail chicken meat products. Benha Vet. Med. J. 2017, 33, 283–291. [Google Scholar] [CrossRef]
- Tschirdewahn, B.; Notermans, S.; Wernars, K.; Untermann, F. The presence of enterotoxigenic Clostridium perfringens strains in faeces of various animals. Int. J. Food Microbiol. 1991, 14, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Elchaghaby, M.A.; Rashad, S.; Wassef, N.M. Bioactivity and antibacterial effect of star anise biosynthesized silver nanoparticles against Streptococcus mutans: An in vitro study. BMC Complement. Med. Ther. 2024, 24, 259. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Aly, S.E.; Sabry, B.A.; Shaheen, M.S.; Hathout, A.S. Assessment of antimycotoxigenic and antioxidant activity of star anise (Illicium verum) in vitro. J. Saudi Soc. Agric. Sci. 2016, 15, 20–27. [Google Scholar] [CrossRef]
- Syukur, M.; Prahasiwi, M.S.; Nurkhasanah; Yuliani, S.; Purwaningsih, Y.; Indriyanti, E. Profiling of Active Compounds of Extract Ethanol, n-Hexane, Ethyl Acetate and Fraction Ethanol of Star Anise (Illicium verum Hook. f.) and Determination of Total Flavonoids, Total Phenolics and Their Potential as Antioxidants. Sci. Technol. Indones. 2023, 8, 219–226. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Bose, S.; Banerjee, S.; Vishnuprasad, C.N.; del Pilar Rodriguez-Torres, M.; Shin, H.S. Star anise (Illicium verum): Chemical compounds, antiviral properties, and clinical relevance. Phytother. Res. 2020, 34, 1248–1267. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Luís, Â.; Sousa, S.; Wackerlig, J.; Dobusch, D.; Duarte, A.P.; Pereira, L.; Domingues, F. Star anise (Illicium verum Hook. f.) essential oil: Antioxidant properties and antibacterial activity against Acinetobacter baumannii. Flavour Fragr. J. 2019, 34, 260–270. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food chemistry 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Nickavar, B.; Esbati, N. Evaluation of the antioxidant capacity and phenolic content of three Thymus species. J. Acupunct. Meridian Stud. 2012, 5, 119–125. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Li, X.; Cao, X.; Li, Y.; Cao, H.; Men, Y. Multistage extraction of star anise and black pepper derivatives for antibacterial, antioxidant, and anticancer activity. Front. Chem. 2021, 9, 660138. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid.-Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A. Utilization of pomegranate peels to increase the shelf life of chicken burger during cold storage. Egypt. J. Food Sci. 2019, 47, 1–10. [Google Scholar] [CrossRef]
- Xueqin, G.; Li, H.; Xuan, M.; Li, F.; Weishuai, L. Effect of star anise extract on the stability of cooked pork during refrigerated storage. China Food Addit. 2022, 33, 148–156. [Google Scholar] [CrossRef]
- Darwish, S.M.; El-Geddawy, M.A.; Khalifa, R.M.; Mohamed, R.A. Antioxidant activities of some spices and herbs added to frozen chicken burger. Front. Sci. 2012, 2, 144–152. [Google Scholar] [CrossRef][Green Version]
- Mahmoud, E.A. Effect of rosemary, basil, and mint leaves extracts on quality of chilled chicken burger. J. Food Dairy Sci. 2017, 8, 151–161. [Google Scholar] [CrossRef]


| Ingredients (%) | Control | Treatment | ||
|---|---|---|---|---|
| 0.5% Mix Extract | 1% Mix Extract | 2% Mix Extract | ||
| Brest Chicken | 88 | 88 | 88 | 88 |
| Breadcrumbs | 5.0 | 4.5 | 4.0 | 3.0 |
| Whole egg | 5.0 | 5.0 | 5.0 | 5.0 |
| Salt | 1.0 | 1.0 | 1.0 | 1.0 |
| Black pepper | 1.0 | 1.0 | 1.0 | 1.0 |
| Mix extract | 0.0 | 0.5 | 1.0 | 2.0 |
| Extracts | Total Phenolic Concentration (mg GAE/g) | Total Flavonoids Concentartaion (mg Quercetin/g) |
|---|---|---|
| Star anise extract | 97.02 ± 6.63 b | 56.74 ± 5.37 b |
| Thymus serpyllum extract | 23.24 ± 0.84 c | 5.41 ± 1.04 c |
| Mix extract | 123.88 ± 7.42 a | 69.04 ± 6.37 a |
| Phenolic and Flavonoid Compounds | Star Anise Extract [µg/g] | Thymus serpyllum [µg/g] |
|---|---|---|
| Gallic acid | 35.35 ± 4.10 A | 2.02 ± 0.33 B |
| Chlorogenic acid | 8.74 ± 2.21 A | 0.00 ± 0.00 B |
| Vanillic acid | 8.84 ± 1.66 A | 0.00 ± 0.00 B |
| Catechin | 85.53 ± 5.30 A | 0.00 ± 0.00 B |
| Cinnamic acid | 10.84 ± 2.10 A | 7.91 ± 0.60 B |
| Salicylic acid | 3.71 ± 0.56 A | 0.00 ± 0.00 B |
| Syringic acid | 0.73 ± 0.42 A | 0.00 ± 0.00 B |
| Ferulic acid | 6.46 ± 2.06 B | 13.55 ± 2.15 A |
| Rutin | 67.91 ± 7.46 A | 0.00 ± 0.00 B |
| Rosmarinic acid | 0.00 ± 0.00 B | 150.44 ± 9.49 A |
| Quercetin | 5.36 ± 1.07 A | 0.00 ± 0.00 B |
| Naringinin | 0.00 ± 0.00 B | 2.90 ± 0.78 A |
| Kaempferol | 2.43 ± 0.68 A | 0.93 ± 0.37 B |
| Apigenin | 0.00 ± 0.00 B | 9.10 ± 0.20 A |
| Caffeic acid | 1.88 ± 0.55 B | 15.52 ± 1.06 A |
| Ellagic acid | 0.96 ± 0.56 A | 0.00 ± 0.00 B |
| 4-hydroxybenzoic acid | 2.90 ± 0.45 A | 0.00 ± 0.00 B |
| Coumaric acid | 74.47 ± 9.19 A | 6.66 ± 2.15 B |
| Sinapic | 0.95 ± 0.39 A | 0.00 ± 0.00 B |
| Luteolin | 0.00 ± 0.00 B | 2.40 ± 0.50 A |
| Benzoic acid | 0.97± 0.49 A | 0.00 ± 0.00 B |
| Myricetin | 0.42 ± 1.11 A | 0.00 ± 0.00 B |
| Epicatechin | 0.00 ± 0.00 B | 0.50 ± 0.09 A |
| Salvianolic acid | 0.00 ± 0.00 B | 27.26 ± 1.56 A |
| Extracts/Antibiotics | Concentration | Inhibition Zone Diameter (mm) Mean ± SD |
|---|---|---|
| Antibacterial activity of star anise and Thymus serpyllum extract | ||
| Star anise extract | 100 mg/mL | 26.07 ± 1.06 c |
| Thymus serpyllum extract | 100 mg/mL | 23.13 ± 1.04 e |
| Mix extract | 100 mg/mL | 31.07 ± 2.01 a |
| Antibacterial activity of antibiotic | ||
| Oxytetracycline (Ox) | 30 µg/disc (0.030 mg) | 22.10 ± 0.85 f |
| Erythromycin (E) | 50 µg/disc (0.050 mg) | 12.13 ± 1.02 f |
| Cefadroxil (CFR) | 30 µg/disc (0.030 mg) | 0.00 ± 0.00 l |
| Cefazolin (CZ) | 30 µg/disc (0.030 mg) | 0.00 ± 0.00 l |
| Doxycycline (DO) | 30 µg/disc (0.030 mg) | 20.13 ± 0.60 g |
| Metronidazole (MTZ) | 25 µg/disc (0.025 mg) | 0.00 ± 0.00 l |
| Roxithromycin (RoX) | 30 µg/disc (0.030 mg) | 17.06 ± 1.10 h |
| Tetracycline (TE) | 30 µg/disc (0.030 mg) | 17.96 ± 0.95 h |
| Chloramphencol (C) | 30 µg/disc (0.010 mg) | 11.00 ± 0.80 k |
| Amoxicillin (AX) | 25 µg/disc (0.025 mg) | 0.00 ± 0.00 l |
| Clindamycin (DA) | 2 µg/disc (0.002 mg) | 15.03 ± 0.94 i |
| Cefoxitin (CX) | 30 µg/disc (0.030 mg) | 0.00 ± 0.00 l |
| Cefotoxime (CTX) | 30 µg/disc (0.030 mg) | 0.00 ± 0.00 l |
| Minimum inhibitory concentrations (MICs) of Mix extract | ||
| Mix Extract | 100 mg/mL | 31.07 ± 2.01 a |
| 50 mg/mL | 27.16 ± 0.76 b | |
| 25 mg/mL | 24.14 ± 0.91 d | |
| 12.5 mg/mL | 20.30 ± 1.30 g | |
| 6.25 mg/mL | 15.12 ± 0.96 i | |
| 3.12 mg/mL | 13.06 ± 0.91 j | |
| 1.5 mg/mL | 0.00 ± 0.00 l | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, G.M.; Henry, S.G.M.; El-Rokh, G.E.A.; Ramadan, N.H.A.; Raoof, H.S.A.; Sulaiman, A.M.; El-Mesallamy, A.M.; Elshaer, S.E.; Gaber, S.M.; Rabah, I.M.; et al. Detection and Inhibition of Clostridium perfringens by Cocktail of Star Anise and Thymus Extracts in Chicken Meat Products. Pathogens 2025, 14, 552. https://doi.org/10.3390/pathogens14060552
Hamad GM, Henry SGM, El-Rokh GEA, Ramadan NHA, Raoof HSA, Sulaiman AM, El-Mesallamy AM, Elshaer SE, Gaber SM, Rabah IM, et al. Detection and Inhibition of Clostridium perfringens by Cocktail of Star Anise and Thymus Extracts in Chicken Meat Products. Pathogens. 2025; 14(6):552. https://doi.org/10.3390/pathogens14060552
Chicago/Turabian StyleHamad, Gamal M., Shenoda Gaber Monir Henry, Gamal E. A. El-Rokh, Nadia H. A. Ramadan, Hany S. Abdel Raoof, Ahmed M. Sulaiman, Ahmed M. El-Mesallamy, Samy E. Elshaer, Sara M. Gaber, Ibrahim M. Rabah, and et al. 2025. "Detection and Inhibition of Clostridium perfringens by Cocktail of Star Anise and Thymus Extracts in Chicken Meat Products" Pathogens 14, no. 6: 552. https://doi.org/10.3390/pathogens14060552
APA StyleHamad, G. M., Henry, S. G. M., El-Rokh, G. E. A., Ramadan, N. H. A., Raoof, H. S. A., Sulaiman, A. M., El-Mesallamy, A. M., Elshaer, S. E., Gaber, S. M., Rabah, I. M., Mahmoud, A. R., Salama, M. S. A., Mehany, T., & Abdelfttah, H. E. A. (2025). Detection and Inhibition of Clostridium perfringens by Cocktail of Star Anise and Thymus Extracts in Chicken Meat Products. Pathogens, 14(6), 552. https://doi.org/10.3390/pathogens14060552









