Influenza-Related Encephalopathy in Children: Epidemiology and Literature Review from a Tertiary Hospital in Northern Italy (Winter 2023–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Diagnostic Methods
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savagner, J.; Trémeaux, P.; Parizel, L.; Boudali, L.; Cahn-Sellem, F.; Oualha, M. Neurological involvement related to the influenza virus in children: A 5-year single-centre retrospective study. Eur. J. Paediatr. Neurol. 2024, 51, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, W.; Chen, J.; Zhang, Q.; Ma, H.; He, L. Identification of neurological complications in childhood influenza: A random forest model. BMC Pediatr. 2024, 24, 347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; O’Brien, K.L.; Madhi, S.A.; Widdowson, M.A.; Byass, P.; Omer, S.B.; Abbas, Q.; Ali, A.; Amu, A.; et al. Respiratory Virus Global Epidemiology Network. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: A systematic review and modelling study. Lancet Glob. Health 2020, 8, e497–e510. [Google Scholar] [CrossRef] [PubMed]
- Fazal, A.; Reinhart, K.; Curns, A.T.; Mustaquim, D.; Reed, C.; Budd, A.P.; Hall, A.J.; Brammer, L.; Dahl, R.M. Reports of encephalopathy among children with influenza-associated mortality—United States, 2010–11 through 2024–25 influenza seasons. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 91–95. [Google Scholar] [CrossRef]
- Mastrolia, M.V.; Rubino, C.; Esposito, S.; Berioli, M.G.; Campagna, I.; Vitale, A.; Pettoello-Mantovani, M. Characteristics and outcome of influenza-associated encephalopathy/encephalitis among children in a tertiary pediatric hospital in Italy, 2017–2019. BMC Infect. Dis. 2019, 19, 1012. [Google Scholar] [CrossRef]
- Jantarabenjakul, W.; Paprad, T.; Sophonphan, J.; Pluemjit, P.; Wongsrisang, L.; Wongsawat, J.; Techasatian, L. Neurological complications associated with influenza in hospitalized children. Influenza Other Respir. Viruses 2023, 17, e13075. [Google Scholar] [CrossRef]
- Yang, M.; Yi, L.; Zhao, Y.; Wu, S.; Zheng, J.; Tang, Y.; Liu, L. Characteristics and outcome of influenza-associated encephalopathy/encephalitis among children in China. Clinics 2024, 79, 100475. [Google Scholar] [CrossRef]
- Italian National Institute of Health. Flu surveillance portal (RespiVirNet). Available online: https://respivirnet.iss.it/ (accessed on 2 April 2025).
- Antoon, J.W.; Hall, M.; Herndon, A.; Johnson, D.P.; Brown, C.M.; Browning, W.L.; Florin, T.A.; Howard, L.M.; Grijalva, C.G.; Williams, D.J. Prevalence, risk factors, and outcomes of influenza-associated neurologic complications in children. J. Pediatr. 2021, 239, 32–38.e5. [Google Scholar] [CrossRef]
- Rao, S.; Martin, J.; Ahearn, M.A.; Osborne, C.; Moss, A.; Dempsey, A.; Dominguez, S.R.; Weinberg, A.; Messacar, K.B. Neurologic manifestations of influenza A(H3N2) infection in children during the 2016–2017 season. J. Pediatr. Infect. Dis. Soc. 2020, 9, 71–74. [Google Scholar] [CrossRef]
- Zhang, R.; Wen, J.; Wu, K.; Lin, S.; Tan, K.; Bi, J.; Deng, J. Influenza-associated neurologic complications in children from an H3N2 outbreak in Shenzhen, China during COVID-19 lockdown. Int. J. Infect. Dis. 2023, 134, 91–94. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, J.; Li, Y.; Zhou, X.; Niu, J.; Chen, J.; Sheng, H.; Wu, X.; Yuan, Y. Bibliometric analysis of publication trends and topics of influenza-related encephalopathy from 2000 to 2022. Immun. Inflamm. Dis. 2023, 11, e1013. [Google Scholar] [CrossRef] [PubMed]
- Alsolami, A.; Shiley, K. Successful treatment of influenza-associated acute necrotizing encephalitis in an adult using high-dose Oseltamivir and methylprednisolone: Case report and literature review. Open Forum Infect. Dis. 2017, 4, ofx145. [Google Scholar] [CrossRef] [PubMed]
- Cleuziou, P.; Renaldo, F.; Renolleau, S.; Javouhey, E.; Tissieres, P.; Léger, P.L.; Bergounioux, J.; Desguerre, I.; Dauger, S.; Levy, M.; et al. Mortality and neurologic sequelae in influenza-associated encephalopathy: Retrospective multicenter PICU cohort in France. Pediatr. Crit. Care Med. 2021, 22, e582–e587. [Google Scholar] [CrossRef]
- Quertermous, B.P.; Williams, D.J.; Bruce, J.; Sekmen, M.; Zhu, Y.; Grijalva, C.G.; Antoon, J.W. Serious neurologic events with seasonal influenza in young children. Acad. Pediatr. 2025, 25, 102801. [Google Scholar] [CrossRef]
- Choi, G.J.; Park, J.Y.; Choi, J.S.; Choi, S.R.; Kim, D.; Lee, J.H.; Woo, Y.J.; Lee, J.; Kim, Y.J. Influenza-associated neurologic complications in hospitalized pediatric patients: A multicenter retrospective study in Republic of Korea. Pediatr. Infect. Dis. J. 2021, 40, e466–e471. [Google Scholar] [CrossRef]
- Yu, M.K.L.; Leung, C.P.P.; Wong, W.H.S.; Ho, A.C.C.; Chiu, A.T.G.; Zhi, H.H.; Chan, G.C.F.; Chan, S.H.S. Clinical spectrum and burden of influenza-associated neurological complications in hospitalised paediatric patients. Front. Pediatr. 2022, 9, 752816. [Google Scholar] [CrossRef]
- Frankl, S.; Coffin, S.E.; Harrison, J.B.; Swami, S.K.; McGuire, J.L. Influenza-associated neurologic complications in hospitalized children. J. Pediatr. 2021, 239, 24–31.e1. [Google Scholar] [CrossRef]
- Dharmagadda, A.; Tambolkar, S.; Mane, S.V.; Singh, S. Clinical and etiological profile of children with acute viral encephalitis in a tertiary care hospital: A cross-sectional study. Cureus 2024, 16, e66588. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, W.; Zeng, M.; Shen, G.; Sun, C.; Liu, G.; Gong, H.; Wang, C.; Ge, M.; Xu, J.; et al. Clinical features and risk factors for severe influenza in children: A study from multiple hospitals in Shanghai. Pediatr. Neonatol. 2021, 62, 428–436. [Google Scholar] [CrossRef]
- Bartolini, L.; Ricci, S.; Azzari, C.; Moriondo, M.; Nieddu, F.; L’erario, M.; Ricci, Z.; Simonini, G.; Mortilla, M.; Indolfi, G.; et al. Severe A(H1N1)pdm09 influenza acute encephalopathy outbreak in children in Tuscany, Italy, December 2023 to January 2024. Euro Surveill. 2024, 29, 2400199. [Google Scholar] [CrossRef]
- Britton, P.N.; Dale, R.C.; Blyth, C.C.; Clark, J.E.; Crawford, N.; Marshall, H.; Elliott, E.J.; Macartney, K.; Booy, R.; Jones, C.A. Causes and clinical features of childhood encephalitis: A multicenter, prospective cohort study. Clin. Infect. Dis. 2020, 70, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Nie, Z.; Huang, L.; Fan, H.; Lu, G.; Yang, D.; Zhang, D. Mortality risk factors in children with severe influenza virus infection admitted to the pediatric intensive care unit. Medicine 2019, 98, e16861. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population incidence of Guillain–Barré syndrome: A systematic review and meta-analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Seehusen, D.A.; Reeves, M.M.; Fomin, D.A. Cerebrospinal fluid analysis. Am. Fam. Physician 2003, 68, 1103–1108. [Google Scholar] [PubMed]
- Dadak, M.; Pul, R.; Lanfermann, H.; Hartmann, H.; Hehr, U.; Donnerstag, F.; Michels, D.; Tryc, A.B. Varying patterns of CNS imaging in influenza A encephalopathy in childhood. Clin. Neuroradiol. 2020, 30, 243–249. [Google Scholar] [CrossRef]
- Toovey, S. Influenza-associated central nervous system dysfunction: A literature review. Travel Med. Infect. Dis. 2008, 6, 114–124. [Google Scholar] [CrossRef]
- Li, S.; Hu, D.; Li, P.; Xiao, W.; Li, H.; Liu, G.; Song, Y.; Ning, S.; Peng, Q.; Zhao, D.; et al. Parameters indicating development of influenza-associated acute necrotizing encephalopathy: Experiences from a single center. Med. Sci. Monit. 2021, 27, e930688. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Ngo, T.H.T.; Nguyen, T.V.H.; Do, T.H. Severe neurological complications with influenza in Vietnamese children. Influenza Other Respir. Viruses 2024, 18, e70035. [Google Scholar] [CrossRef]
- Paksu, M.S.; Aslan, K.; Kendirli, T.; Akyildiz, B.N.; Yener, N.; Yildizdas, R.D.; Davutoglu, M.; Yaman, A.; Isikay, S.; Sensoy, G.; et al. Neuroinfluenza: Evaluation of seasonal influenza associated severe neurological complications in children (a multicenter study). Childs Nerv. Syst. 2018, 34, 335–347. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Influenza Portal. Available online: https://www.salute.gov.it/portale/influenza/homeInfluenza.jsp (accessed on 2 April 2025).
- Centers for Disease Control and Prevention (CDC). National Center for Immunization and Respiratory Diseases. Available online: https://www.cdc.gov/ncird/index.html (accessed on 2 April 2025).

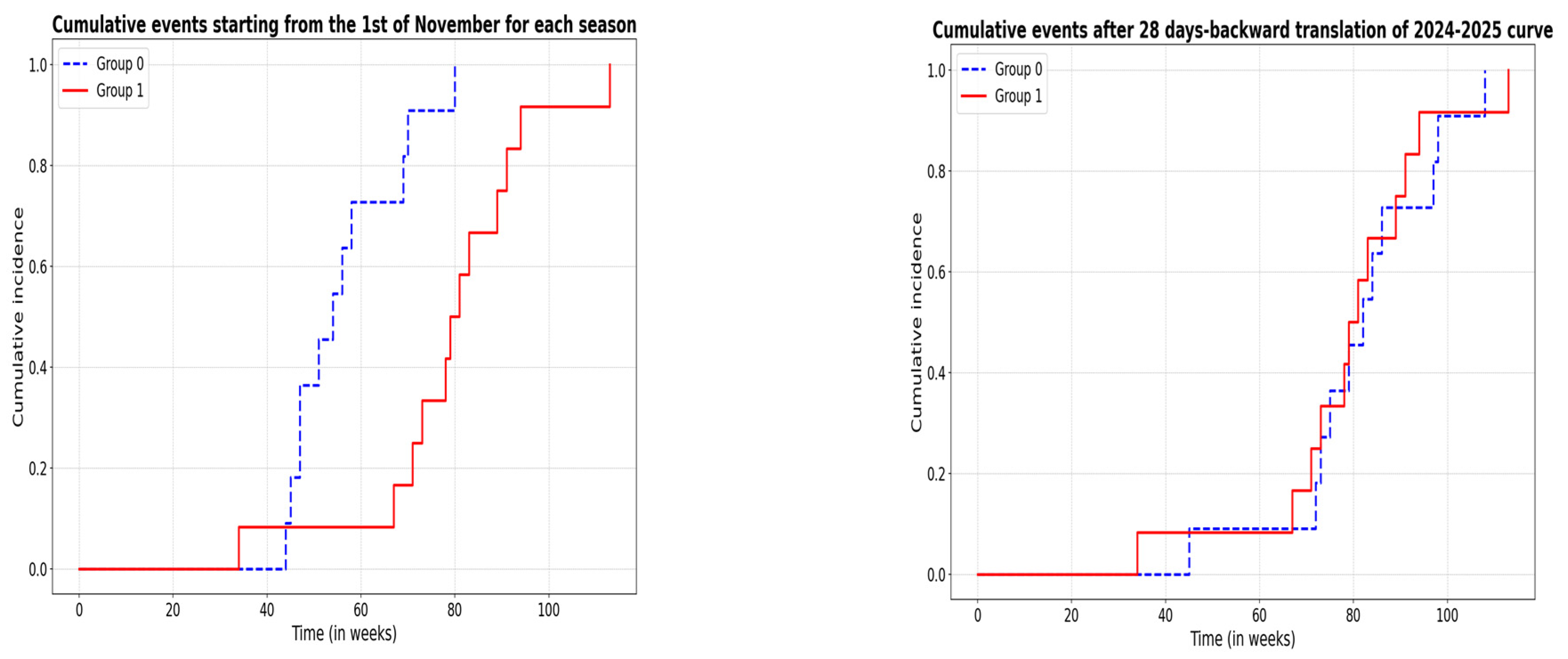
| All Patients | 2023–2024 Winter Season | 2024–2025 Winter Season | Statistics */** | |
|---|---|---|---|---|
| n (%) | 25 (100) | 11 (44) | 14 (56) | |
| Age at admission (in years), median (IQR) | 3.69 (3.86) | 5 (5.5) | 4 (3.5) | ** p.80 |
| Sex (male/female): n /n (%/%) | 14/11 (56/44) | 6/4 (32/24) | 8/6 (41.5/58.5) | * p.89 |
| Neurological comorbidity § n (%) | 10 (40) | 3 (12) | 7 (28) | * p.25 |
| Influenza B type | 4 (16) | 0 (0) | 4 (28.5) | * p.53 |
| Underlying neurological conditions at admission: | * p.87 | |||
| • Hyporeactivity n (%) | 9 (36) | 3 (12) | 6 (24) | |
| • Hyperreactivity n (%) | 5 (20) | 2 (8) | 3 (12) | |
| • Seizures n (%) | 11 (40) | 6 (24) | 5 (20) | |
| Time (days) from flu-like to neurological symptoms, median (IQR) | 1 (3) | 1 (1) | 2 (3.7) | ** p.768 |
| Respiratory distress/involvement ‡ n (%) | 9 (36) | 4 (16) | 5 (20) | * p.648 |
| Gastrointestinal symptoms n (%) | 7 (28) | 2 (8) | 5 (20) | * p.332 |
| C-Reactive protein mg/dl, median (IQR) | 4.3 (16.3) | 11 (21) | 8.85 (11.4) | ** p.312 |
| White blood cells/mmc, median (IQR) | 8000 (6815) | 8120 (4255) | 7420 (6515) | ** p.384 |
| Neutrophils/mmc, median (IQR) | 4105 (5390) | 4860 (4630) | 2800 (4160) | ** p.536 |
| Lymphocytes/mmc, median (IQR) | 1300 (1502) | 1020 (1143) | 1400 (2130) | ** p.151 |
| Lumbar tap n (%) | 10 (40) | 5 (20) | 5 (20) | * p.69 |
| Pathologic findings ⁋ | 3 (33) | 3 (100) | 0 (0) | |
| EEG anomalies at admission: | 25 (100) | * p.177 | ||
| • Slow patterns n (%) | 18 (72) | 6 (24) | 12 (48) | |
| • Epileptic features n (%) | 7 (28) | 5 (20) | 2 (8) | |
| Brain imaging n (%) | 14 (56) | 8 (72) | 6 (43) | * p.144 |
| Brain TC n (%) | 4 (31) | 3 (27) | 1 (7) | * p.144 |
| Only brain MRI n (%) | 9 (36) | 4 (36) | 5 (36) | * p.144 |
| Pathologic findings n (%) | 2 (15) | 2 (100) | 0 (0) | * p.5 |
| Acyclovir n (%) | 11 (44) | 6 (24) | 5 (20) | * p.435 |
| Acyclovir duration (days), median (IQR) | 6 (5) | 3.5 (6) | 7 (1) | ** p.792 |
| Oseltamivir duration (days), median (IQR) | 5 (0) | 5 (0) | 5 (0) | ** p.525 |
| Glucocorticoids n (%) | 16 (64) | 8 (3) | 8 (32) | * p.39 |
| Glucocorticoids duration (days), median (IQR) | 12 (3.75) | 12.5 (7) | 10 (6.5) | ** p.022 |
| Intravenous immunoglobulin n (%) | 3 (12) | 2 (66.6) | 1 (33.3) | * p.56 |
| Antibiotics n (%) | 9 (37) | 5 (21) | 4 (16.67) | * p.285 |
| P-ICU admission n (%) | 3 (12) | 2 (18) | 1 (6.6) | * p.28 |
| Serious neurological complication (ataxia, ADEM, GBS, epilepticus status) | 4 (16) | 3 (12) | 1 (4) | * p.28 |
| Antiepileptic drugs at discharge n (%) | 3 (12) | 2 (18) | 1 (6) | * p.6 |
| Length of stay in days, median (IQR) | 8.5 (7.24) | 9 (7) | 89 (6) | ** p.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, R.; Denina, M.; Badiali, L.; Sandei, M.; Mazzetti, G.; Canavese, C.; Tocchet, A.; Versace, A.; Garazzino, S. Influenza-Related Encephalopathy in Children: Epidemiology and Literature Review from a Tertiary Hospital in Northern Italy (Winter 2023–2024). Pathogens 2025, 14, 551. https://doi.org/10.3390/pathogens14060551
Vitale R, Denina M, Badiali L, Sandei M, Mazzetti G, Canavese C, Tocchet A, Versace A, Garazzino S. Influenza-Related Encephalopathy in Children: Epidemiology and Literature Review from a Tertiary Hospital in Northern Italy (Winter 2023–2024). Pathogens. 2025; 14(6):551. https://doi.org/10.3390/pathogens14060551
Chicago/Turabian StyleVitale, Raffaele, Marco Denina, Laura Badiali, Matteo Sandei, Giulia Mazzetti, Carlotta Canavese, Aba Tocchet, Antonia Versace, and Silvia Garazzino. 2025. "Influenza-Related Encephalopathy in Children: Epidemiology and Literature Review from a Tertiary Hospital in Northern Italy (Winter 2023–2024)" Pathogens 14, no. 6: 551. https://doi.org/10.3390/pathogens14060551
APA StyleVitale, R., Denina, M., Badiali, L., Sandei, M., Mazzetti, G., Canavese, C., Tocchet, A., Versace, A., & Garazzino, S. (2025). Influenza-Related Encephalopathy in Children: Epidemiology and Literature Review from a Tertiary Hospital in Northern Italy (Winter 2023–2024). Pathogens, 14(6), 551. https://doi.org/10.3390/pathogens14060551








