Genomic and Virulence Characteristics of Brucella intermedia Isolated from Hospital Wastewater in Ghana
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Source and Identification
2.2. DNA Extraction and Whole-Genome Sequencing
2.3. Identification and Antimicrobial Susceptibility Testing
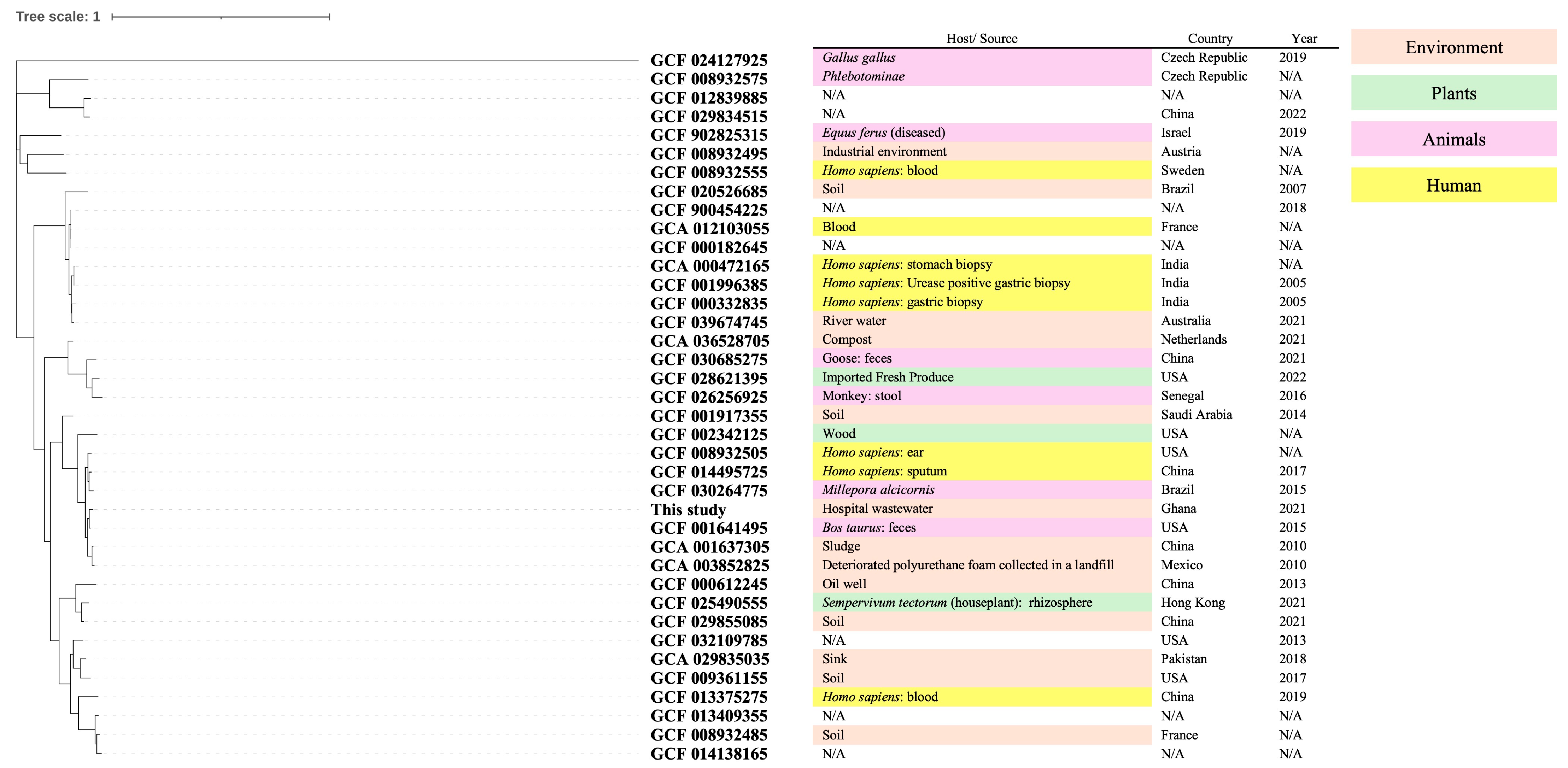
2.4. Phylogenetic Analysis
2.5. AMR Genes and Virulence Factor Analysis
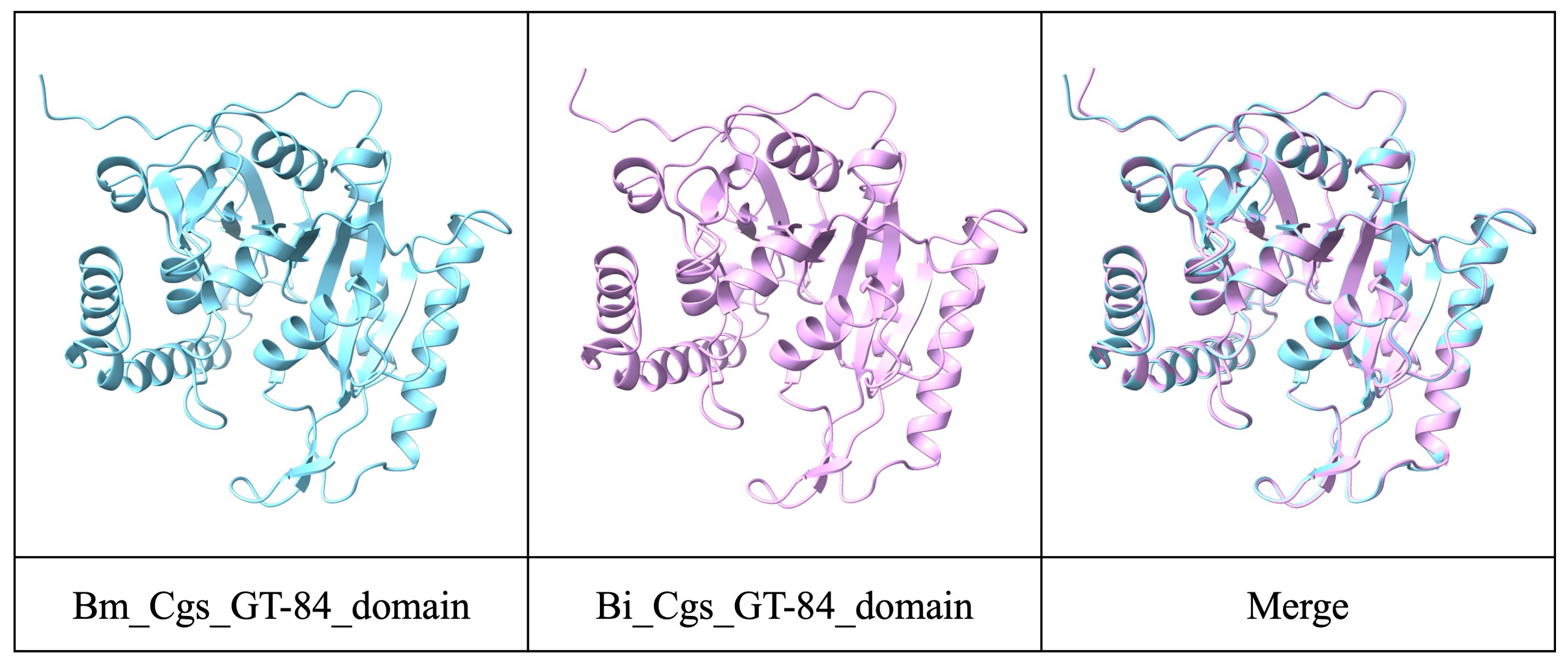
2.6. Structure Prediction of Cgs
3. Results
3.1. Identification of a Colistin-Resistant Strain from Hospital Wastewater
3.2. Phylogenetic Characteristics
3.3. Antimicrobial Susceptibility Testing and AMR Gene Analysis
3.4. Virulence Factor Analysis and Putative Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| ANI | Average Nucleotide Identity |
| BBS | Brucellosis-Causing Brucella Species |
| BLAST | Basic Local Alignment Search Tool |
| Cgs | Cyclic ß-1,2 Glucan Synthase |
| CLSI | Clinical and Laboratory Standards Institute |
| DNBSEQ | DNA Nanoball Sequencing |
| NBBS | Non-Brucellosis-Causing Brucella Species |
| PCR | Polymerase Chain Reaction |
| RGI | Resistance Gene Identifier |
| VFDB | Virulence Factor Database |
References
- Hördt, A.; López, M.G.; Meier-Kolthoff, J.P.; Schleuning, M.; Weinhold, L.-M.; Tindall, B.J.; Gronow, S.; Kyrpides, N.C.; Woyke, T.; Göker, M. Analysis of 1,000+ Type-Strain Genomes Substantially Improves Taxonomic Classification of Alphaproteobacteria. Front. Microbiol. 2020, 11, 468. [Google Scholar] [CrossRef]
- Yagel, Y.; Sestito, S.; Motro, Y.; Shnaiderman-Torban, A.; Khalfin, B.; Sagi, O.; Navon-Venezia, S.; Steinman, A.; Moran-Gilad, J. Genomic Characterization of Antimicrobial Resistance, Virulence, and Phylogeny of the Genus Ochrobactrum. Antibiotics 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Rajput, V.; Dharne, M. Pan-Genomics of Ochrobactrum Species from Clinical and Environmental Origins Reveals Distinct Populations and Possible Links. Genomics 2020, 112, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- She, R.; Anglewicz, C.; Jerke, K.; Relich, R.; Glazier, M.; Filkins, L.; Schuetz, A. Brucella and Ochrobactrum Taxonomic Updates for Laboratories; American Society for Microbiology: Washington, DC, USA, 2023. [Google Scholar]
- Ryan, M.P.; Pembroke, J.T. The Genus Ochrobactrum as Major Opportunistic Pathogens. Microorganisms 2020, 8, 1797. [Google Scholar] [CrossRef]
- Chau, K.K.; Barker, L.; Budgell, E.P.; Vihta, K.D.; Sims, N.; Kasprzyk-Hordern, B.; Harriss, E.; Crook, D.W.; Read, D.S.; Walker, A.S.; et al. Systematic Review of Wastewater Surveillance of Antimicrobial Resistance in Human Populations. Environ. Int. 2022, 162, 107171. [Google Scholar] [CrossRef]
- Mahazu, S.; Prah, I.; Ota, Y.; Hayashi, T.; Suzuki, M.; Yoshida, M.; Hoshino, Y.; Akeda, Y.; Suzuki, T.; Ishino, T.; et al. Colistin Resistance Mediated by Mcr-3-Related Phosphoethanolamine Transferase Genes in Aeromonas Species Isolated from Aquatic Environments in Avaga and Pakro Communities in the Eastern Region of Ghana. IDR 2024, 17, 3011–3023. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement; CLSI Guideline M100-S24; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2014. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing CLSI Guideline M100-Ed32; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2022. [Google Scholar]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput ANI Analysis of 90K Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Hall, B.G.; Nisbet, J. Building Phylogenetic Trees from Genome Sequences With kSNP4. Mol. Biol. Evol. 2023, 40, msad235. [Google Scholar] [CrossRef]
- Tanizawa, Y.; Fujisawa, T.; Kaminuma, E.; Nakamura, Y.; Arita, M. DFAST and DAGA: Web-Based Integrated Genome Annotation Tools and Resources. Biosci. Microbiota Food Health 2016, 35, 173–184. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for Predictions of Phenotypes from Genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.W.; Alcock, B.P.; French, S.; Farha, M.A.; Raphenya, A.R.; Brown, E.D.; McArthur, A.G. A Standardized Nomenclature for Resistance-Modifying Agents in the Comprehensive Antibiotic Resistance Database. Microbiol. Spectr. 2023, 11, e02744-23. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, B.; Zheng, D.; Chen, L.; Yang, J. VFDB 2025: An Integrated Resource for Exploring Anti-Virulence Compounds. Nucleic Acids Res. 2025, 53, D871–D877. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for Structure Building and Analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Hirai, J.; Yamagishi, Y.; Sakanashi, D.; Koizumi, Y.; Suematsu, H.; Mikamo, H. A Case of Bacteremia Caused by Ochrobactrum intermedium. J. Jpn. Assoc. Infect. Dis. 2017, 90, 129. [Google Scholar]
- Mantur, B.; Amarnath, S.; Shinde, R. Review of Clinical and Laboratory Features of Human Brucellosis. Indian J. Med. Microbiol. 2007, 25, 188–202. [Google Scholar] [CrossRef]
- Ciocchini, A.E.; Roset, M.S.; Briones, G.; De Iannino, N.I.; Ugalde, R.A. Identification of Active Site Residues of the Inverting Glycosyltransferase Cgs Required for the Synthesis of Cyclic β-1,2-Glucan, a Brucella abortus Virulence Factor. Glycobiology 2006, 16, 679–691. [Google Scholar] [CrossRef]
- Leclercq, S.O.; Cloeckaert, A.; Zygmunt, M.S. Taxonomic Organization of the Family Brucellaceae Based on a Phylogenomic Approach. Front. Microbiol. 2020, 10, 3083. [Google Scholar] [CrossRef]
- Huovinen, P. Resistance to Trimethoprim-Sulfamethoxazole. Clin. Infect. Dis. 2001, 32, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Xu, W.; Huang, D.; Liu, C.; Lu, J.; Zhu, M.; Bao, Q.; Pan, W. Aac(6’)-Iaq, a Novel Aminoglycoside Acetyltransferase Gene Identified from an Animal Isolate Brucella Intermedia DW0551. Front. Cell. Infect. Microbiol. 2025, 15, 1551240. [Google Scholar] [CrossRef] [PubMed]
- Sedzicki, J.; Ni, D.; Lehmann, F.; Wu, N.; Zenobi, R.; Jung, S.; Stahlberg, H.; Dehio, C. Mechanism of Cyclic β-Glucan Export by ABC Transporter Cgt of Brucella. Nat. Struct. Mol. Biol. 2022, 29, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Blasco, J.M.; Letesson, J.J.; Gorvel, J.P.; Moriyón, I. Pathogenicity and Its Implications in Taxonomy: The Brucella and Ochrobactrum Case. Pathogens 2022, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chai, Z.; Wang, X.; Zhang, Z.; Zhang, F.; Kang, F.; Liu, W.; Ren, H.; Jin, Y.; Yue, J. Comparative Genomic Analysis Provides Insights into the Genetic Diversity and Pathogenicity of the Genus Brucella. Front. Microbiol. 2024, 15, 1389859. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Zhao, Z.; Cao, Y.; Li, B. Hospital Wastewater as a Reservoir for Antibiotic Resistance Genes: A Meta-Analysis. Front. Public Health 2020, 8, 574968. [Google Scholar] [CrossRef]
- Thiebault, T. Sulfamethoxazole/Trimethoprim Ratio as a New Marker in Raw Wastewaters: A Critical Review. Sci. Total Environ. 2020, 715, 136916. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The Threat of Antimicrobial Resistance in Developing Countries: Causes and Control Strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
| Antimicrobials | MIC (μg/mL) | Interpretation |
|---|---|---|
| Piperacillin | >64 | R |
| Piperacillin-tazobactam | >64 | R |
| Ceftazidime | >16 | R |
| Cefepime | >16 | R |
| Aztreonam | >16 | R |
| Imipenem | ≤2 | S |
| Meropenem | ≤2 | S |
| Gentamicin | 4 | S |
| Tobramycin | >8 | R |
| Amikacin | 32 | I |
| Minocycline | ≤2 | S |
| Ciprofloxacin | 0.5 | S |
| Levofloxacin | ≤0.5 | S |
| Trimethoprim-sulfamethoxazole | >2/38 | R |
| Colistin | >4 | R |
| ARO Term | AMR Gene Family | Drug Class | Resistance Mechanism | % Identity of Matching Region | % Length of Reference Sequence |
|---|---|---|---|---|---|
| adeF | resistance-nodulation-cell division (RND) antibiotic efflux pump | fluoroquinolone antibiotic, tetracycline antibiotic | antibiotic efflux | 48.12 | 99.24 |
| ANT(9)-Ic | ANT(9) | aminoglycoside antibiotic | antibiotic inactivation | 97.68 | 100 |
| qacG | small multidrug resistance (SMR) antibiotic efflux pump | disinfecting agents and antiseptics | antibiotic efflux | 43.27 | 102.8 |
| adeF | resistance-nodulation-cell division (RND) antibiotic efflux pump | fluoroquinolone antibiotic, tetracycline antibiotic | antibiotic efflux | 71.29 | 100.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuya, R.; Takei, S.; Tabe, Y.; Ablordey, A.; Saito, R. Genomic and Virulence Characteristics of Brucella intermedia Isolated from Hospital Wastewater in Ghana. Pathogens 2025, 14, 522. https://doi.org/10.3390/pathogens14060522
Furuya R, Takei S, Tabe Y, Ablordey A, Saito R. Genomic and Virulence Characteristics of Brucella intermedia Isolated from Hospital Wastewater in Ghana. Pathogens. 2025; 14(6):522. https://doi.org/10.3390/pathogens14060522
Chicago/Turabian StyleFuruya, Runa, Satomi Takei, Yoko Tabe, Anthony Ablordey, and Ryoichi Saito. 2025. "Genomic and Virulence Characteristics of Brucella intermedia Isolated from Hospital Wastewater in Ghana" Pathogens 14, no. 6: 522. https://doi.org/10.3390/pathogens14060522
APA StyleFuruya, R., Takei, S., Tabe, Y., Ablordey, A., & Saito, R. (2025). Genomic and Virulence Characteristics of Brucella intermedia Isolated from Hospital Wastewater in Ghana. Pathogens, 14(6), 522. https://doi.org/10.3390/pathogens14060522







