Temperature Influences Antimicrobial Resistance and Virulence of Vibrio parahaemolyticus Clinical Isolates from Quebec, Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Growth Curves and Minimal Inhibitory Concentrations
2.3. Biofilm Formation Assays
2.4. Hemolysin Production and Detection of tdh and trh by PCR
2.5. Swimming Motility Assays
2.6. Membrane Vesicle Production
2.7. Statistical Analysis
3. Results
3.1. V. parahaemolyticus Grows Faster at 37 °C than at 20 °C
3.2. Temperature Affects V. parahaemolyticus Sensitivity to Antimicrobials
3.3. V. parahaemolyticus Produces More Biofilm at a Lower Temperature
3.4. Temperature Has an Impact on V. parahaemolyticus’s Swimming Motility
3.5. Temperature Has No Effect on Membrane Vesicle Formation
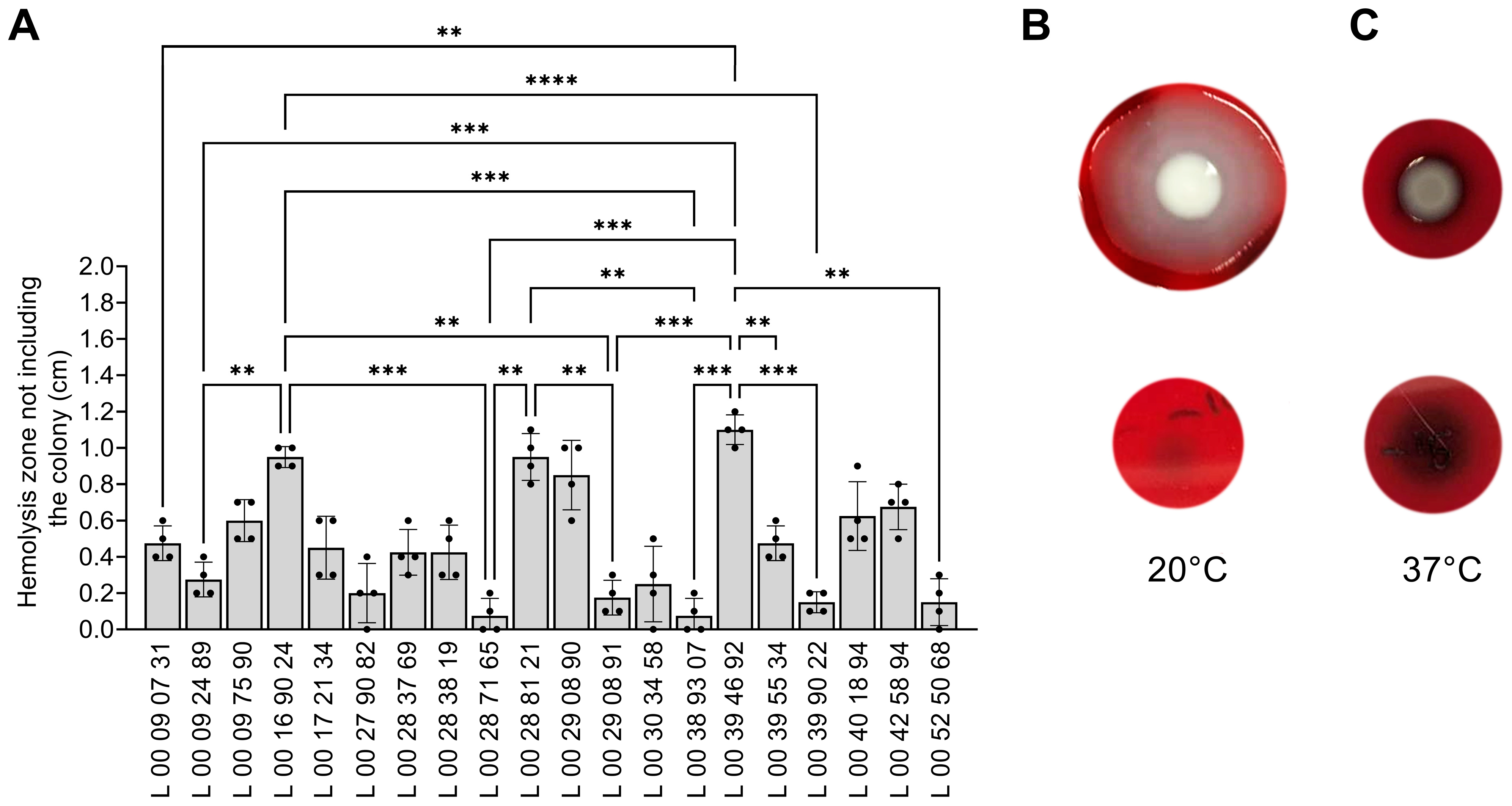
3.6. Effect of Temperature on Hemolysin Production
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker-Austin, C.; Trinanes, J.; Gonzalez-Escalona, N.; Martinez-Urtaza, J. Non-cholera Vibrios: The microbial barometer of climate change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef]
- CDC. Cholera and Other Vibrio Illness Surveillance: Annual Summary. 2019. Available online: https://www.cdc.gov/vibrio/php/surveillance/annual-summary-2019.html (accessed on 6 December 2024).
- National Center for Emerging and Zoonotic Infectious Diseases (U.S.); Division of Foodborne, Waterborne, and Environmental Diseases. National Enteric Disease Surveillance: COVIS Annual Summary, 2012; U.S. Centers For Disease Control And Prevention: Atlanta, GA, USA, 2014; p. 9.
- National Center for Emerging and Zoonotic Infectious Diseases (U.S.); Division of Foodborne, Waterborne, and Environmental Diseases. National Enteric Disease Surveillance: COVIS Annual Summary, 2013; U.S. Centers For Disease Control And Prevention: Atlanta, GA, USA, 2015.
- National Center for Emerging and Zoonotic Infectious Diseases (U.S.); Division of Foodborne, Waterborne, and Environmental Diseases. National Enteric Disease Surveillance: COVIS Annual Summary, 2014; U.S. Centers For Disease Control and Prevention: Atlanta, GA, USA, 2016; p. 11.
- Seelman, S.L.; Whitney, B.M.; Stokes, E.K.; Elliot, E.L.; Griswold, T.; Patel, K.; Bloodgood, S.; Jones, J.L.; Cripe, J.; Cornell, J.; et al. An outbreak investigation of Vibrio parahaemolyticus infections in the United States linked to crabmeat imported from venezuela: 2018. Foodborne Pathog. Dis. 2023, 20, 123–131. [Google Scholar] [CrossRef]
- Dechet, A.M.; Yu, P.A.; Koram, N.; Painter, J. Nonfoodborne Vibrio infections: An important cause of morbidity and mortality in the United States, 1997-2006. Clin. Infect. Dis. 2008, 46, 970–976. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Public Health Notice: Outbreak of Vibrio parahaemolyticus Infections Linked to Shellfish; Public Health Agency of Canada: Ottawa, ON, Canada, 2020.
- Public Health Agency of Canada. Public Health Notice—Outbreak of Vibrio parahaemolyticus Linked to Raw Shellfish; Public Health Agency of Canada: Ottawa, ON, Canada, 2017.
- Centers for Disease Control and Prevention (CDC). Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters--Pacific Northwest, 1997. MMWR Morb. Mortal. Wkly. Rep. 1998, 47, 457–462. [Google Scholar]
- Ma, J.Y.; Zhu, X.K.; Hu, R.G.; Qi, Z.Z.; Sun, W.C.; Hao, Z.P.; Cong, W.; Kang, Y.H. A systematic review, meta-analysis and meta-regression of the global prevalence of foodborne Vibrio spp. infection in fishes: A persistent public health concern. Mar. Pollut. Bull. 2023, 187, 114521. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Aldridge, K.E.; Sanders, C.V. Infections related to the ingestion of seafood Part I: Viral and bacterial infections. Lancet Infect. Dis. 2004, 4, 201–212. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Public health aspects of Vibrio spp. related to the consumption of seafood in the EU. EFSA J. 2024, 22, e8896. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Stockley, L.; Rangdale, R.; Martinez-Urtaza, J. Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: A European perspective. Environ. Microbiol. Rep. 2010, 2, 7–18. [Google Scholar] [CrossRef]
- Mahoney, J.C.; Gerding, M.J.; Jones, S.H.; Whistler, C.A. Comparison of the pathogenic potentials of environmental and clinical Vibrio parahaemolyticus strains indicates a role for temperature regulation in virulence. Appl. Environ. Microbiol. 2010, 76, 7459–7465. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Iida, T. The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev. Med. Microbiol. 1993, 4, 106–113. [Google Scholar] [CrossRef]
- Theethakaew, C.; Feil, E.J.; Castillo-Ramirez, S.; Aanensen, D.M.; Suthienkul, O.; Neil, D.M.; Davies, R.L. Genetic relationships of Vibrio parahaemolyticus isolates from clinical, human carrier, and environmental sources in Thailand, determined by multilocus sequence analysis. Appl. Environ. Microbiol. 2013, 79, 2358–2370. [Google Scholar] [CrossRef]
- Gutierrez West Casandra, K.; Klein Savannah, L.; Lovell Charles, R. High frequency of virulence factor genes tdh, trh, and tlh in Vibrio parahaemolyticus strains isolated from a Pristine estuary. Appl. Environ. Microbiol. 2013, 79, 2247–2252. [Google Scholar] [CrossRef]
- Park, K.S.; Ono, T.; Rokuda, M.; Jang, M.H.; Okada, K.; Iida, T.; Honda, T. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 2004, 72, 6659–6665. [Google Scholar] [CrossRef]
- Han, N.; Mizan, M.F.R.; Jahid, I.K.; Ha, S.-D. Biofilm formation by Vibrio parahaemolyticus on food and food contact surfaces increases with rise in temperature. Food Control 2016, 70, 161–166. [Google Scholar] [CrossRef]
- Aagesen, A.M.; Phuvasate, S.; Su, Y.C.; Hase, C.C. Characterizing the adherence profiles of virulent Vibrio parahaemolyticus isolates. Microb. Ecol. 2018, 75, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Josenhans, C.; Suerbaum, S. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 2002, 291, 605–614. [Google Scholar] [CrossRef]
- Rajkowski, K.T. Biofilms in fish processing. In Biofilms in the Food and Beverage Industries; Elsevier: Cambridge, UK, 2009; pp. 499–516. [Google Scholar]
- Galanis, E.; Otterstatter, M.; Taylor, M. Measuring the impact of sea surface temperature on the human incidence of Vibrio sp. infection in British Columbia, Canada, 1992–2017. Environ. Health 2020, 19, 58. [Google Scholar] [CrossRef]
- Taylor, M.; Cheng, J.; Sharma, D.; Bitzikos, O.; Gustafson, R.; Fyfe, M.; Greve, R.; Murti, M.; Stone, J.; Honish, L.; et al. Outbreak of Vibrio parahaemolyticus associated with consumption of raw oysters in Canada, 2015. Foodborne Pathog. Dis. 2018, 15, 554–559. [Google Scholar] [CrossRef]
- Ibáñez, A.; Garrido-Chamorro, S.; Barreiro, C. Microorganisms and climate change: A not so invisible effect. Microbiol. Res. 2023, 14, 918–947. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Trinanes, J.A.; Taylor, N.G.H.; Hartnell, R.; Siitonen, A.; Martinez-Urtaza, J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Chang. 2013, 3, 73–77. [Google Scholar] [CrossRef]
- Vezzulli, L.; Brettar, I.; Pezzati, E.; Reid, P.C.; Colwell, R.R.; Hofle, M.G.; Pruzzo, C. Long-term effects of ocean warming on the prokaryotic community: Evidence from the vibrios. ISME J. 2012, 6, 21–30. [Google Scholar] [CrossRef]
- Climate change exacerbates almost two-thirds of pathogenic diseases affecting humans. Nat. Clim. Chang. 2022, 12, 791–792. [CrossRef]
- Fleischmann, S.; Herrig, I.; Wesp, J.; Stiedl, J.; Reifferscheid, G.; Strauch, E.; Alter, T.; Brennholt, N. Prevalence and distribution of potentially human pathogenic Vibrio spp. on German North and Baltic sea coasts. Front. Cell Infect. Microbiol. 2022, 12, 846819. [Google Scholar] [CrossRef]
- Pauze-Foixet, J.; Mathieu-Denoncourt, A.; Duperthuy, M. Elevated concentrations of polymyxin B elicit a biofilm-specific resistance mechanism in Vibrio cholerae. Res. Microbiol. 2024, 175, 104179. [Google Scholar] [CrossRef]
- Bej, A.K.; Patterson, D.P.; Brasher, C.W.; Vickery, M.C.L.; Jones, D.D.; Kaysner, C.A. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. J. Microbiol. Methods 1999, 36, 215–225. [Google Scholar] [CrossRef]
- Giacomucci, S.; Cros, C.D.; Perron, X.; Mathieu-Denoncourt, A.; Duperthuy, M. Flagella-dependent inhibition of biofilm formation by sub-inhibitory concentration of polymyxin B in Vibrio cholerae. PLoS ONE 2019, 14, e0221431. [Google Scholar] [CrossRef]
- Giacomucci, S.; Mathieu-Denoncourt, A.; Vincent, A.T.; Jannadi, H.; Duperthuy, M. Experimental evolution of Vibrio cholerae identifies hypervesiculation as a way to increase motility in the presence of polymyxin B. Front. Microbiol. 2022, 13, 932165. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Juodeikis, R.; Carding Simon, R. Outer membrane vesicles: Biogenesis, functions, and issues. Microbiol. Mol. Biol. Rev. 2022, 86, e00032-22. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, P. Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus. Front. Microbiol. 2014, 5, 805. [Google Scholar] [CrossRef] [PubMed]
- Billaud, M.; Seneca, F.; Tambutté, E.; Czerucka, D. An Increase of Seawater Temperature Upregulates the Expression of Vibrio parahaemolyticus Virulence Factors Implicated in Adhesion and Biofilm Formation. Front. Microbiol. 2022, 13, 840628. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, S.H.; Hwang, I.G.; Yoon, K.S. Effect of temperature on growth of Vibrio parahaemolyticus [corrected] and Vibrio vulnificus in flounder, salmon sashimi and oyster meat. Int. J. Environ. Res. Public Health 2012, 9, 4662–4675. [Google Scholar] [CrossRef]
- Janecko, N.; Bloomfield, S.J.; Palau, R.; Mather, A.E. Whole genome sequencing reveals great diversity of Vibrio spp in prawns at retail. Microb. Genom. 2021, 7, 000647. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Urtaza, J.; Cabrera-Gumbau, J.M. Screening of AMR-related genes in the genomes of Vibrio parahaemolyticus strains isolated in Europe from clinical, environmental and other sources. Zenodo 2024. [Google Scholar] [CrossRef]
- De Silva, P.M.; Chong, P.; Fernando Dinesh, M.; Westmacott, G.; Kumar, A. Effect of incubation temperature on antibiotic resistance and virulence factors of Acinetobacter baumannii ATCC 17978. Antimicrob. Agents Chemother. 2017, 62, e01514-17. [Google Scholar] [CrossRef]
- Mackowiak, P.A.; Marling-Cason, M.; Cohen, R.L. Effects of temperature on antimicrobial susceptibility of bacteria. J. Infect. Dis. 1982, 145, 550–553. [Google Scholar] [CrossRef]
- Wheat, P.F.; Winstanley, T.G.; Spencer, R.C. Effect of temperature on antimicrobial susceptibilities of Pseudomonas maltophilia. J. Clin. Pathol. 1985, 38, 1055–1058. [Google Scholar] [CrossRef]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef]
- Nenninger, A.; Mastroianni, G.; Robson, A.; Lenn, T.; Xue, Q.; Leake, M.C.; Mullineaux, C.W. Independent mobility of proteins and lipids in the plasma membrane of Escherichia coli. Mol. Microbiol. 2014, 92, 1142–1153. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Ginez, L.D.; Osorio, A.; Vazquez-Ramirez, R.; Arenas, T.; Mendoza, L.; Camarena, L.; Poggio, S. Changes in fluidity of the E. coli outer membrane in response to temperature, divalent cations and polymyxin-B show two different mechanisms of membrane fluidity adaptation. FEBS J. 2022, 289, 3550–3567. [Google Scholar] [CrossRef]
- Chiang, M.-L.; Yu, R.-C.; Chou, C.-C. Fatty acid composition, cell morphology and responses to challenge by organic acid and sodium chloride of heat-shocked Vibrio parahaemolyticus. Int. J. Food Microbiol. 2005, 104, 179–187. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Rahmati-Bahram, A.; Magee, J.T.; Jackson, S.K. Effect of temperature on aminoglycoside binding sites in Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 1997, 39, 19–24. [Google Scholar] [CrossRef]
- Detweiler, C.S.; Monack, D.M.; Brodsky, I.E.; Mathew, H.; Falkow, S. virK, somA and rcsC are important for systemic Salmonella enterica serovar Typhimurium infection and cationic peptide resistance. Mol. Microbiol. 2003, 48, 385–400. [Google Scholar] [CrossRef]
- Huang, L.; Ahmed, S.; Gu, Y.; Huang, J.; An, B.; Wu, C.; Zhou, Y.; Cheng, G. The effects of natural products and environmental conditions on antimicrobial resistance. Molecules 2021, 26, 4277. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Skurnik, M. Temperature-regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia. Mol. Microbiol. 2000, 37, 67–80. [Google Scholar] [CrossRef]
- Cullmann, W.; Dick, W. Influence of temperature on beta-lactamase production and outer membrane proteins in Gram-negative rods. Chemotherapy 2009, 36, 277–286. [Google Scholar] [CrossRef]
- Aagesen, A.M.; Hase, C.C. Seasonal effects of heat shock on bacterial populations, including artificial Vibrio parahaemolyticus exposure, in the Pacific oyster, Crassostrea gigas. Food Microbiol. 2014, 38, 93–103. [Google Scholar] [CrossRef]
- McCarter, L.L. Dual flagellar systems enable motility under different circumstances. J. Mol. Microbiol. Biotechnol. 2004, 7, 18–29. [Google Scholar] [CrossRef]
- Khan, F.; Tabassum, N.; Anand, R.; Kim, Y.-M. Motility of Vibrio spp.: Regulation and controlling strategies. Appl. Microbiol. Biotechnol. 2020, 104, 8187–8208. [Google Scholar] [CrossRef]
- Stewart, B.J.; McCarter, L.L. Lateral flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 2003, 185, 4508–4518. [Google Scholar] [CrossRef]
- Aagesen, A.M.; Phuvasate, S.; Su, Y.C.; Hase, C.C. Persistence of Vibrio parahaemolyticus in the Pacific oyster, Crassostrea gigas, is a multifactorial process involving pili and flagella but not type III secretion systems or phase variation. Appl. Environ. Microbiol. 2013, 79, 3303–3305. [Google Scholar] [CrossRef]
- Yildiz, F.H.; Visick, K.L. Vibrio biofilms: So much the same yet so different. Trends Microbiol. 2009, 17, 109–118. [Google Scholar] [CrossRef]
- Gode-Potratz, C.J.; Kustusch, R.J.; Breheny, P.J.; Weiss, D.S.; McCarter, L.L. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol. Microbiol. 2011, 79, 240–263. [Google Scholar] [CrossRef]
- Merino, S.; Shaw, J.G.; Tomás, J.M. Bacterial lateral flagella: An inducible flagella system. FEMS Microbiol. Lett. 2006, 263, 127–135. [Google Scholar] [CrossRef]
- McCarter, L.; Silverman, M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol. Microbiol. 1990, 4, 1057–1062. [Google Scholar] [CrossRef]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef]
- Song, X.; Ma, Y.; Fu, J.; Zhao, A.; Guo, Z.; Malakar, P.K.; Pan, Y.; Zhao, Y. Effect of temperature on pathogenic and non-pathogenic Vibrio parahaemolyticus biofilm formation. Food Control 2017, 73, 485–491. [Google Scholar] [CrossRef]
- Velez, K.E.C.; Leighton, R.E.; Decho, A.W.; Pinckney, J.L.; Norman, R.S. Modeling pH and temperature effects as climatic hazards in Vibrio vulnificus and Vibrio parahaemolyticus planktonic growth and biofilm formation. Geohealth 2023, 7, e2022GH000769. [Google Scholar] [CrossRef]
- Liu, M.; Nie, H.; Luo, X.; Yang, S.; Chen, H.; Cai, P. A polysaccharide biosynthesis Locus in Vibrio parahaemolyticus important for biofilm formation has homologs widely distributed in aquatic bacteria mainly from Gammaproteobacteria. mSystems 2022, 7, e0122621. [Google Scholar] [CrossRef]
- Leighton, R.E.; Correa Vélez, K.E.; Xiong, L.; Creech, A.G.; Amirichetty, K.P.; Anderson, G.K.; Cai, G.; Norman, R.S.; Decho, A.W. Vibrio parahaemolyticus and Vibrio vulnificus in vitro colonization on plastics influenced by temperature and strain variability. Front. Microbiol. 2023, 13, 1099502. [Google Scholar] [CrossRef]
- Srivastava, D.; Waters, C.M. A tangled web: Regulatory connections between quorum sensing and cyclic Di-GMP. J. Bacteriol. 2012, 194, 4485–4493. [Google Scholar] [CrossRef]
- Duddy, O.P.; Silpe, J.E.; Fei, C.; Bassler, B.L. Natural silencing of quorum-sensing activity protects Vibrio parahaemolyticus from lysis by an autoinducer-detecting phage. PLoS Genet. 2023, 19, e1010809. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, Y.; Gao, H.; Sun, J.; Li, X.; Zhang, M.; Xue, X.; Yang, W.; Ni, B.; Hu, L.; et al. OpaR controls the metabolism of c-di-GMP in Vibrio parahaemolyticus. Front. Microbiol. 2021, 12, 676436. [Google Scholar] [CrossRef]
- Zamorano-Sánchez, D.; Alejandre-Sixtos Jesús, E.; Arredondo-Hernández, A.; Martínez-Méndez, R. OpaR exerts a dynamic control over c-di-GMP homeostasis and cpsA expression in Vibrio parahaemolyticus through its Rregulation of ScrC and the trigger phosphodiesterase TpdA. Microbiol. Spectr. 2023, 11, e00872-23. [Google Scholar] [CrossRef]
- Gode-Potratz, C.J.; McCarter, L.L. Quorum sensing and silencing in Vibrio parahaemolyticus. J. Bacteriol. 2011, 193, 4224–4237. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ono, T.; Rokuda, M.; Jang, M.H.; Iida, T.; Honda, T. Cytotoxicity and enterotoxicity of the thermostable direct hemolysin-deletion mutants of Vibrio parahaemolyticus. Microbiol. Immunol. 2004, 48, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Kubomura, S.; Hirano, H.; Mizue, K.; Ogawa, M.; Mizuguchi, Y. Cloning and characterization of a gene encoding a new thermostable hemolysin from Vibrio parahaemolyticus. FEMS Microbiol. Lett. 1990, 67, 339–345. [Google Scholar] [CrossRef]
- Zhang, X.H.; Austin, B. Haemolysins in Vibrio species. J. Appl. Microbiol. 2005, 98, 1011–1019. [Google Scholar] [CrossRef]
- Taniguchi, H.; Ohta, H.; Ogawa, M.; Mizuguchi, Y. Cloning and expression in Escherichia coli of Vibrio parahaemolyticus thermostable direct hemolysin and thermolabile hemolysin genes. J. Bacteriol. 1985, 162, 510–515. [Google Scholar] [CrossRef]
- Gotoh, K.; Kodama, T.; Hiyoshi, H.; Izutsu, K.; Park, K.S.; Dryselius, R.; Akeda, Y.; Honda, T.; Iida, T. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS ONE 2010, 5, e13365. [Google Scholar] [CrossRef]
- Schmitt, P.; Rosa, R.D.; Duperthuy, M.; de Lorgeril, J.; Bachere, E.; Destoumieux-Garzon, D. The antimicrobial defense of the Pacific oyster, Crassostrea gigas. How diversity may compensate for scarcity in the regulation of resident/pathogenic microflora. Front. Microbiol. 2012, 3, 160. [Google Scholar] [CrossRef]
- Roux, A.; Payne, S.M.; Gilmore, M.S. Microbial telesensing: Probing the environment for friends, foes, and food. Cell Host Microbe 2009, 6, 115–124. [Google Scholar] [CrossRef]
- Pratama, A.; Ishii, E.; Kodama, T.; Iida, T.; Matsuda, S. The xenogeneic silencer Histone-Like Nucleoid-Structuring protein mediates the temperature and salinity-dependent regulation of the type III secretion system 2 in Vibrio parahaemolyticus. J. Bacteriol. 2022, 205, e00266-22. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Zhan, P.; Qiu, Q.; Chen, J.; Xiong, J. RNA-seq analysis unveils temperature and nutrient adaptation mechanisms relevant for pathogenicity in Vibrio parahaemolyticus. Aquaculture 2022, 558, 738397. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, Y.; Tang, H.; Hu, L.F.; Yang, W.H.; Zhu, X.J.; Huang, X.X.; Wang, T.; Zhang, Y.Q. ToxR is required for biofilm formation and motility of Vibrio parahaemolyticus. Biomed. Environ. Sci. 2018, 31, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Sun, J.; Qiu, Y.; Zhang, M.; Xue, X.; Li, X.; Yang, W.; Zhou, D.; Hu, L.; Zhang, Y. The quorum sensing regulator OpaR is a repressor of polar flagellum genes in Vibrio parahaemolyticus. J. Microbiol. 2021, 59, 651–657. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Chodur, D.M.; Antunes, L.C.; Trimble, M.J.; McCarter, L.L. Output targets and transcriptional regulation by a cyclic dimeric GMP-responsive circuit in the Vibrio parahaemolyticus Scr network. J. Bacteriol. 2012, 194, 914–924. [Google Scholar] [CrossRef]
- Li, X.; Lian, W.; Zhang, M.; Luo, X.; Zhang, Y.; Lu, R. QsvR and OpaR coordinately regulate the transcription of cpsS and cpsR in Vibrio parahaemolyticus. Can. J. Microbiol. 2024, 70, 128–134. [Google Scholar] [CrossRef]
- Spaur, M.; Davis, B.J.K.; Kivitz, S.; DePaola, A.; Bowers, J.C.; Curriero, F.C.; Nachman, K.E. A systematic review of post-harvest interventions for Vibrio parahaemolyticus in raw oysters. Sci. Total Environ. 2020, 745, 140795. [Google Scholar] [CrossRef]
- Love, D.C.; Kuehl, L.M.; Lane, R.M.; Fry, J.P.; Harding, J.; Davis, B.J.K.; Clancy, K.; Hudson, B. Performance of cold chains and modeled growth of Vibrio parahaemolyticus for farmed oysters distributed in the United States and internationally. Int. J. Food Microbiol. 2020, 313, 108378. [Google Scholar] [CrossRef]
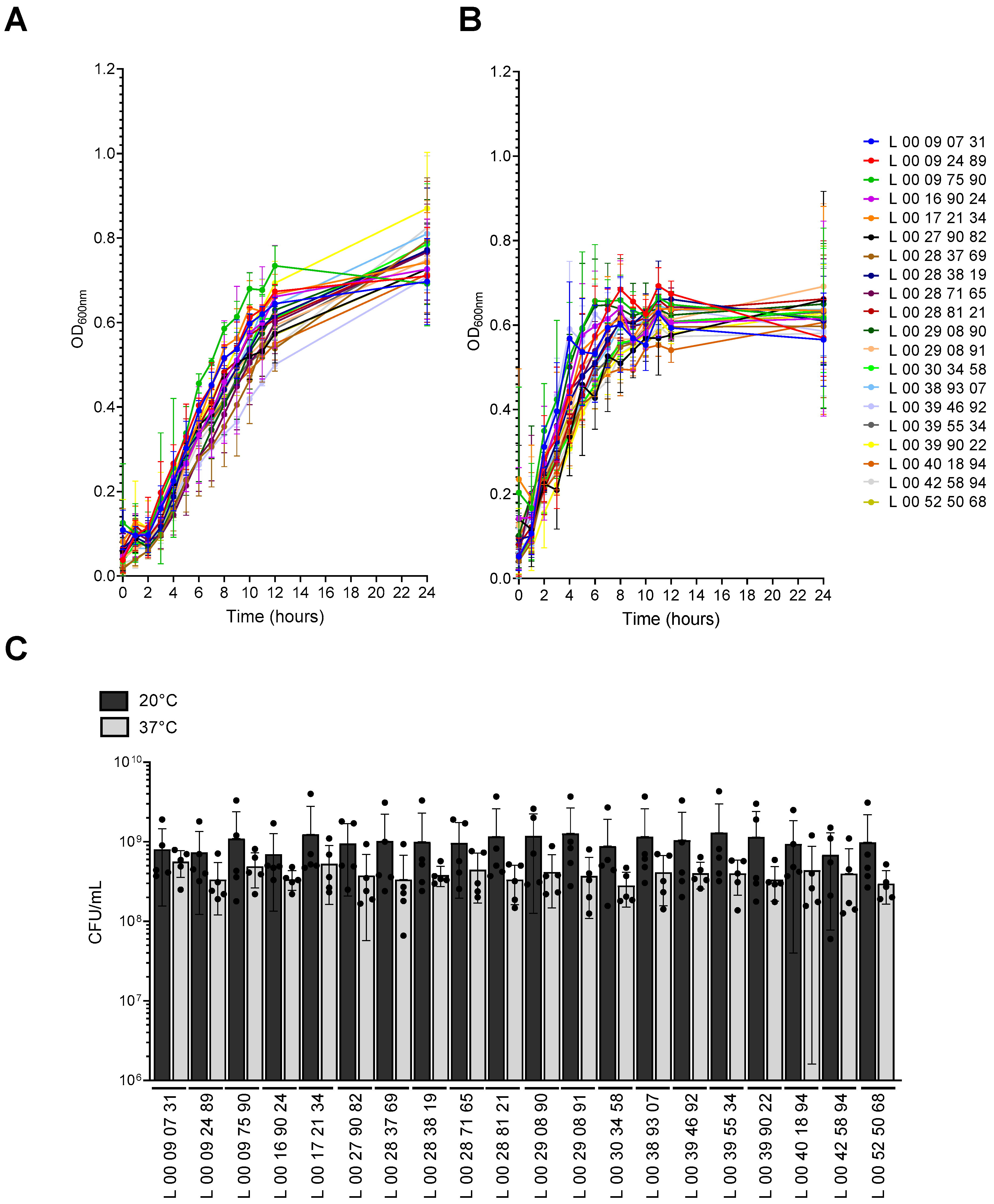
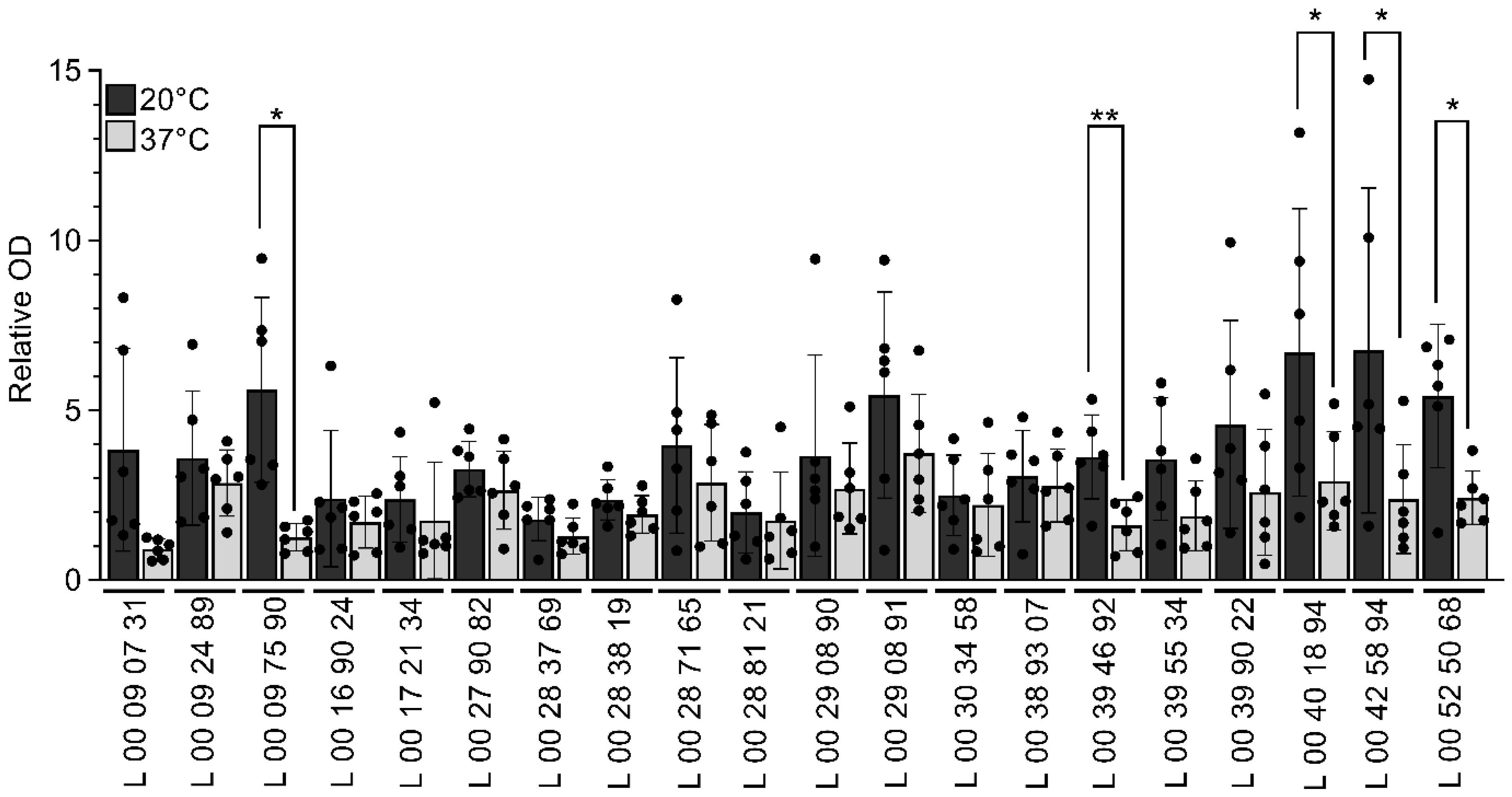
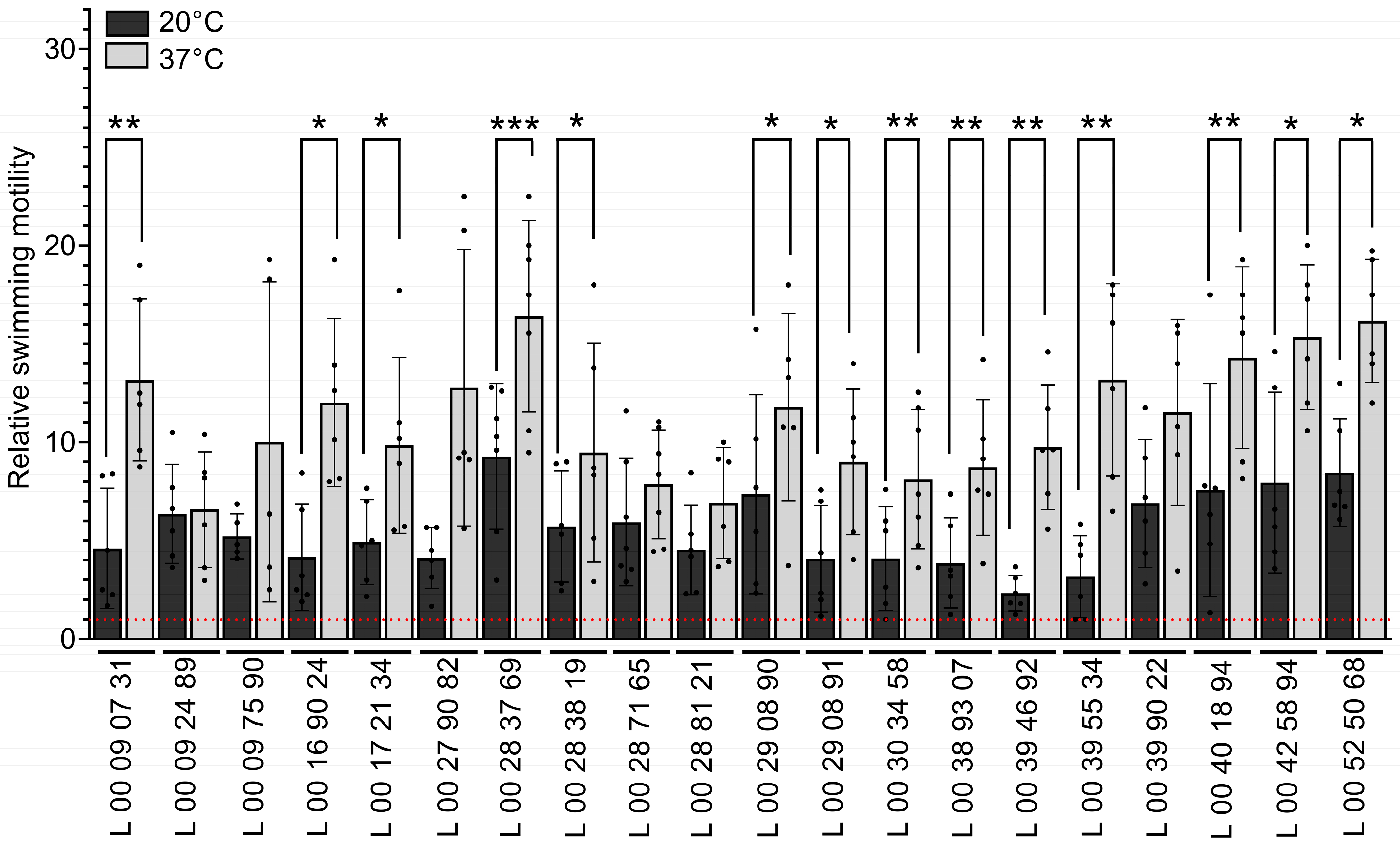
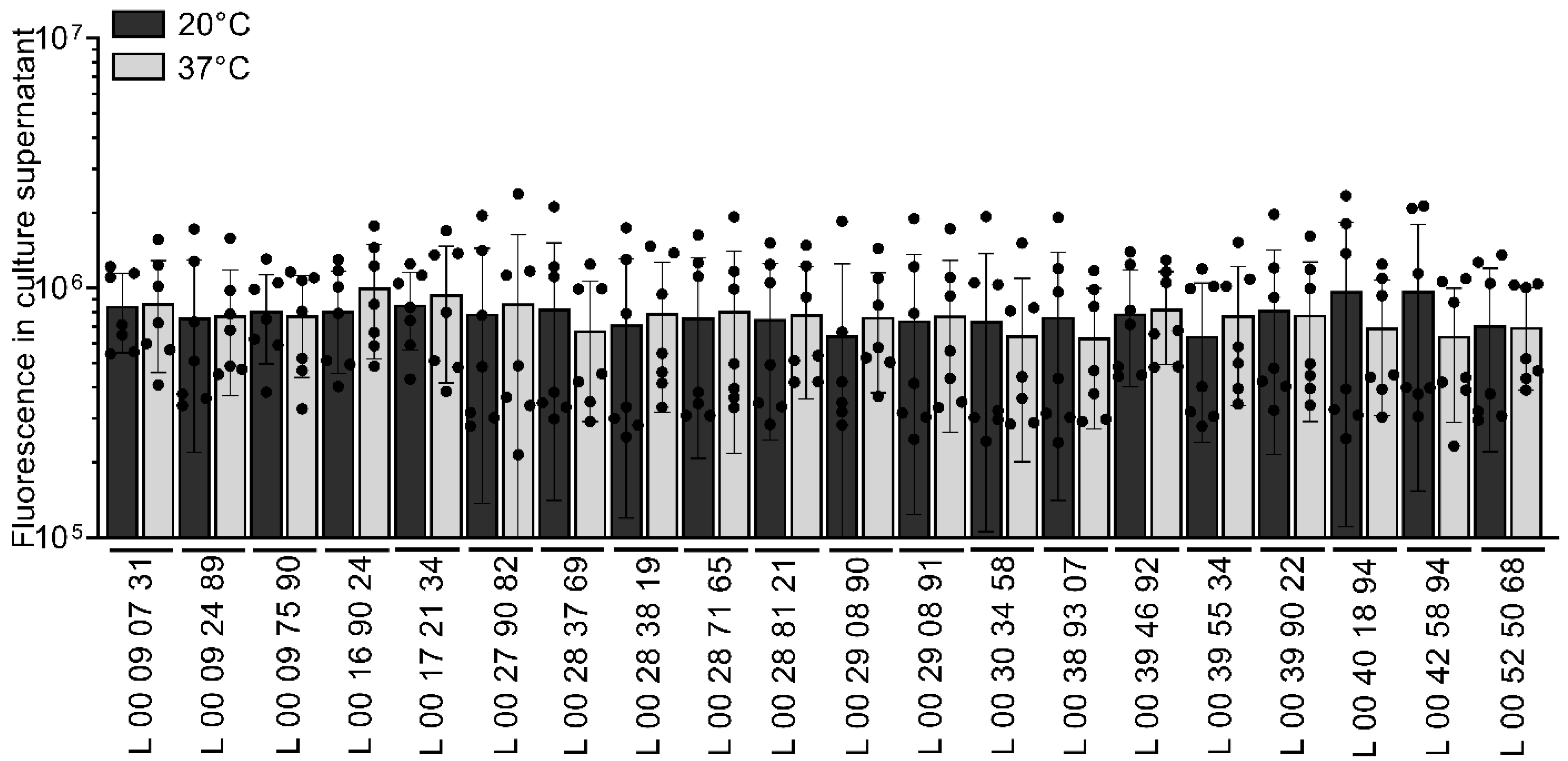
| Strain | Sampling Date |
|---|---|
| V. parahaemolyticus | |
| L 00 09 07 31 | August 2018 |
| L 00 09 24 89 | August 2018 |
| L 00 09 75 90 | September 2018 |
| L 00 16 90 24 | August 2019 |
| L 00 17 21 34 | September 2019 |
| L 00 27 90 82 | July 2020 |
| L 00 28 37 69 | August 2020 |
| L 00 28 38 19 | August 2020 |
| L 00 28 71 65 | September 2020 |
| L 00 28 81 21 | September 2020 |
| L 00 29 08 90 | September 2020 |
| L 00 29 08 91 | September 2020 |
| L 00 30 34 58 | October 2020 |
| L 00 38 93 07 | September 2021 |
| L 00 39 46 92 | September 2021 |
| L 00 39 55 34 | September 2021 |
| L 00 39 90 22 | October 2021 |
| L 00 40 18 94 | October 2021 |
| L 00 42 58 94 | December 2021 |
| L 00 52 50 68 | August 2022 |
| MIC (µg/mL) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATB | PmB | Carb | Rifampicin | Strep | Kana | Tetra | Chloramp | ||||||||
| Target | Membrane | Cell Wall | RNA Synthesis | Protein Synt. | Protein Synt. | Protein Synt. | Protein Synt. | ||||||||
| T° | 20 °C | 37 °C | 20 °C | 37 °C | 20 °C | 37 °C | 20 °C | 37 °C | 20 °C | 37 °C | 20 °C | 37 °C | 20 °C | 37 °C | |
| Strain | Sampling Date | ||||||||||||||
| L 00 09 07 31 | August 2018 | 50 | 25 | 100 | 50 | 6.25 | 6.25 | 25 | 25 | 25 | 12.5 | 1.56 | 0.78 | 0.78 | 0.78 |
| L 00 09 24 89 | August 2018 | 100 | 50 | 100 | 50 | 6.25 | 12.5 | 50 | 25 | 25 | 50 | 1.56 | 0.78 | 0.78 | 0.78 |
| L 00 09 75 90 | September 2018 | 50 | 50 | 100 | 50 | 6.25 | 6.25 | 50 | 25 | 25 | 100 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 16 90 24 | August 2019 | 50 | 25 | 100 | 50 | 6.25 | 6.25 | 50 | 25 | 25 | 12.5 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 17 21 34 | September 2019 | 50 | 25 | 100 | 50 | 6.25 | 6.25 | 50 | 50 | 25 | 25 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 27 90 82 | July 2020 | 100 | 50 | 50 | 50 | 6.25 | 6.25 | 25 | 50 | 25 | 50 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 28 37 69 | August 2020 | 100 | 50 | 50 | 50 | 6.25 | 6.25 | 50 | 25 | 25 | 25 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 28 38 19 | August 2020 | 100 | 50 | 50 | 25 | 12.5 | 3.13 | 50 | 25 | 25 | 25 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 28 71 65 | September 2020 | 100 | 50 | 50 | 50 | 6.25 | 3.13 | 50 | 25 | 25 | 25 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 28 81 21 | September 2020 | 100 | 50 | 50 | 50 | 6.25 | 3.13 | 50 | 50 | 25 | 25 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 29 08 90 | September 2020 | 100 | 50 | 100 | 50 | 6.25 | 3.13 | 50 | 25 | 25 | 25 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 29 08 91 | September 2020 | 100 | 50 | 100 | 25 | 6.25 | 3.13 | 50 | 25 | 25 | 25 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 30 34 58 | October 2020 | 100 | 50 | 50 | 25 | 6.25 | 6.25 | 50 | 50 | 25 | 50 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 38 93 07 | September 2021 | 100 | 100 | 50 | 25 | 6.25 | 6.25 | 50 | 25 | 50 | 12.5 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 39 46 92 | September 2021 | 50 | 50 | 100 | 50 | 6.25 | 3.13 | 25 | 25 | 12.5 | 6.26 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 39 55 34 | September 2021 | 100 | 50 | 100 | 50 | 6.25 | 6.25 | 50 | 50 | 25 | 25 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 39 90 22 | October 2021 | 100 | 50 | 100 | 50 | 6.25 | 12.5 | 50 | 25 | 25 | 100 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 40 18 94 | October 2021 | 100 | 50 | 100 | 50 | 6.25 | 6.25 | 50 | 25 | 25 | 50 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 42 58 94 | December 2021 | 100 | 50 | 100 | 50 | 6.25 | 6.25 | 50 | 25 | 25 | 50 | 0.78 | 0.78 | 0.78 | 0.78 |
| L 00 52 50 68 | August 2022 | 100 | 50 | 100 | 25 | 6.25 | 6.25 | 50 | 25 | 50 | 12.5 | 0.78 | 0.78 | 0.78 | 0.78 |
| Strain | TDH | TRH |
|---|---|---|
| L 00 09 07 31 | − | − |
| L 00 09 24 89 | + | + |
| L 00 09 75 90 | − | + |
| L 00 16 90 24 | − | + |
| L 00 17 21 34 | − | + |
| L 00 27 90 82 | + | + |
| L 00 28 37 69 | + | + |
| L 00 28 38 19 | + | + |
| L 00 28 71 65 | + | + |
| L 00 28 81 21 | + | + |
| L 00 29 08 90 | + | + |
| L 00 29 08 91 | + | + |
| L 00 30 34 58 | + | + |
| L 00 38 93 07 | + | + |
| L 00 39 46 92 | − | + |
| L 00 39 55 34 | + | + |
| L 00 39 90 22 | + | + |
| L 00 40 18 94 | + | + |
| L 00 42 58 94 | + | + |
| L 00 52 50 68 | + | + |
| 20 °C | 37 °C | |
|---|---|---|
| Growth | ||
| Speed | Slow | Fast |
| Maximum OD600nm | High | Low |
| CFU/mL | High | Low |
| Biofilm biomass | High | Low |
| Motility | ||
| Swimming | Low | High |
| Swarming | High | Low |
| Resistance to antimicrobials | More resistant | Less resistant |
| Hemolysis | None | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahieddine, F.C.; Mathieu-Denoncourt, A.; Duperthuy, M. Temperature Influences Antimicrobial Resistance and Virulence of Vibrio parahaemolyticus Clinical Isolates from Quebec, Canada. Pathogens 2025, 14, 521. https://doi.org/10.3390/pathogens14060521
Mahieddine FC, Mathieu-Denoncourt A, Duperthuy M. Temperature Influences Antimicrobial Resistance and Virulence of Vibrio parahaemolyticus Clinical Isolates from Quebec, Canada. Pathogens. 2025; 14(6):521. https://doi.org/10.3390/pathogens14060521
Chicago/Turabian StyleMahieddine, Feriel C., Annabelle Mathieu-Denoncourt, and Marylise Duperthuy. 2025. "Temperature Influences Antimicrobial Resistance and Virulence of Vibrio parahaemolyticus Clinical Isolates from Quebec, Canada" Pathogens 14, no. 6: 521. https://doi.org/10.3390/pathogens14060521
APA StyleMahieddine, F. C., Mathieu-Denoncourt, A., & Duperthuy, M. (2025). Temperature Influences Antimicrobial Resistance and Virulence of Vibrio parahaemolyticus Clinical Isolates from Quebec, Canada. Pathogens, 14(6), 521. https://doi.org/10.3390/pathogens14060521






