Identification of Virulence Genes and Antibiotic Resistance in Extraintestinal Pathogenic Escherichia coli Isolated from Broiler Carcasses Using MALDI-TOF MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and Identification
2.2. Virulence Gene Detection
2.3. Antibiotic Resistance Determination
2.4. Data Analysis
3. Results
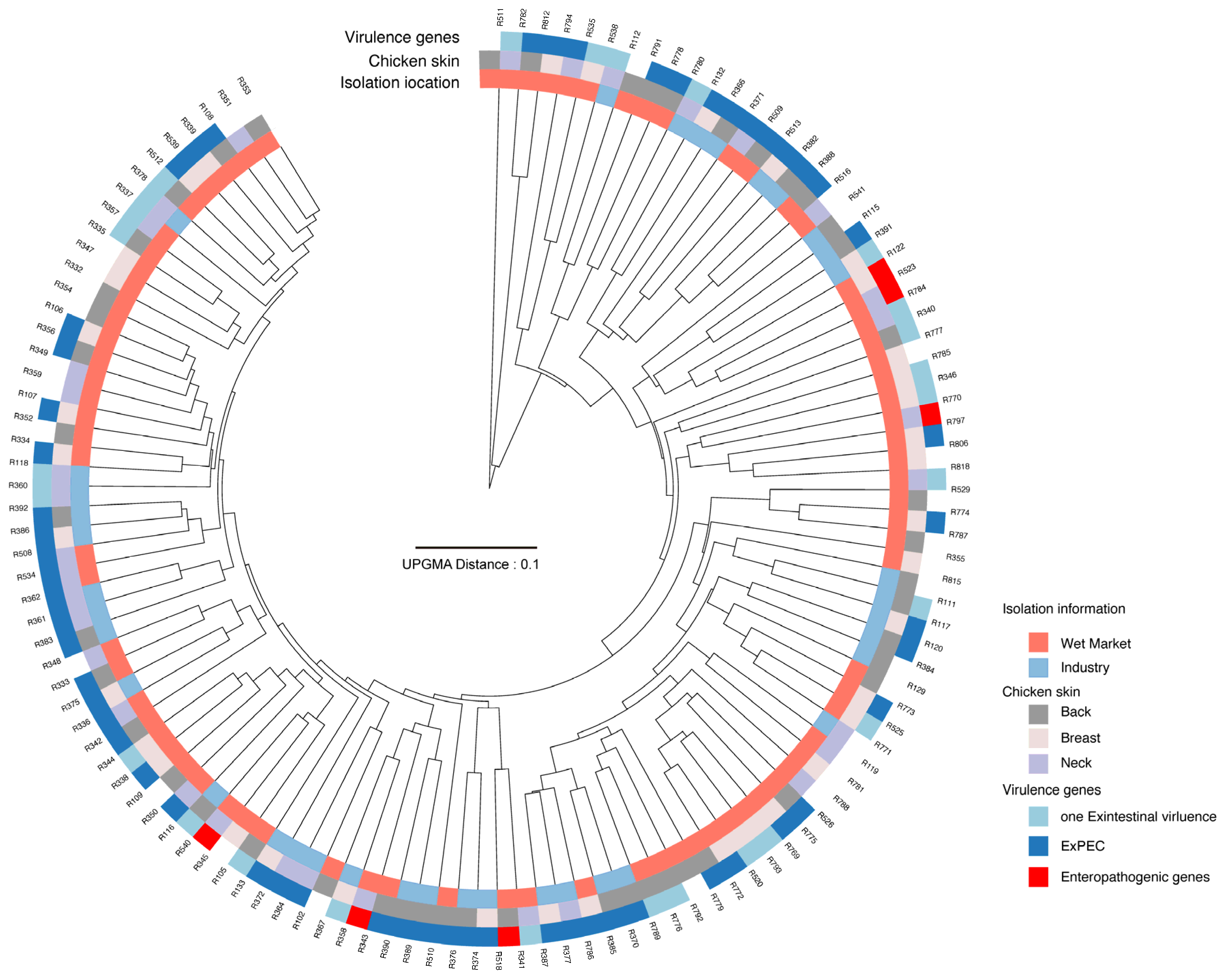
3.1. Determination of ExPEC in Chicken Carcasses Processed in Different Environments
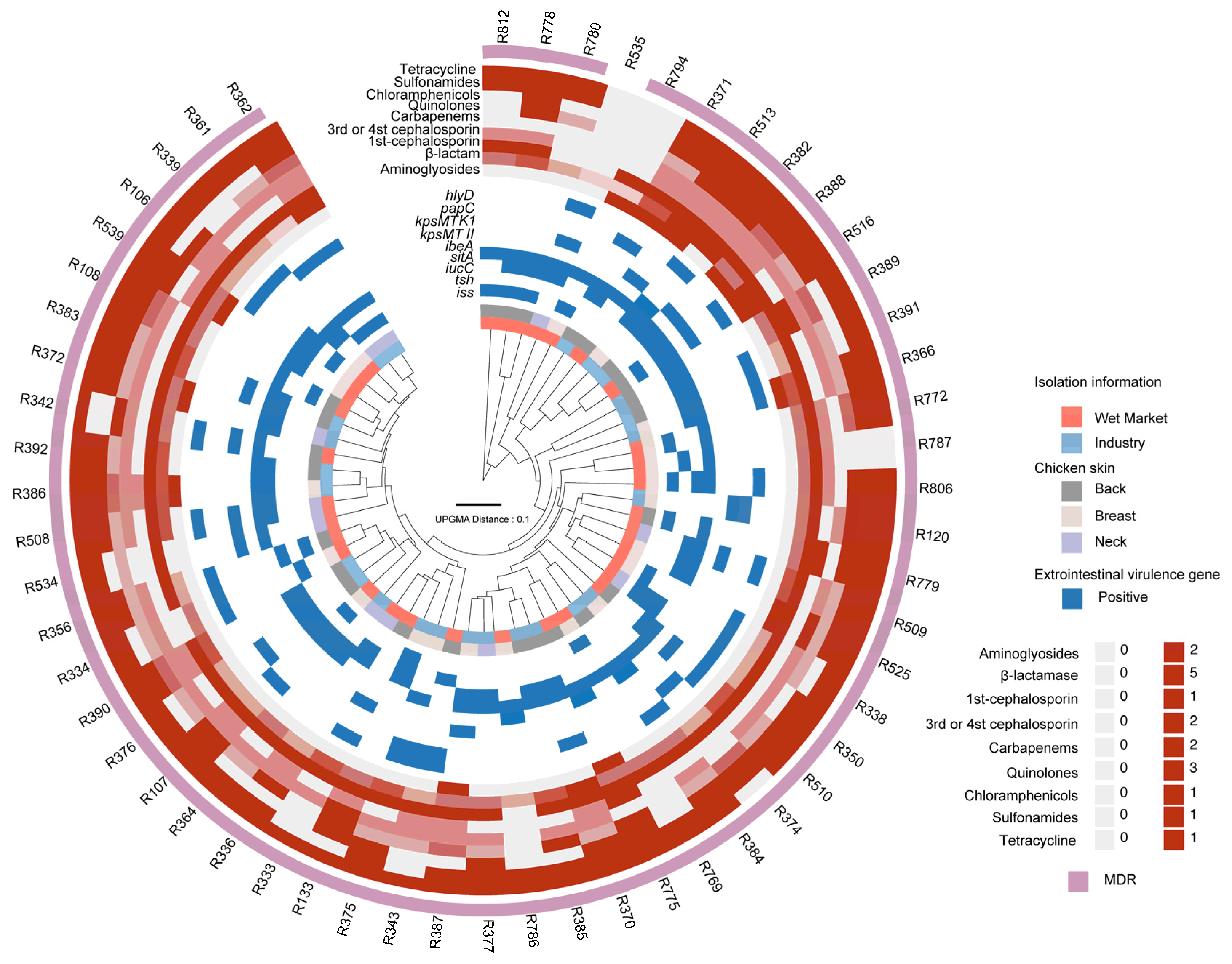
3.2. Multidrug Resistance in ExPEC Isolates
3.3. PMF Analysis Reveals Heterogeneity in Virulence and Antibiotic Resistance Profiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jakobsen, L.; Spangholm, D.J.; Pedersen, K.; Jensen, L.B.; Emborg, H.-D.; Agersø, Y.; Aarestrup, F.M.; Hammerum, A.M.; Frimodt-Møller, N. Broiler Chickens, Broiler Chicken Meat, Pigs and Pork as Sources of ExPEC Related Virulence Genes and Resistance in Escherichia coli Isolates from Community-Dwelling Humans and UTI Patients. Int. J. Food Microbiol. 2010, 142, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Jamali, H.; Akrami, F.; Bouakkaz, S.; Dozois, C.M. Prevalence of Specific Serogroups, Antibiotic Resistance and Virulence Factors of Avian Pathogenic Escherichia coli (APEC) Isolated from Clinical Cases: A Systematic Review and Meta-Analysis. Microb. Pathog. 2024, 194, 106843. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Ma, P.-P.; Liu, W.-S.; Liang, X.; Li, X.-Y.; Li, Y.-Z.; Liu, B.-T. Prevalence and Antibiotic Resistance Characteristics of Extraintestinal Pathogenic Escherichia coli among Healthy Chickens from Farms and Live Poultry Markets in China. Animals 2021, 11, 1112. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, N.; Cen, J.; Han, J.; Tang, Y.; Xu, Z.; Zeng, H.; Houf, K.; Yu, Z. Comparative Analyses of Bacterial Contamination and Microbiome of Broiler Carcasses in Wet Market and Industrial Processing Environments. Int. J. Food Microbiol. 2025, 426, 110937. [Google Scholar] [CrossRef]
- Mitchell, N.M.; Johnson, J.R.; Johnston, B.; Curtiss, R.; Mellata, M. Zoonotic Potential of Escherichia coli Isolates from Retail Chicken Meat Products and Eggs. Appl. Environ. Microbiol. 2015, 81, 1177–1187. [Google Scholar] [CrossRef]
- Lamar, F.; Mondlane-Milisse, A.; Brito, D.R.A.; Mucache, H.N.; Jesser, K.J.; Fagnant-Sperati, C.S.; Victor, C.; Shioda, K.; Fafetine, J.M.; Saíde, J.Â.O.; et al. Accumulation of Microbial Hazards and Assessment of Food Hygiene Associated with Broiler Chicken Processing at Open Air Food Markets in Maputo, Mozambique. Int. J. Food Microbiol. 2025, 427, 110960. [Google Scholar] [CrossRef]
- Elshebrawy, H.A.; Kasem, N.G.; Sallam, K.I. Methicillin- and Vancomycin-Resistant Staphylococcus aureus in Chicken Carcasses, Ready-to-Eat Chicken Meat Sandwiches, and Buffalo Milk. Int. J. Food Microbiol. 2025, 427, 110968. [Google Scholar] [CrossRef]
- Sanches, M.S.; Baptista, A.A.S.; de Souza, M.; Menck-Costa, M.F.; Koga, V.L.; Kobayashi, R.K.T.; Rocha, S.P.D. Genotypic and Phenotypic Profiles of Virulence Factors and Antimicrobial Resistance of Proteus mirabilis Isolated from Chicken Carcasses: Potential Zoonotic Risk. Braz. J. Microbiol. 2019, 50, 685–694. [Google Scholar] [CrossRef]
- Pavlickova, S.; Klancnik, A.; Dolezalova, M.; Mozina, S.S.; Holko, I. Antibiotic Resistance, Virulence Factors and Biofilm Formation Ability in Escherichia coli Strains Isolated from Chicken Meat and Wildlife in the Czech Republic. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2017, 52, 570–576. [Google Scholar] [CrossRef]
- Gharaibeh, M.H.; Al Sheyab, S.Y.; Malkawi, I.M.; Al Qudsi, F.R. Phenotypic and Genotypic Characterization of Escherichia coli Isolated from the Chicken Liver in Relation to Slaughterhouse Conditions. Heliyon 2024, 10, e27759. [Google Scholar] [CrossRef]
- Pitout, J. Extraintestinal Pathogenic Escherichia coli: A Combination of Virulence with Antibiotic Resistance. Front. Microbiol. 2012, 3, 9. [Google Scholar] [CrossRef]
- Koskella, B.; Hall, L.J.; Metcalf, C.J.E. The Microbiome beyond the Horizon of Ecological and Evolutionary Theory. Nat. Ecol. Evol. 2017, 1, 1606–1615. [Google Scholar] [CrossRef]
- Yu, Z.; Joossens, M.; Houf, K. Analyses of the Bacterial Contamination on Belgian Broiler Carcasses at Retail Level. Front. Microbiol. 2020, 11, 21–51. [Google Scholar] [CrossRef]
- Sumbana, J.J.; Santona, A.; Fiamma, M.; Taviani, E.; Deligios, M.; Zimba, T.; Lucas, G.; Sacarlal, J.; Rubino, S.; Paglietti, B. Extraintestinal Pathogenic Escherichia coli ST405 Isolate Coharboring BlaNDM-5 and BlaCTXM-15: A New Threat in Mozambique. Microb. Drug Resist. 2021, 27, 1633–1640. [Google Scholar] [CrossRef]
- Wozniak-Biel, A.; Bugla-Płoskońska, G.; Kielsznia, A.; Korzekwa, K.; Tobiasz, A.; Korzeniowska-Kowal, A.; Wieliczko, A. High Prevalence of Resistance to Fluoroquinolones and Tetracycline Campylobacter Spp. Isolated from Poultry in Poland. Microb. Drug Resist. 2018, 24, 314–322. [Google Scholar] [CrossRef]
- Yu, Z.J.; Peruzy, M.F.; Dumolin, C.; Joossens, M.; Houf, K.; Yu, Z.; Peruzy, M.F.; Dumolin, C.; Joossens, M.; Houf, K. Assessment of Food Microbiological Indicators Applied on Poultry Carcasses by Culture Combined MALDI-TOF MS Identification and 16S rRNA Amplicon Sequencing. Food Microbiol. 2019, 82, 53–61. [Google Scholar] [CrossRef]
- Edwards, U.; Rogall, T.; Blöcker, H.; Emde, M.; Böttger, E.C. Isolation and Direct Complete Nucleotide Determination of Entire Genes. Characterization of a Gene Coding for 16S Ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef]
- Johnson, J.R.; Murray, A.C.; Gajewski, A.; Sullivan, M.; Snippes, P.; Kuskowski, M.A.; Smith, K.E. Isolation and Molecular Characterization of Nalidixic Acid-Resistant Extraintestinal Pathogenic Escherichia coli from Retail Chicken Products. Antimicrob. Agents Chemother. 2003, 47, 2161–2168. [Google Scholar] [CrossRef]
- Weinstein, M.P. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; M100-S25; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Clark, C.M.; Costa, M.S.; Sanchez, L.M.; Murphy, B.T. Coupling MALDI-TOF Mass Spectrometry Protein and Specialized Metabolite Analyses to Rapidly Discriminate Bacterial Function. Proc. Natl. Acad. Sci. USA 2018, 115, 4981–4986. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.-X.; Huang, L. Image GP: An Easy-to-Use Data Visualization Web Server for Scientific Researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef]
- GB 29921-2021; National Food Safety Standards Limits of Pathogenic Bacteria in Prepackaged Foods. National Standard of the People’s Republic of China: Beijing, China, 2021.
- Riley, L.W. Distinguishing Pathovars from Nonpathovars: Escherichia coli. Microbiol. Spectr. 2020, 8, 10–1128. [Google Scholar] [CrossRef]
- Antão, E.-M.; Glodde, S.; Li, G.; Sharifi, R.; Homeier, T.; Laturnus, C.; Diehl, I.; Bethe, A.; Philipp, H.-C.; Preisinger, R.; et al. The Chicken as a Natural Model for Extraintestinal Infections Caused by Avian Pathogenic Escherichia coli (APEC). Microb. Pathog. 2008, 45, 361–369. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, W.; Luo, L.; Wang, H.; Shao, H.; Zhang, T.; Luo, Q. Genetic Diversity and Multidrug Resistance of Phylogenic Groups B2 and D in InPEC and ExPEC Isolated from Chickens in Central China. BMC Microbiol. 2022, 22, 60. [Google Scholar] [CrossRef]
- Li, D.; Reid, C.J.; Kudinha, T.; Jarocki, V.M.; Djordjevic, S.P. Genomic Analysis of Trimethoprim Resistant Extraintestinal Pathogenic Escherichia coli (ExPEC) and Recurrent Urinary Tract Infections. Microb. Genom. 2020, 6, e000475. [Google Scholar]
- Harwalkar, A.; Gupta, S.; Rao, A.; Srinivasa, H. Lower Prevalence of HlyD, PapC and Cnf-1 Genes in Ciprofloxacin-Resistant Uropathogenic Escherichia coli than Their Susceptible Counterparts Isolated from Southern India. J. Infect. Public Health 2014, 7, 413–419. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Z.; Li, T.; Cheng, M.; Sun, H.; Cui, M.; Zhang, C.; Xu, S.; Wang, H.; Wu, C. Current Status and Trends in Antimicrobial Use in Food Animals in China, 2018–2020. One Health Adv. 2023, 1, 29. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A Systematic Review on Antibiotics Misuse in Livestock and Aquaculture and Regulation Implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Da’na, D.A.; Al-Ghouti, M.A. Application of MALDI-TOF MS for Identification of Environmental Bacteria: A Review. J. Environ. Manag. 2022, 305, 114359. [Google Scholar] [CrossRef]
- Yoon, E.-J.; Jeong, S.H. MALDI-TOF Mass Spectrometry Technology as a Tool for the Rapid Diagnosis of Antimicrobial Resistance in Bacteria. Antibiotics 2021, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Flores-Treviño, S.; Garza-González, E.; Mendoza-Olazarán, S.; Morfín-Otero, R.; Camacho-Ortiz, A.; Rodríguez-Noriega, E.; Martínez-Meléndez, A.; Bocanegra-Ibarias, P. Screening of Biomarkers of Drug Resistance or Virulence in ESCAPE Pathogens by MALDI-TOF Mass Spectrometry. Sci. Rep. 2019, 9, 18945. [Google Scholar] [CrossRef] [PubMed]
- Feucherolles, M.; Cauchie, H.-M.; Penny, C. MALDI-TOF Mass Spectrometry and Specific Biomarkers: Potential New Key for Swift Identification of Antimicrobial Resistance in Foodborne Pathogens. Microorganisms 2019, 7, 593. [Google Scholar] [CrossRef]
- Parvin, M.S.; Talukder, S.; Ali, M.Y.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Antimicrobial Resistance Pattern of Escherichia coli Isolated from Frozen Chicken Meat in Bangladesh. Pathogens 2020, 9, 420. [Google Scholar] [CrossRef]
- Bai, J.; Shi, X.; Nagaraja, T.G. A Multiplex PCR Procedure for the Detection of Six Major Virulence Genes in Escherichia coli O157:H7. J. Microbiol. Methods 2010, 82, 85–89. [Google Scholar] [CrossRef]
- Xia, X.; Meng, J.; Zhao, S.; Bodeis-Jones, S.; Gaines, S.A.; Ayers, S.L.; McDermott, P.F. Identification and Antimicrobial Resistance of Extraintestinal Pathogenic Escherichia coli from Retail Meats. J. Food Prot. 2011, 74, 38–44. [Google Scholar] [CrossRef]
| Classes | Antimicrobial Agents | Susceptible | Intermediate | Resistant | Resistant for the Class |
|---|---|---|---|---|---|
| Aminoglycosides | AMK | 51 | 1 | 4 | 11/56 |
| GEN | 39 | 8 | 9 | ||
| β-lactams | AMC | 17 | 12 | 27 | 55/56 |
| AMP | 6 | 2 | 48 | ||
| AZI | 27 | 7 | 22 | ||
| PIP | 47 | 7 | 2 | ||
| SAM | 31 | 11 | 14 | ||
| TZP | 2 | 1 | 53 | ||
| Cephalosporins | CZ | 4 | 0 | 52 | 52/56 |
| FEP | 41 | 13 | 2 | ||
| CTX | 34 | 3 | 19 | ||
| CAZ | 40 | 11 | 5 | ||
| Carbapenems | IPM | 13 | 2 | 41 | 41/56 |
| MEM | 51 | 3 | 2 | ||
| Quinolones | CIP | 21 | 12 | 23 | 47/56 |
| LEV | 12 | 29 | 15 | ||
| MXF | 8 | 3 | 45 | ||
| Others | C | 12 | 11 | 33 | 33/56 |
| TE | 2 | 2 | 52 | 52/56 | |
| SXT | 7 | 1 | 48 | 49/56 |
| Resistance | ExPEC Counts | |
|---|---|---|
| ≤5 agents (5/20) | 10.71% (6/56) | |
| ≥10 agents (10/20) | 53.7% (30/56) | |
| ≥14 agents (14/20) | 5.56% (3/56) | |
| MDR | ≥3 classes (8) | 94.6% (54/56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.-T.; Tang, Y.-X.; Wu, S.-Y.; Chen, Y.-R.; Zou, Z.-P.; Zeng, H.; Yu, Z. Identification of Virulence Genes and Antibiotic Resistance in Extraintestinal Pathogenic Escherichia coli Isolated from Broiler Carcasses Using MALDI-TOF MS. Pathogens 2025, 14, 501. https://doi.org/10.3390/pathogens14050501
Han J-T, Tang Y-X, Wu S-Y, Chen Y-R, Zou Z-P, Zeng H, Yu Z. Identification of Virulence Genes and Antibiotic Resistance in Extraintestinal Pathogenic Escherichia coli Isolated from Broiler Carcasses Using MALDI-TOF MS. Pathogens. 2025; 14(5):501. https://doi.org/10.3390/pathogens14050501
Chicago/Turabian StyleHan, Jia-Tong, Yu-Xuan Tang, Si-Yi Wu, Yi-Ran Chen, Zhan-Peng Zou, Hang Zeng, and Zhongjia Yu. 2025. "Identification of Virulence Genes and Antibiotic Resistance in Extraintestinal Pathogenic Escherichia coli Isolated from Broiler Carcasses Using MALDI-TOF MS" Pathogens 14, no. 5: 501. https://doi.org/10.3390/pathogens14050501
APA StyleHan, J.-T., Tang, Y.-X., Wu, S.-Y., Chen, Y.-R., Zou, Z.-P., Zeng, H., & Yu, Z. (2025). Identification of Virulence Genes and Antibiotic Resistance in Extraintestinal Pathogenic Escherichia coli Isolated from Broiler Carcasses Using MALDI-TOF MS. Pathogens, 14(5), 501. https://doi.org/10.3390/pathogens14050501








