The Translationally Controlled Tumor Protein (TCTP), a Novel Antigen of Babesia bovis, Participates in the Establishment of Acute Infection and Contains Neutralizing B-Cell Epitopes
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of tctp Gene in the Genome of B. bovis
2.2. DNA Extraction, Amplification and Sequencing of tctp Gene
2.3. Transcription Analysis by RT-PCR
2.4. TCTP Topology Analysis
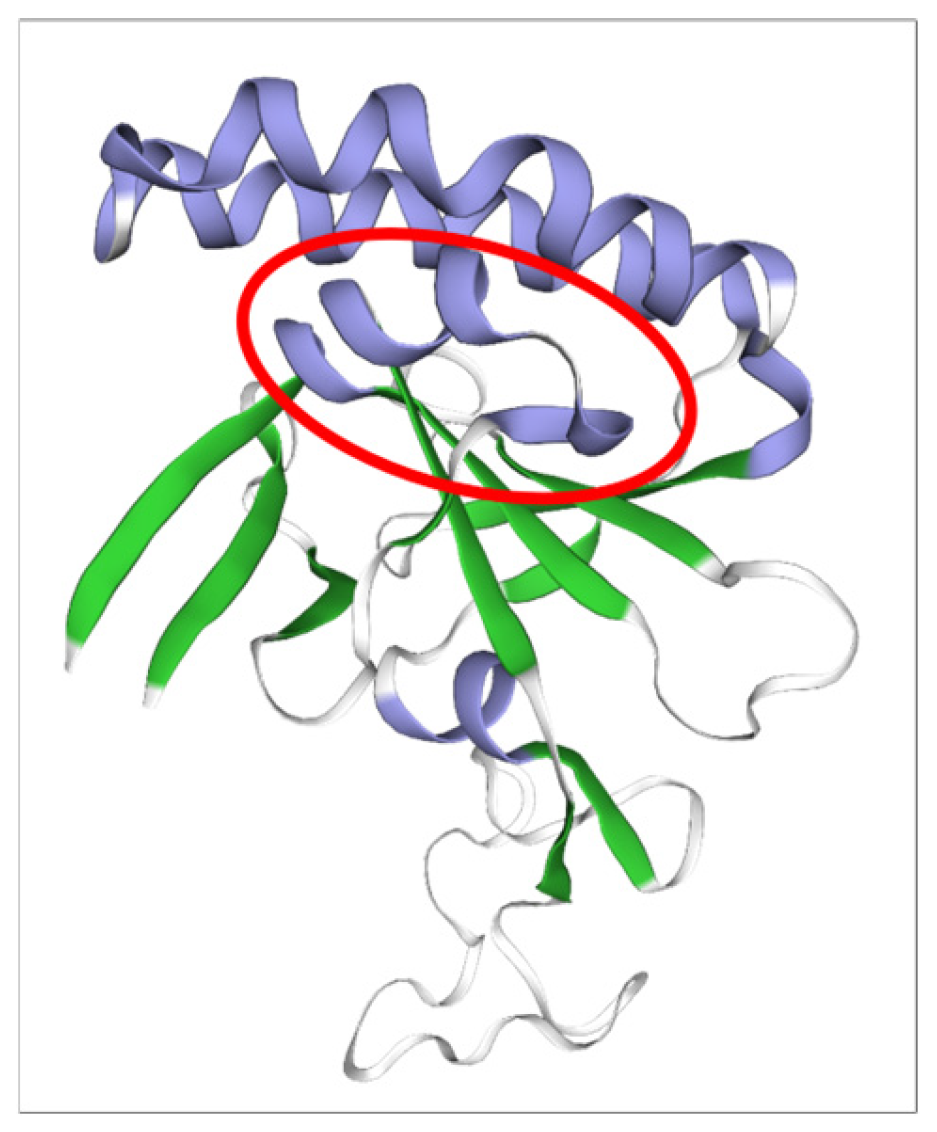
2.5. Prediction of the Three-Dimensional Structure of B. bovis TCTP
2.6. Prediction of Peptides Containing B-Cell Epitopes
2.7. Expression of TCTP in Babesia bovis
2.8. Generation of Specific Antibodies Anti-B. bovis TCTP in Rabbits
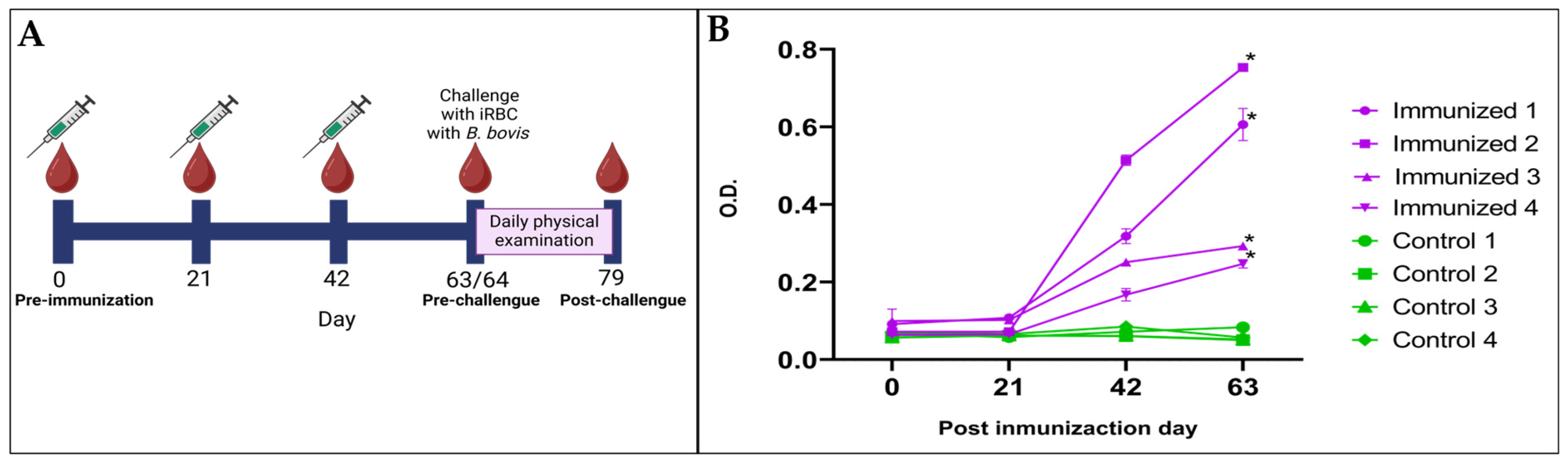
2.9. Immunization of Cattle with TCTP Peptides and Challenge with Virulent B. bovis
2.10. Confocal Microscopy
2.11. Assessment of Specific Antibody Response to TCTP and Antibody Titration of Sera in Immunized Cattle
2.12. Determination of Total Antibodies in Bovine Sera
2.13. Neutralization Assay
3. Results
3.1. TCTP Is a Highly Conserved Gene in Babesia bovis
3.2. Identification of B-Cell Epitope Candidates from BboTCTP
3.3. TCTP Is Expressed in Intraerythrocytic Stages
3.4. BboTCTP Interferes with the Host Immune Response to Infection
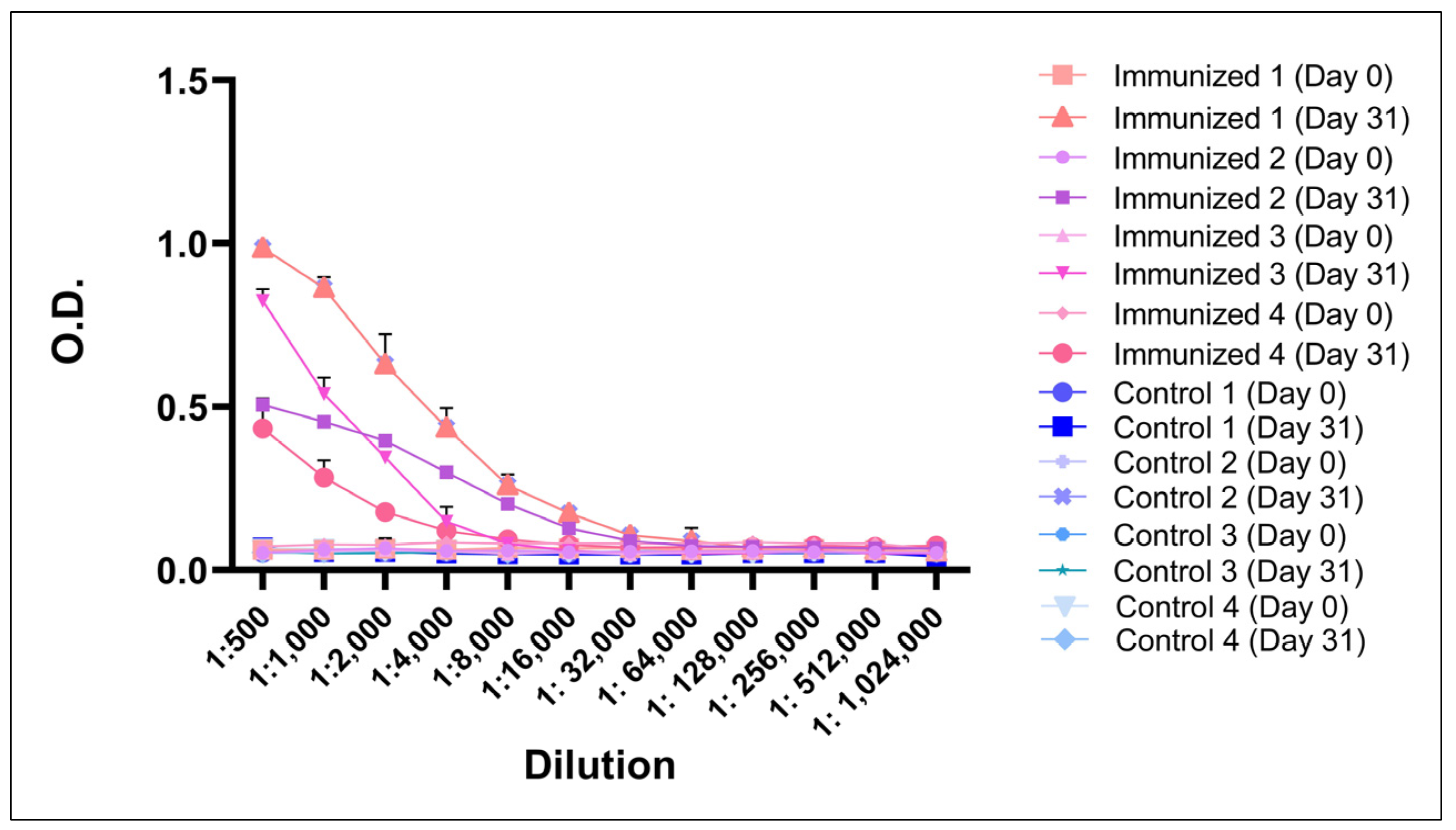
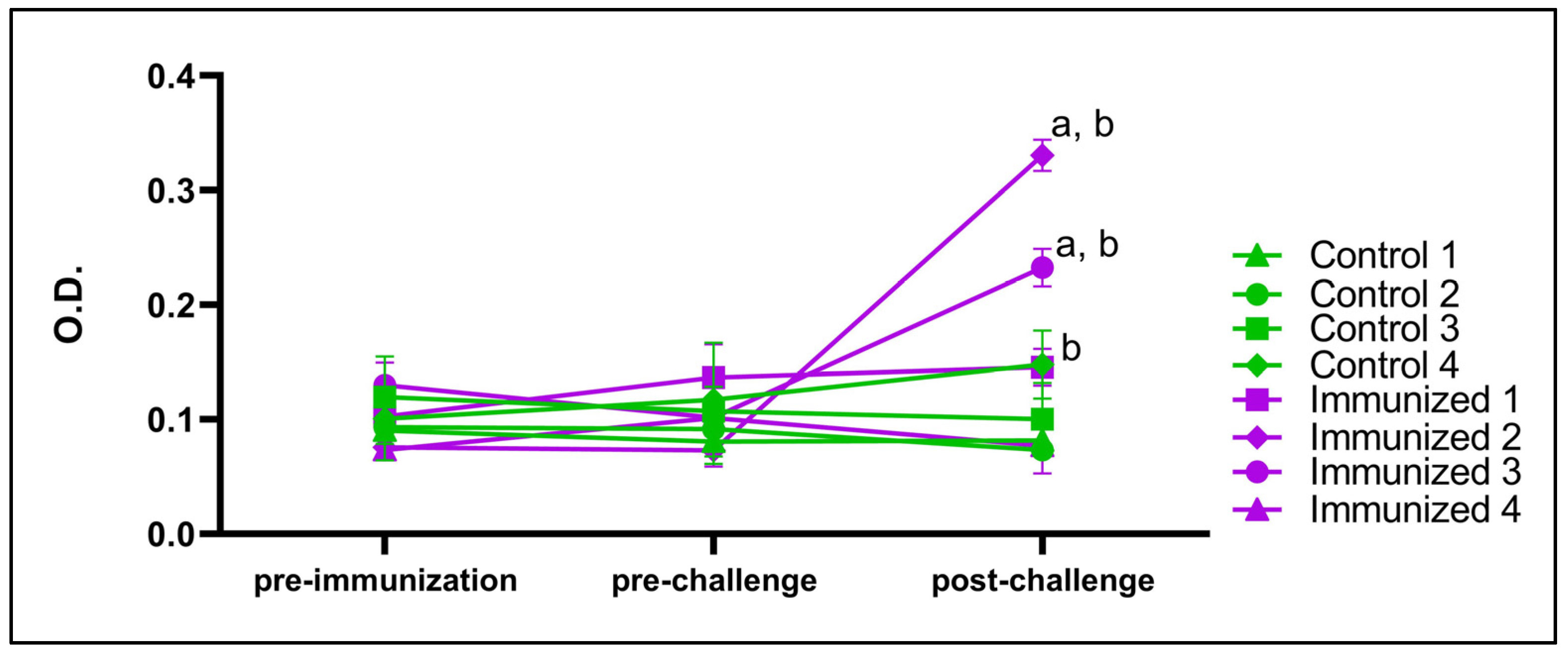
3.5. BboTCTP Interferes with the Immune Response of Cattle
3.6. Antibodies Anti rBboTCTP Block Invasion of Merozoites to Erythrocytes In Vitro
4. Discussion
4.1. TCTP Is a Highly Conserved
4.2. BboTCTP Expression During the Erythrocytic Stage
4.3. Potential Inhibitory Effect of Anti-TCTP Antibodies on Parasite Invasion
4.4. Protective Effects of TCTP Immunization in Challenged Cattle
4.5. Antibody Levels and Possible Immunomodulatory Role of BboTCT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B. bovis | Babesia bovis |
| B. bigemina | Babesia bigemina |
| B. divergens | Babesia divergens |
| IL | Interleukin |
| BLAST | Basic Local Aligment Search Tool |
| DNA | Deoxyribunuclei chain reaction |
| PCR | Polymerase chain reaction |
| s | second |
| °C | Degrees Celcius |
| UNAM | National Autonomous University of Mexico |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RNA | Ribonucleic Acid |
| µg | Micrograms |
| cDNA | Complementary DNA |
| µL | Microliter |
| bp | Base pairs |
| WB | Western blot |
| iRBC | Infected red blood cells |
| SDS | Sodium dodecyl sulfate |
| PBS | Phosphate buffered saline |
| mM | Milimolar |
| h | Hour |
| TBS | Tris-buffered Saline |
| MAPS | Multiple antigenic peptide |
| rpm | Revolution per minute |
| mL | Milliliter |
| OPD | O-phenylenediamine dihydrochloride |
| ELISA | Enzyme-liked immunosorbent assay |
| µm | Micrometer |
| nRBC | Non-infected red blood cells |
| ANOVA | Analysis of variance |
| NCBI | National Center for Biotechnology Information |
| mRNA | Messenger RNA |
References
- De Waal, D.T.; Combrink, M.P. Live Vaccines against Bovine Babesiosis. Vet. Parasitol. 2006, 138, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A World Emerging. Infect. Genet. Evol. 2012, 12, 1788–1809. [Google Scholar] [CrossRef] [PubMed]
- Gohil, S.; Herrmann, S.; Günther, S.; Cooke, B.M. Bovine Babesiosis in the 21st Century: Advances in Biology and Functional Genomics. Int. J. Parasitol. 2013, 43, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.; Palmer, G.H. Designing Blood-Stage Vaccines against Babesia Bovis and B. Bigemina. Parasitol. Today 1999, 15, 275–281. [Google Scholar] [CrossRef]
- Bommer, U.-A.; Thiele, B.-J. The Translationally Controlled Tumour Protein (TCTP). Int. J. Biochem. Cell Biol. 2004, 36, 379–385. [Google Scholar] [CrossRef]
- Bhisutthibhan, J.; Philbert, M.A.; Fujioka, H.; Aikawa, M.; Meshnick, S.R. The Plasmodium Falciparum Translationally Controlled Tumor Protein: Subcellular Localization and Calcium Binding. Eur. J. Cell Biol. 1999, 78, 665–670. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Bhisutthibhan, J.; Shapiro, T.A.; Rogerson, S.J.; Taylor, T.E.; Tembo, M.; Langdon, J.M.; Meshnick, S.R. Immune Mimicry in Malaria: Plasmodium Falciparum Secretes a Functional Histamine-Releasing Factor Homolog in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 10829–10832. [Google Scholar] [CrossRef]
- Pelleau, S.; Diop, S.; Dia Badiane, M.; Vitte, J.; Beguin, P.; Nato, F.; Diop, B.M.; Bongrand, P.; Parzy, D.; Jambou, R. Enhanced Basophil Reactivities during Severe Malaria and Their Relationship with the Plasmodium Falciparum Histamine-Releasing Factor Translationally Controlled Tumor Protein. Infect. Immun. 2012, 80, 2963–2970. [Google Scholar] [CrossRef]
- Mathieu, C.; Demarta-Gatsi, C.; Porcherie, A.; Brega, S.; Thiberge, S.; Ronce, K.; Smith, L.; Peronet, R.; Amino, R.; Ménard, R.; et al. Plasmodium Berghei Histamine-Releasing Factor Favours Liver-Stage Development via Inhibition of IL-6 Production and Associates with a Severe Outcome of Disease: Malaria Parasite HRF Controls Pathogenicity. Cell. Microbiol. 2015, 17, 542–558. [Google Scholar] [CrossRef]
- Amzallag, N.; Passer, B.J.; Allanic, D.; Segura, E.; Théry, C.; Goud, B.; Amson, R.; Telerman, A. TSAP6 Facilitates the Secretion of Translationally Controlled Tumor Protein/Histamine-Releasing Factor via a Nonclassical Pathway. J. Biol. Chem. 2004, 279, 46104–46112. [Google Scholar] [CrossRef]
- Eichhorn, T.; Winter, D.; Büchele, B.; Dirdjaja, N.; Frank, M.; Lehmann, W.-D.; Mertens, R.; Krauth-Siegel, R.L.; Simmet, T.; Granzin, J.; et al. Molecular Interaction of Artemisinin with Translationally Controlled Tumor Protein (TCTP) of Plasmodium Falciparum. Biochem. Pharmacol. 2013, 85, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Bommer, U.-A. The Translational Controlled Tumour Protein TCTP: Biological Functions and Regulation. In TCTP/tpt1-Remodeling Signaling from Stem Cell to Disease; Results and Problems in Cell Differentiation; Springer International Publishing: Cham, Switzerland, 2017; Volume 64, pp. 69–126. ISBN 978-3-319-67590-9. [Google Scholar]
- Calderón-Pérez, B.; Xoconostle-Cázares, B.; Lira-Carmona, R.; Hernández-Rivas, R.; Ortega-López, J.; Ruiz-Medrano, R. The Plasmodium Falciparum Translationally Controlled Tumor Protein (TCTP) Is Incorporated More Efficiently into B Cells than Its Human Homologue. PLoS ONE 2014, 9, e85514. [Google Scholar] [CrossRef][Green Version]
- Walker, D.J.; Pitsch, J.L.; Peng, M.M.; Robinson, B.L.; Peters, W.; Bhisutthibhan, J.; Meshnick, S.R. Mechanisms of Artemisinin Resistance in the Rodent Malaria Pathogen Plasmodium Yoelii. Antimicrob. Agents Chemother. 2000, 44, 344–347. [Google Scholar] [CrossRef]
- Thaw, P.; Baxter, N.J.; Hounslow, A.M.; Price, C.; Waltho, J.P.; Craven, C.J. Structure of TCTP Reveals Unexpected Relationship with Guanine Nucleotide- Free Chaperones. Nat. Struct. Biol. 2001, 8, 701–704. [Google Scholar] [CrossRef]
- Hinojosa-Moya, J.; Xoconostle-Cázares, B.; Piedra-Ibarra, E.; Méndez-Tenorio, A.; Lucas, W.J.; Ruiz-Medrano, R. Phylogenetic and Structural Analysis of Translationally Controlled Tumor Proteins. J. Mol. Evol. 2008, 66, 472–483. [Google Scholar] [CrossRef]
- Ruiz-Medrano, R. Structure-Function Relationship of TCTP. In TCTP/tpt1-Remodeling Signaling from Stem Cell to Disease; Xoconostle-Cazares, B., Ed.; Results and Problems in Cell Differentiation; Springer International Publishing: Cham, Switzerland, 2017; Volume 64, pp. 47–68. ISBN 978-3-319-67590-9. [Google Scholar]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI Search and Sequence Analysis Tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Möller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of Methods for the Prediction of Membrane Spanning Regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam Protein Families Database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New Features and Functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef]
- Saha, S.; Raghava, G.P.S. BcePred: Prediction of Continuous B-Cell Epitopes in Antigenic Sequences Using Physico-Chemical Properties. In Artificial Immune Systems; Nicosia, G., Cutello, V., Bentley, P.J., Timmis, J., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 3239, pp. 197–204. ISBN 978-3-540-23097-7. [Google Scholar]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving Sequence-Based B-Cell Epitope Prediction Using Conformational Epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 Update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.G.; Dighe, V.D.; Thakuria, D.; Malik, Y.S.; Kumar, S. Multiple Antigenic Peptide (MAP): A Synthetic Peptide Dendrimer for Diagnostic, Antiviral and Vaccine Strategies for Emerging and Re-Emerging Viral Diseases. Indian J. Virol. 2013, 24, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Lillehoj, H.S.; Lee, S.H.; Lee, K.W.; Lillehoj, E.P.; Bertrand, F.; Dupuis, L.; Deville, S. MontanideTM ISA 71 VG Adjuvant Enhances Antibody and Cell-Mediated Immune Responses to Profilin Subunit Antigen Vaccination and Promotes Protection against Eimeria Acervulina and Eimeria Tenella. Exp. Parasitol. 2011, 127, 178–183. [Google Scholar] [CrossRef]
- Alarcón, G.J.C.; Martínez, J.A.Á.; Ramírez, E.E.R.; Aragón, J.A.R.; Gualito, J.J.M. Protección contra babesiosis bovina con una vacuna mixta de Babesia bovis y Babesia bigemina derivada de cultivo in vitro bajo una confrontación de campo. Inmunización en un área libre de la enfermedad. Vet. Mex. 2003, 34, 323–332. [Google Scholar]
- Demarta-Gatsi, C.; Smith, L.; Thiberge, S.; Peronet, R.; Commere, P.-H.; Matondo, M.; Apetoh, L.; Bruhns, P.; Ménard, R.; Mécheri, S. Protection against Malaria in Mice Is Induced by Blood Stage–Arresting Histamine-Releasing Factor (HRF)–Deficient Parasites. J. Exp. Med. 2016, 213, 1419–1428. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, Y.; Li, Z.; Cao, S.; Zhang, Z.; Jia, H. Translationally Controlled Tumor Protein Is Required for the Fast Growth of Toxoplasma Gondii and Maintenance of Its Intracellular Development. FASEB J. 2018, 32, 906–919. [Google Scholar] [CrossRef]
- Taylor, K.J.; Van, T.T.H.; MacDonald, S.M.; Meshnick, S.R.; Fernley, R.T.; Macreadie, I.G.; Smooker, P.M. Immunization of Mice with Plasmodium TCTP Delays Establishment of Plasmodium Infection. Parasite Immunol. 2015, 37, 23–31. [Google Scholar] [CrossRef]





| Peptide | Sequence | Amino Acid | Position | Antigenicity Value | Allergenicity Value |
|---|---|---|---|---|---|
| Peptide TCTP I | GDEVCSDAYTHLNPFDNPE | 19 | 10–28 | 0.3626 (non-predicted) | 0.1147 (predicted) |
| Peptide TCTP II | TGYIKKYIKRVTAHLEENAPD | 21 | 92–112 | −0.1684 (non-predicted) | 0.3931 (predicted) |
| Peptide TCTP III | SSKVAKGNEDYGIACNDDEEGGG | 23 | 38–60 | 1.6397 (predicted) | 0.1443 (predicted) |
| Peptide TCTP IV | CSDAYTHLNPFDNPEFASVAFE | 22 | 14–35 | 0.3216 (non-predicted) | 0.2153 (predicted) |
| Maximum Parasitemia in Peripheral Blood | Temperature > 40.5 °C More Than 3 Days | Decrease in Mean Corpuscular Volume > 40% | Lethargy, Depression | Anorexia | Experimental Death | |
|---|---|---|---|---|---|---|
| Control | 0.05% | + | + | + | + | x |
| 0.45% | + | + | + | x | ||
| 0.45% | + | + | ||||
| 0.25% | + | + | + | + | x | |
| Immunized | 0.10% | + | ||||
| 0.35% | + | |||||
| 0.25% | ||||||
| 0.20% | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Almeida, C.; Hernández-Silva, D.J.; Hernández-Arvizu, E.E.; Asada, M.; Kawazu, S.-i.; Ueti, M.W.; Gomez-Soto, J.G.; Dávila-Montero, U.M.; Vega y Murguía, C.A.; Mosqueda, J. The Translationally Controlled Tumor Protein (TCTP), a Novel Antigen of Babesia bovis, Participates in the Establishment of Acute Infection and Contains Neutralizing B-Cell Epitopes. Pathogens 2025, 14, 502. https://doi.org/10.3390/pathogens14050502
Pérez-Almeida C, Hernández-Silva DJ, Hernández-Arvizu EE, Asada M, Kawazu S-i, Ueti MW, Gomez-Soto JG, Dávila-Montero UM, Vega y Murguía CA, Mosqueda J. The Translationally Controlled Tumor Protein (TCTP), a Novel Antigen of Babesia bovis, Participates in the Establishment of Acute Infection and Contains Neutralizing B-Cell Epitopes. Pathogens. 2025; 14(5):502. https://doi.org/10.3390/pathogens14050502
Chicago/Turabian StylePérez-Almeida, Chyntia, Diego Josimar Hernández-Silva, Edwin Esaú Hernández-Arvizu, Masahito Asada, Shin-ichiro Kawazu, Massaro W. Ueti, José Guadalupe Gomez-Soto, Urso Martín Dávila-Montero, Carlos A. Vega y Murguía, and Juan Mosqueda. 2025. "The Translationally Controlled Tumor Protein (TCTP), a Novel Antigen of Babesia bovis, Participates in the Establishment of Acute Infection and Contains Neutralizing B-Cell Epitopes" Pathogens 14, no. 5: 502. https://doi.org/10.3390/pathogens14050502
APA StylePérez-Almeida, C., Hernández-Silva, D. J., Hernández-Arvizu, E. E., Asada, M., Kawazu, S.-i., Ueti, M. W., Gomez-Soto, J. G., Dávila-Montero, U. M., Vega y Murguía, C. A., & Mosqueda, J. (2025). The Translationally Controlled Tumor Protein (TCTP), a Novel Antigen of Babesia bovis, Participates in the Establishment of Acute Infection and Contains Neutralizing B-Cell Epitopes. Pathogens, 14(5), 502. https://doi.org/10.3390/pathogens14050502









