High Prevalence and Genetic Heterogeneity of Anaplasma marginale in Smallholder Bovine Populations of Pakistan, and Its Implications
Abstract
1. Introduction
2. Materials and Methods
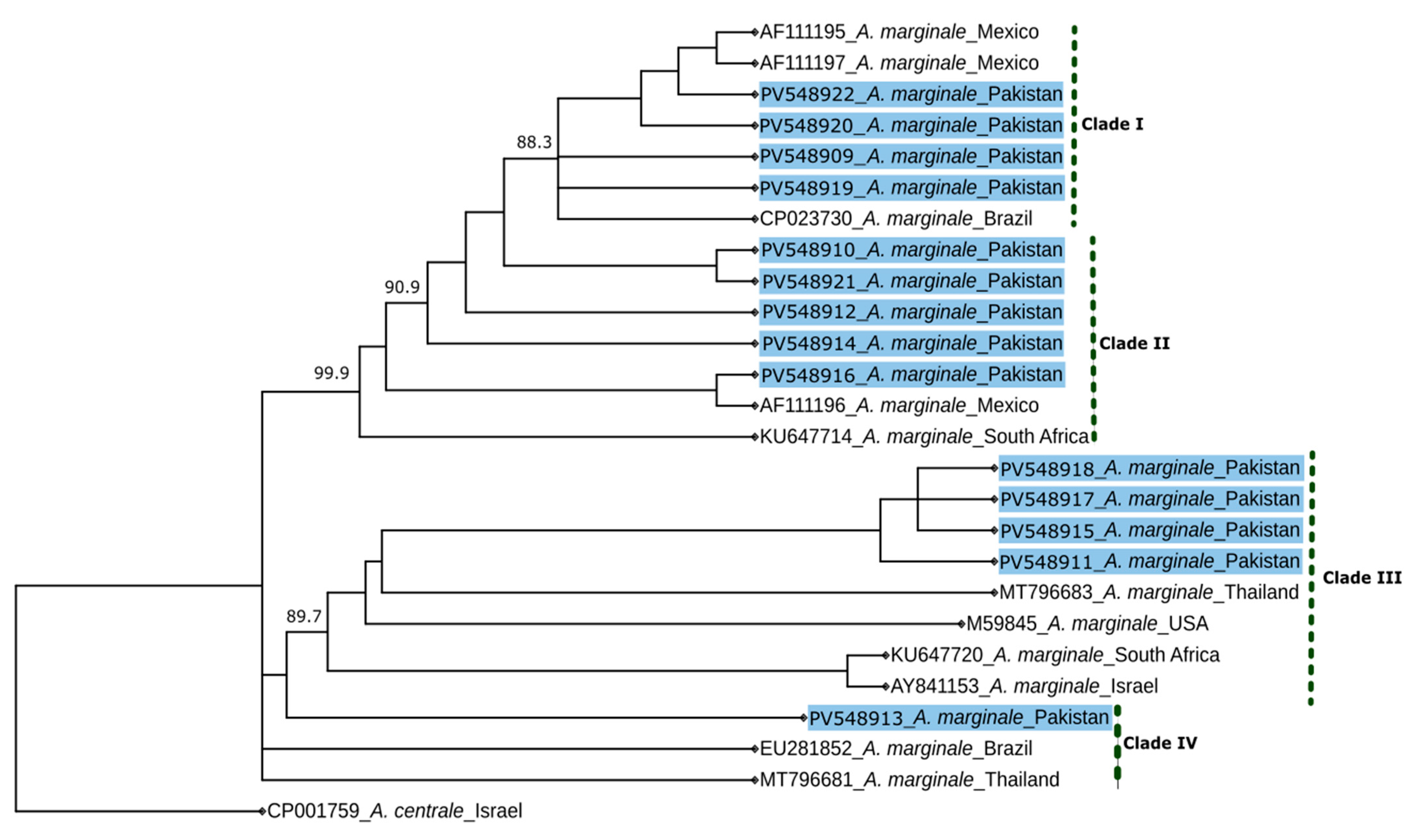
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aubry, P.; Geale, D. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; de la Fuente, J.; Guglielmone, A.A.; Meléndez, R.D. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin. Microbiol. Rev. 2003, 16, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S. The natural history of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef]
- Theiler, A. Anaplasma marginale. Ann. Transvaal Mus. 1910, 2, 53–55. [Google Scholar]
- De La Fournière, S.; Guillemi, E.C.; Paoletta, M.S.; Pérez, A.; Obregón, D.; Cabezas-Cruz, A.; Sarmiento, N.F.; Farber, M.D. Transovarial transmission of Anaplasma marginale in Rhipicephalus (Boophilus) microplus ticks results in a bottleneck for strain diversity. Pathogens 2023, 12, 1010. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Chapter 3.4.1. Bovine anaplasmosis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health (WOAH): Paris, France, 2024; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.04.01_Bovine_Anaplasmosis.pdf (accessed on 1 April 2025).
- Kuttler, K. Anaplasma infections in wild and domestic ruminants: A review. J. Wildl. Dis. 1984, 20, 12–20. [Google Scholar] [CrossRef]
- Ghafar, A.; Gasser, R.B.; Abbas, T.; Rehman, A.; Gauci, C.G.; Jabbar, A. Ticks and tick-borne diseases of bovines in a smallholder livestock context: The Pakistani example. Adv. Parasitol. 2021, 114, 167–244. [Google Scholar]
- Ghafar, A.; Abbas, T.; Rehman, A.; Sandhu, Z.-u.-D.; Cabezas-Cruz, A.; Jabbar, A. Systematic review of ticks and tick-borne pathogens of small ruminants in Pakistan. Pathogens 2020, 9, 937. [Google Scholar] [CrossRef]
- Ghafar, A.; Cabezas-Cruz, A.; Galon, C.; Obregon, D.; Gasser, R.B.; Moutailler, S.; Jabbar, A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasites Vectors 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Ghafar, A.; Gasser, R.B.; Rashid, I.; Ghafoor, A.; Jabbar, A. Exploring the prevalence and diversity of bovine ticks in five agro-ecological zones of Pakistan using phenetic and genetic tools. Ticks Tick-Borne Dis. 2020, 11, 101472. [Google Scholar] [CrossRef]
- Ghafar, A.; Khan, A.; Cabezas-Cruz, A.; Gauci, C.G.; Niaz, S.; Ayaz, S.; Mateos-Hernández, L.; Galon, C.; Nasreen, N.; Moutailler, S. An assessment of the molecular diversity of ticks and tick-borne microorganisms of small ruminants in Pakistan. Microorganisms 2020, 8, 1428. [Google Scholar] [CrossRef] [PubMed]
- Government of Pakistan. Pakistan Economic Survey 2023–2024; Ministry of Finance: Islamabad, Pakistan, 2024; pp. 21–43. Available online: https://www.finance.gov.pk/survey_2024.html (accessed on 16 April 2025).
- Jabbar, A.; Abbas, T.; Sandhu, Z.-u.-D.; Saddiqi, H.A.; Qamar, M.F.; Gasser, R.B. Tick-borne diseases of bovines in Pakistan: Major scope for future research and improved control. Parasites Vectors 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Molad, T.; Mazuz, M.; Fleiderovitz, L.; Fish, L.; Savitsky, I.; Krigel, Y.; Leibovitz, B.; Molloy, J.; Jongejan, F.; Shkap, V. Molecular and serological detection of A. centrale-and A. marginale-infected cattle grazing within an endemic area. Vet. Microbiol. 2006, 113, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, A.; Koehler, A.V.; Hall, R.S.; Gauci, C.G.; Gasser, R.B.; Jabbar, A. Targeted next-generation sequencing and informatics as an effective tool to establish the composition of bovine piroplasm populations in endemic regions. Microorganisms 2020, 9, 21. [Google Scholar] [CrossRef]
- Werle, E.; Schneider, C.; Renner, M.; Völker, M.; Fiehn, W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 1994, 22, 4354. [Google Scholar] [CrossRef]
- Edgar, R.C. Muscle5: High-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat. Commun. 2022, 13, 6968. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Proceedings of Nucleic Acids Symposium Series; Oxford University Press: Oxford, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Simonsen, M.; Mailund, T.; Pedersen, C.N. Rapid neighbour-joining. In Proceedings of the Algorithms in Bioinformatics: 8th International Workshop, WABI 2008, Proceedings 8, Karlsruhe, Germany, 15–19 September 2008; pp. 113–122. [Google Scholar]
- Yang, Z. Phylogenetic analysis using parsimony and likelihood methods. J. Mol. Evol. 1996, 42, 294–307. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Martinez, B.A.F.; Leotti, V.B.; Silva, G.d.S.e.; Nunes, L.N.; Machado, G.; Corbellini, L.G. Odds ratio or prevalence ratio? An overview of reported statistical methods and appropriateness of interpretations in cross-sectional studies with dichotomous outcomes in veterinary medicine. Front. Vet. Sci. 2017, 4, 193. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.; Martin, W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; VER Inc.: Charlottetown, PE, Canada, 2010. [Google Scholar]
- Ali, S.; Hasan, M.; Ashraf, K.; Ahmad, A.; Khan, J.; Rashid, M. Molecular prevalence of Anaplasma marginale in ruminants and Rhipicephalus ticks in northern Pakistan. Trop. Biomed. 2023, 40, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Parveen, A.; Muhammad Awais, M.; Gillani, Q.; Aktas, M.; Ozubek, S.; Iqbal, F. A report on molecular detection and phylogenetic evaluation of Anaplasma marginale in ticks and blood samples collected from cattle in district Layyah in Punjab (Pakistan). Curr. Microbiol. 2021, 78, 274–281. [Google Scholar] [CrossRef]
- Asif, M.; Ben Said, M.; Vinueza, R.L.; Leon, R.; Ahmad, N.; Parveen, A.; Khan, A.; Ejaz, A.; Ali, M.; Khan, A.U. Seasonal investigation of Anaplasma marginale infection in Pakistani cattle reveals hematological and biochemical changes, multiple associated risk factors and msp5 gene conservation. Pathogens 2022, 11, 1261. [Google Scholar] [CrossRef]
- Atif, F.A.; Abbas, R.Z.; Mehnaz, S.; Qamar, M.F.; Hussain, K.; Nazir, M.U.; Zaman, M.A.; Khan, A.U.; Said, M.B. First report on molecular surveillance based on duplex detection of Anaplasma marginale and Theileria annulata in dairy cattle from Punjab, Pakistan. Trop. Anim. Health Prod. 2022, 54, 155. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Hussain, A.; Aziz, M.U.; Song, B.; Zeb, J.; Moutailler, S.; Foucault-Simonin, A.; Smith, R.L.; Cabezas-Cruz, A.; George, D. Exploring the coinfection and genetic diversity of multiple tick-borne pathogens in livestock population of Punjab, Pakistan. Transbound. Emerg. Dis. 2024, 2024, 9958535. [Google Scholar] [CrossRef] [PubMed]
- Jamil, S.; Chiou, C.-C.; Muqaddas, H.; Ullah, H.; Asif, M.; Rao, S.; Hussain, H.; Fatima, Q.; Nasreen, N.; Niaz, S. Simultaneous molecular detection of Anaplasma marginale and Theileria annulata in cattle blood samples collected from Pakistan-Afghanistan border region. PLoS ONE 2023, 18, e0288050. [Google Scholar] [CrossRef]
- Rehman, A.; Conraths, F.J.; Sauter-Louis, C.; Krücken, J.; Nijhof, A.M. Epidemiology of tick-borne pathogens in the semi-arid and the arid agro-ecological zones of Punjab province, Pakistan. Transbound. Emerg. Dis. 2019, 66, 526–536. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Bertagnoli, S.; Falchi, A.; Figoni, J.; Fite, J.; Hoch, T.; Quillery, E.; Moutailler, S.; Raffetin, A.; René-Martellet, M. An update of evidence for pathogen transmission by ticks of the genus Hyalomma. Pathogens 2023, 12, 513. [Google Scholar] [CrossRef]
- Ali, S.; Ahmad, A.S.; Ashraf, K.; Khan, J.A.; Rashid, M.I. Insights into the involvement of male Hyalomma anatolicum ticks in transmitting Anaplasma marginale, lumpy skin disease virus and Theileria annulata. Trop. Anim. Health Prod. 2024, 56, 167. [Google Scholar] [CrossRef]
- Ashraf, Q.U.; Khan, A.U.; Khattak, R.M.; Ali, M.; Shaikh, R.S.; Iqbal, F. A report on the high prevalence of Anaplasma sp. in buffaloes from two provinces in Pakistan. Ticks Tick-Borne Dis. 2013, 4, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Obregón, D.; Corona, B.G.; De La Fuente, J.; Cabezas-Cruz, A.; Gonçalves, L.R.; Matos, C.A.; Armas, Y.; Hinojosa, Y.; Alfonso, P.; Oliveira, M.C.; et al. Molecular evidence of the reservoir competence of water buffalo (Bubalus bubalis) for Anaplasma marginale in Cuba. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rajput, Z.; Hu, S.-H.; Arijo, A.; Habib, M.; Khalid, M. Comparative study of Anaplasma parasites in tick carrying buffaloes and cattle. J. Zhejiang Univ.-Sci. B 2005, 6, 1057–1062. [Google Scholar] [CrossRef]
- Silva, J.B.; Cabezas-Cruz, A.; Fonseca, A.H.; Barbosa, J.D.; de la Fuente, J. Infection of water buffalo in Rio de Janeiro Brazil with Anaplasma marginale strains also reported in cattle. Vet. Parasitol. 2014, 205, 730–734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benitez, D.; Cetrá, B.; Florin-Christensen, M. Rhipicephalus (Boophilus) microplus ticks can complete their life cycle on the water buffalo (Bubalus bubalis). J. Buffalo Sci. 2012, 1, 2. [Google Scholar] [CrossRef]
- Jonsson, N.N.; Bock, R.E.; Jorgensen, W.K.; Morton, J.M.; Stear, M.J. Is endemic stability of tick-borne disease in cattle a useful concept? Trends Parasitol. 2012, 28, 85–89. [Google Scholar] [CrossRef]
- Mahoney, D.F. The application of epizootiological principals in the control of babesiosis in cattle. Bull.-Off. Int. Des épizooties 1974, 81, 123–138. [Google Scholar]
- Yang, J.; Han, R.; Liu, Z.; Niu, Q.; Guan, G.; Liu, G.; Luo, J.; Yin, H. Insight into the genetic diversity of Anaplasma marginale in cattle from ten provinces of China. Parasites Vectors 2017, 10, 565. [Google Scholar] [CrossRef]
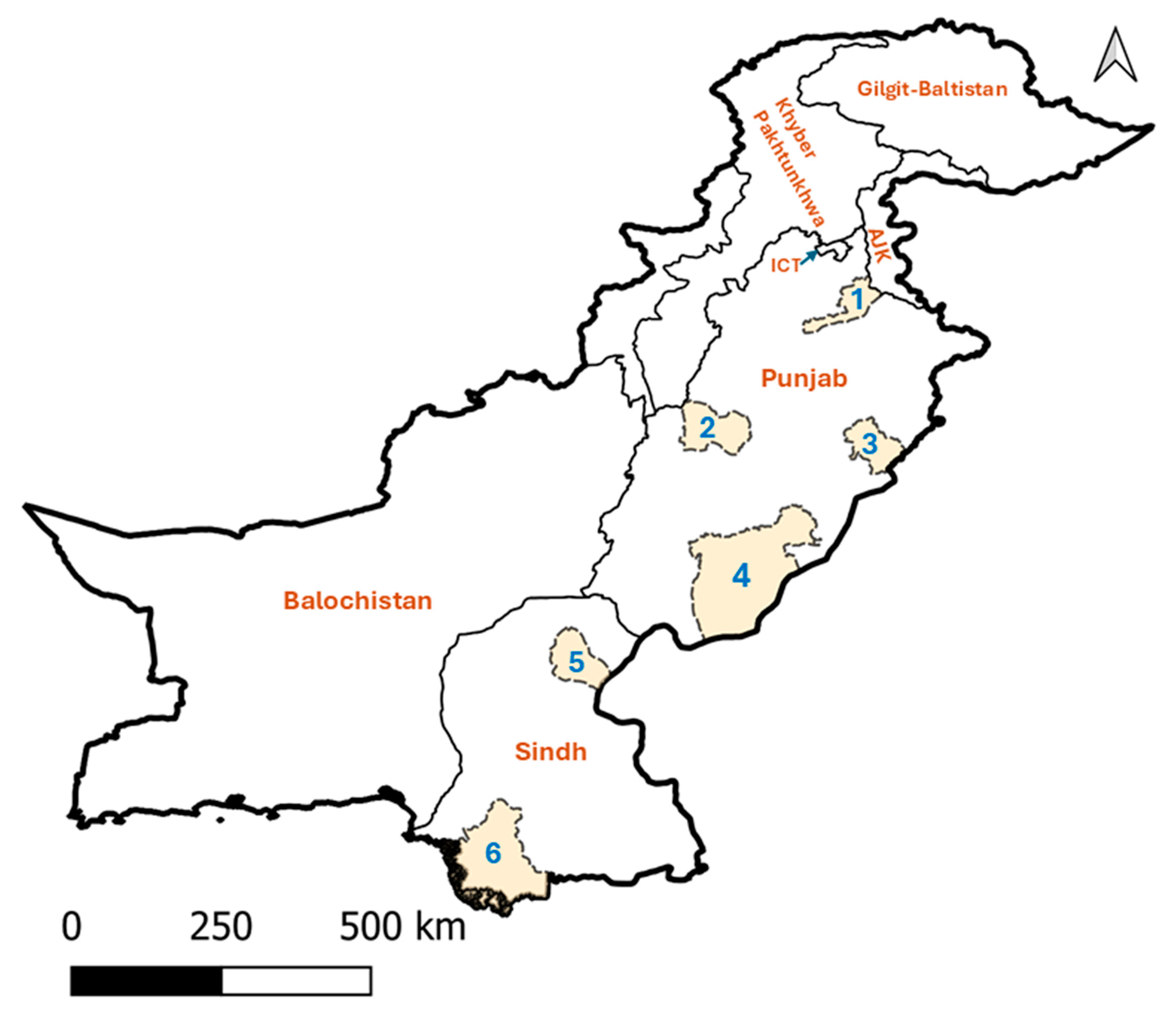
| Province | District | Positive/Tested Herds No. | Positive/Tested Animals No. | |||||
|---|---|---|---|---|---|---|---|---|
| Age Groups | Cattle | Buffalo | ||||||
| Adult | Calf | Female | Male | Female | Male | |||
| Punjab | Bahawalpur | 21/22 | 25/40 | - | 17/21 | 5/5 | 3/11 | 0/3 |
| Jhelum | 7/25 | 10/47 | 0/4 | 10/22 | 0/4 | 0/18 | 0/7 | |
| Layyah | 16/25 | 25/53 | 0/3 | 23/39 | 1/5 | 1/9 | 0/3 | |
| Okara | 19/26 | 36/56 | 2/6 | 21/28 | 7/7 | 9/23 | 1/4 | |
| Sindh | Sukkur | 18/26 | 30/55 | - | 17/19 | 7/8 | 3/19 | 3/9 |
| Thatta | 6/26 | 7/56 | 0/1 | 2/11 | 2/5 | 2/28 | 1/13 | |
| Total | 87/150 | 133/307 | 2/14 | 90/140 | 22/34 | 18/108 | 5/39 | |
| Variable | Category | Animals No. (%) | Prevalence Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Age | Young stock | 14 (4.4) | Referent | |
| Adults | 307 (95.6) | 2.78 (0.79–9.82) | 0.113 | |
| Sex | Male | 73 (22.7) | Referent | |
| Female | 248 (77.3) | 0.95 (0.77–1.17) | 0.635 | |
| Species | Buffaloes | 147 (45.8) | Referent | |
| Cattle | 174 (54.2) | 3.69 (2.23–6.10) | <0.001 | |
| Ticks Recovery | Ticks absent | 201 (62.6) | Referent | |
| Ticks present | 120 (37.4) | 1.39 (1.05–1.84) | 0.021 |
| District | Animals No. (%) | Prevalence Ratio (95% CI) | p-Value |
|---|---|---|---|
| Thatta | 57 (17.8) | Referent | |
| Bahawalpur | 40 (12.5) | 2.78 (1.51–5.11) | 0.001 |
| Jehlum | 51 (15.9) | 1.17 (0.53–2.57) | 0.705 |
| Layyah | 56 (17.5) | 1.93 (0.90–4.16) | 0.093 |
| Okara | 62 (19.3) | 3.08 (1.63–5.82) | 0.001 |
| Sukkur | 55 (17.1) | 3.26 (1.72–6.18) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafar, A.; Shaukat, W.; Waqas, M.; Gauci, C.G.; Gasser, R.B.; Jabbar, A. High Prevalence and Genetic Heterogeneity of Anaplasma marginale in Smallholder Bovine Populations of Pakistan, and Its Implications. Pathogens 2025, 14, 499. https://doi.org/10.3390/pathogens14050499
Ghafar A, Shaukat W, Waqas M, Gauci CG, Gasser RB, Jabbar A. High Prevalence and Genetic Heterogeneity of Anaplasma marginale in Smallholder Bovine Populations of Pakistan, and Its Implications. Pathogens. 2025; 14(5):499. https://doi.org/10.3390/pathogens14050499
Chicago/Turabian StyleGhafar, Abdul, Waseem Shaukat, Muhammad Waqas, Charles G. Gauci, Robin B. Gasser, and Abdul Jabbar. 2025. "High Prevalence and Genetic Heterogeneity of Anaplasma marginale in Smallholder Bovine Populations of Pakistan, and Its Implications" Pathogens 14, no. 5: 499. https://doi.org/10.3390/pathogens14050499
APA StyleGhafar, A., Shaukat, W., Waqas, M., Gauci, C. G., Gasser, R. B., & Jabbar, A. (2025). High Prevalence and Genetic Heterogeneity of Anaplasma marginale in Smallholder Bovine Populations of Pakistan, and Its Implications. Pathogens, 14(5), 499. https://doi.org/10.3390/pathogens14050499







