Improvement of Cryopreservation and Production of Attenuated Babesia Parasites to Prevent Bovine Babesiosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Site of Field Challenge
2.2. Animals
2.3. Culture Media and Supplements
2.4. In Vitro Blood Culture of Parasites
2.5. Cryopreservation
2.6. Recovering Frozen Babesia Bigemina and Babesia bovis from In Vitro Blood Culture
2.7. Antigen Production
2.8. Indirect Fluorescent Antibody Test
2.9. Parasite Detection PCR
2.10. Vaccination and Challenge
2.11. Statistical Analysis
3. Results
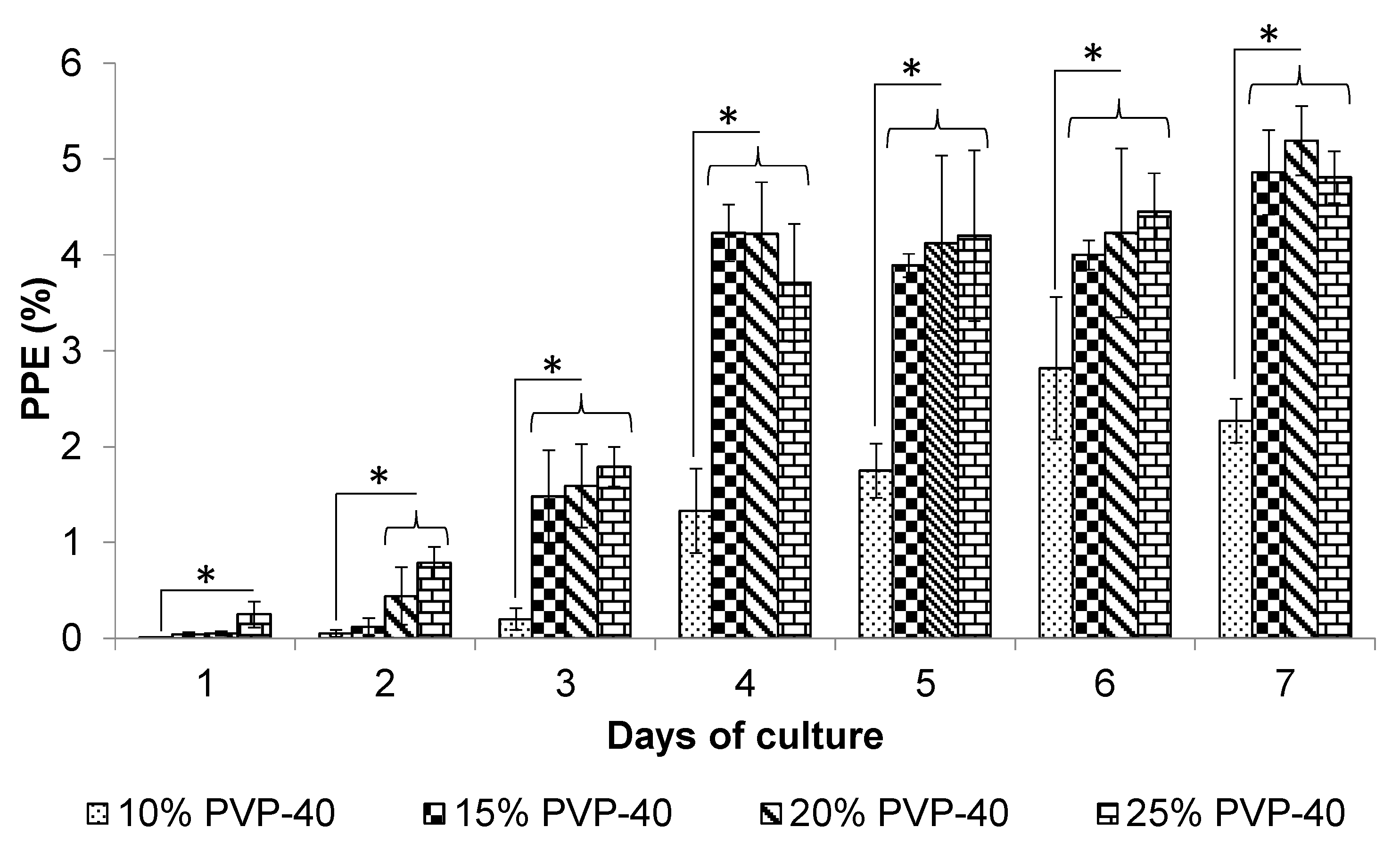
3.1. Effect of PVP-40 Concentration on the Recovery of Parasites
3.2. Prevalence of Babesia Parasites and Tick Infection Rate
3.3. Vaccinated Animals
3.4. Challenged Animals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bock, R.; Jackson, L.; de Vos, A.; Jorgensen, W. Bovine babesiosis. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef]
- Hunfeld, K.P.; Hildebrandt, A.; Gray, J.S. Babesiosis: Recent insights into an ancient disease. Int. J. Parasitol. 2008, 38, 1219–1237. [Google Scholar] [CrossRef]
- Florin-Christensen, M.; Suarez, C.E.; Rodriguez, A.E.; Flores, D.A.; Schnittger, L. Vaccines against bovine babesiosis: Where we are now and possible roads ahead. Parasitology 2014, 141, 1563–1592. [Google Scholar] [CrossRef]
- Grisi, L.; Leite, R.C.; Martins, J.R.; Barros, A.T.; Andreotti, R.; Cançado, P.H.; Pérez de León, A.A.; Pereira, J.B.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 150–156. [Google Scholar] [CrossRef]
- Levy, M.G.; Ristic, M. Babesia bovis: Continuous cultivation in a microaerophilus stationary phase culture. Science 1980, 207, 1218–1220. [Google Scholar] [CrossRef]
- Rojas, M.C.; Rodríguez-Vivas, R.I.; Figueroa, J.V.; Acosta, V.K.Y.; Gutiérrez, R.E.J.; Álvarez, J.A. In vitro culture of Babesia bovis in a bovine serum-free culture medium supplemented with insulin, transferrin, and selenite. Exp. Parasitol. 2016, 170, 214–219. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.I.; Trees, J.A. Utilización del cultivo in vitro de Babesia bovis para evaluar la efectividad de babesicidas. Rev. Biomed. 1994, 5, 133–139. [Google Scholar]
- Rodríguez, S.D.; Buening, G.M.; Green, T.J.; Carson, C.A. Cloning of Babesia bovis by in vitro cultivation. Infect. Immun. 1983, 42, 15–18. [Google Scholar] [CrossRef]
- Rauf, U.; Suleman, M.; Abid, A.; Jamil, H.; Menghwar, H.; Zameer, D.A.; Imran, R.M.; Akbar, H. Humoral and Cell-Mediated Immune Response Validation in Calves after a Live Attenuated Vaccine of Babesia bigemina. Pathogens 2020, 9, 936. [Google Scholar] [CrossRef]
- Mangold, A.J.; Vanzini, V.R.; Echaide, I.E.; de Eschaide, S.T.; Volpogni, M.M.; Guglielmone, A.A. Viability after thawing and dilution of simultaneously cryopreserved vaccinal Babesia bovis and Babesia bigemina strains cultured in vitro. Vet. Parasitol. 1996, 61, 345–348. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.I.; Quiñones-Avila, F.J.; Ramírez-Cruz, G.T.; Cruz, D.; Wagner, G. Aislamiento de una cepa de campo de Babesia bigemina (Piroplasma: Babesiidae) y establecimiento del cultivo in vitro para la producción de antígenos. Rev. Biol. Trop. 2007, 55, 127–133. [Google Scholar] [CrossRef]
- Miyake, Y.; Karanis, P.; Uga, S. Cryopreservation of protozoan parasites. Cryobiology 2004, 48, 1–7. [Google Scholar] [CrossRef]
- Palmer, D.A.; Buening, G.M.; Carson, C.A. Cryopreservation of Babesia bovis for in vitro cultivation. Parasitology 1982, 84, 567–572. [Google Scholar] [CrossRef]
- Schmid, P.; Huvard, J.M.; Lee, S.A.; Byrne, M.K.; Flegel, A.W. Red blood cell preservation by droplet freezing with polyvinilpyrrolidone or sucrose-dextrose and by bulk freezing with glycerol. Transfusion 2011, 51, 2703–2708. [Google Scholar] [CrossRef]
- Vega, C.A.; Buening, G.M.; Rodríguez, S.D.; Carson, C.A.; McLaughlin, K. Cryopreservation of B. bigemina for in vitro cultivation. Am. J. Vet. Res. 1985, 46, 421–423. [Google Scholar] [CrossRef]
- Álvarez, J.A.; Rojas, C.; Figueroa, J.V. An overview of current knowledge on in vitro Babesia cultivation for production of live attenuated vaccines for bovine babesiosis in Mexico. Front. Vet. Sci. 2020, 7, 364. [Google Scholar] [CrossRef]
- Rojas, M.C.; Rodríguez-Vivas, R.I.; Figueroa, J.V.; Acosta, V.K.Y.; Gutiérrez, R.E.J.; Bautista-Garfias, C.R.; Lira-Amaya, J.J.; Polanco-Martínez, D.J.; Álvarez, J.A. Babesia bigemina: Advances in continuous in vitro culture using serum-free medium supplemented with insulin, transferrin, selenite, and putrescine. Parasitol. Int. 2018, 67, 294–301. [Google Scholar] [CrossRef]
- Álvarez, J.A.; Figueroa, J.V.; Ueti, M.W.; Rojas-Martínez, C. Innovative Alternatives for continuous in vitro culture of Babesia bigemina in medium free of components of animal origin. Pathogens 2020, 9, 343. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Lugaresi, C.I.; Volpogni, M.M.; Anziani, O.S.; Vanzini, V.R. Babesial antibody dynamics after cattle immunization with live vaccines, measured with an indirect immunofluorescence test. Vet. Parasitol. 1997, 70, 33–39. [Google Scholar] [CrossRef]
- Figueroa, J.; Chieves, L.; Johnson, G.; Buening, G. Polymerase chain reaction-based assay for the detection of Babesia bigemina, Babesia bovis and Anaplasma marginale DNA in bovine blood. Vet. Parasitol. 1993, 50, 69–81. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Huyen, N.X.; Shinuo, C.; Inpankaew, T.; Maklon, K.; Aboulaila, M.; Ueno, A.; Goo, Y.K.; Yokoyama, N.; Jittapalapong, S.; et al. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet. Parasitol. 2011, 178, 201–207. [Google Scholar] [CrossRef]
- Stevenson, D.J.; Morgan, C.; Goldie, E.; Connel, G.; Grant, M.H. Cryopreservation of viable hepatocyte monolayers in cryoprotectant media with high serum content: Metabolism of testosterone and kaempherol post-cryopreservation. Cryobiology 2004, 49, 97–113. [Google Scholar] [CrossRef]
- Shuttleworth, R.; Higgins, A.Z.; Eroglu, A.; Benson, J.D. Comparison of dilute and nondilute osmotic equilibrium models for erythrocytes. Cryobiology 2022, 109, 72–79. [Google Scholar] [CrossRef]
- Quan, G.; Zhang, L.; Guo, Y.; Liu, M.; Wang, J.; Wang, Y.; Dong, B.; Liu, A.; Zhang, J.; Han, Y. Intracellular sugars improve survival of human red blood cells cryopreserved at -80 degrees C in the presence of polyvinyl pyrrolidone and human serum albumin. CryoLetters 2007, 28, 95–108. [Google Scholar]
- Standfast, N.F.; Jorgensen, W.K. Comparison of the infectivity of Babesia bovis, Babesia bigemina and Anaplasma centrale for cattle after cryopreservation in either dimethylsulphoxide (DMSO) or polyvinylpyrrolidone (PVP). Aust. Vet. J. 1997, 75, 62–63. [Google Scholar] [CrossRef]
- Yu, P.U.; Bobrova, O.M.; Nardid, O.A.; Zubob, P.M.; Kovalenko, I.F.; Kuchkov, V.M.; Vodopianova, L.A. Cryopreservation of equine and bovine erythrocytes using combined protective media. Probl. Cryobiol. Cryomed. 2019, 29, 255–265. [Google Scholar]
- Kusakisako, K.; Masatani, T.; Yada, Y.; Talactac, M.R.; Hernandez, M.P.; Maeda, H.; Mochizuki, M.; Tanaka, T. Improvement of the cryopreservation method for the Babesia gibsoni parasite by using commercial freezing media. Parasitol. Int. 2016, 65, 532–535. [Google Scholar] [CrossRef]
- Hakimi, H.; Asada, M.; Kawazu, S.I. Recent advances in molecular genetic tools for Babesia. Vet. Sci. 2021, 8, 222. [Google Scholar] [CrossRef]
- Bastos, R.G.; Laughery, J.M.; Ozubek, S.; Alzan, H.F.; Taus, N.S.; Ueti, M.W.; Suarez, C.E. Identification of novel immune correlates of protection against acute bovine babesiosis by superinfecting cattle with in vitro culture attenuated and virulent Babesia bovis strains. Front. Immunol. 2022, 13, 1045608. [Google Scholar] [CrossRef]
- Bastos, R.G.; Capelli-Peixoto, J.; Laughery, J.M.; Suarez, C.E.; Ueti, M.W. Vaccination with an in vitro culture attenuated Babesia bovis strain safely protects highly susceptible adult cattle against acute bovine babesiosis. Front. Immunol. 2023, 14, 1219913. [Google Scholar] [CrossRef]
- Oldiges, P.D.; Laughery, M.J.; Tagliari, J.N.; Dabis, C.W.; Knowles, P.D.; da Silva, V.I.; Suarez, E.C. Transfected Babesia bovis Expressing a Tick GSTasaLiveVector Vaccine. PLoS Negl. Trop. Dis. 2016, 10, e0005152. [Google Scholar] [CrossRef] [PubMed]
- Mazuz, M.L.; Laughery, J.M.; Lebovitz, B.; Yasur-Landau, D.; Rot, A.; Bastos, R.G.; Edery, N.; Fleiderovitz, L.; Levi, M.M.; Suarez, C.E. Experimental infection of calves with transfected attenuated Babesia bovis expressing the Rhipicephalus microplus Bm86 antigen and eGFP marker: Preliminary studies towards a dual anti-Tick/Babesia vaccine. Pathogens 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Martínez, C.; Figueroa, M.J.V.; Alvarado, A.; Mejía, P.; Mosqueda, J.J.; Falcon, A.; Vega, M.C.A.; Alvarez, J. Bovine babesiosis live vaccine production: Use of gamma irradiation on the substrate. Ann. N. Y. Acad. Sci. 2006, 1081, 405–416. [Google Scholar] [CrossRef]
- Shkap, V.; de Vos, J.A.; Zweygarth, E.; Jongejan, F. Attenuated vaccines for tropical theileriosis, babesiosis and heartwater: The continuing necessity. Trends Parasitol. 2007, 23, 420–426. [Google Scholar] [CrossRef]
- Chaparro, C.L.; Reyes, C.; Vanessa, D.E.; Moreno, P.D.; Patarroyo, M.A. Developing Anti-Babesia bovis Blood Stage Vaccines: A New Perspective Regarding Synthetic Vaccines. Int. J. Mol. Sci. 2023, 24, 5219. [Google Scholar] [CrossRef]
- Madruga, C.R.; Aycardi, E.; Kessler, R.H.; Moreira, S.M.; Figueiredo, G.R.; Curvo, E.J. Antibody levels anti-Babesia bigemina and Babesia bovis in calves of Nelore and Ibagé breeds and Nelore crosses. Pesq. Agropec. Bras. 1985, 19, 1163–1168. [Google Scholar]
- Hines, A.E.; Palmer, H.G.; Jasmer, P.D.; Goff, L.G.; McElwain, F.T. Immunization of Cattle with Recombinant Babesia bovis Merozoite Surface Antigen-1. Infect. Immun. 1995, 63, 349–352. [Google Scholar] [CrossRef]
- Hidalgo-Ruiz, M.; Suarez, C.E.; Mercado-Uriostegui, M.A.; Hernandez, O.R.; Ramos, J.A.; Galindo, V.E.; Leon, A.G.; Hernandez, J.M.; Mosqueda, J. Babesia bovis RON2 contains conserved B-cell epitopes that induce an invasion-blocking humoral immune response in immunized cattle. Parasit Vectors 2018, 11, 575. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Ratthanophart, J.; Salama, A.; AbouLaila, M.; Asada, M.; Ueno, A.; Alhasan, H.; Guswanto, A.; Masatani, T.; Yokoyama, N.; et al. Molecular Characterization of a New Babesia bovis Thrombospondin-Related Anonymous Protein (BbTRAP2). PLoS ONE 2013, 8, e83305. [Google Scholar] [CrossRef]
| Primer Sequence | Amplicon Size | ||
|---|---|---|---|
| B. bovis | Single | CGAGGAAGGAACTACCGATG | 354 bp |
| GGAGCTTCAACGTACGAGGT | |||
| nPCR | TGGCTACCATGAACTACAAGACTTA | 275 bp | |
| GAGCAGAACCTTCTTCACCAT | |||
| B. bigemina | Single | GAGTCTGCCAAATCCTTAC | 879 bp |
| TCCTCTACAGCTGCTTCG | |||
| nPCR | AGCTTGCTTTCACAACTCGCC | 412 bp | |
| TTGGTGCTTTGACCGACGACAT |
| Days Post-Vaccination | |||||
|---|---|---|---|---|---|
| 8 Days | 17 Days | ||||
| Treatment Group | B. bovis | B. bigemina | B. bovis | B. bigemina | B. bovis and B. bigemina |
| Group I | 4/6 | 3/6 | 5/6 | 5/6 | 4/6 |
| Group II | 3/6 | 1/6 | 3/6 | 3/6 | 3/6 |
| Group III | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Days Post Challenge | |||||
|---|---|---|---|---|---|
| 8 Days | 13 Days | ||||
| Treatment Group | B. bovis | B. bigemina | B. bovis | B. bigemina | B. bovis and B. bigemina |
| Group I | 6/6 | 2/6 | 5/6 | 4/6 | 3/6 |
| Group II | 6/6 | 2/6 | 6/6 | 3/6 | 2/6 |
| Group III | 5/5 | 0/5 | 5/5 | 3/5 | 3/6 |
| Group | |||
|---|---|---|---|
| Parameter | I | II | III |
| Fever day 7 (>40 °C) | 0/6 | 0/6 | 5/5 |
| Reduction hematocrit (%) | 24.1 | 35.5 | 40 |
| Parasitemia | 3/6 | 4/6 | 5/5 |
| Severe disease a | 0/6 | 0/6 | 5/5 |
| Drug treatment | 0/6 | 0/6 | 5/5 |
| Protection (%) b | 100 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Martínez, C.; Lira-Amaya, J.J.; Ueti, M.W.; Castañeda-Arriola, R.O.; Millán, J.V.F.; Martínez, J.A.Á. Improvement of Cryopreservation and Production of Attenuated Babesia Parasites to Prevent Bovine Babesiosis. Pathogens 2025, 14, 498. https://doi.org/10.3390/pathogens14050498
Rojas-Martínez C, Lira-Amaya JJ, Ueti MW, Castañeda-Arriola RO, Millán JVF, Martínez JAÁ. Improvement of Cryopreservation and Production of Attenuated Babesia Parasites to Prevent Bovine Babesiosis. Pathogens. 2025; 14(5):498. https://doi.org/10.3390/pathogens14050498
Chicago/Turabian StyleRojas-Martínez, Carmen, José J. Lira-Amaya, Massaro W. Ueti, Roberto O. Castañeda-Arriola, Julio V. Figueroa Millán, and Jesús A. Álvarez Martínez. 2025. "Improvement of Cryopreservation and Production of Attenuated Babesia Parasites to Prevent Bovine Babesiosis" Pathogens 14, no. 5: 498. https://doi.org/10.3390/pathogens14050498
APA StyleRojas-Martínez, C., Lira-Amaya, J. J., Ueti, M. W., Castañeda-Arriola, R. O., Millán, J. V. F., & Martínez, J. A. Á. (2025). Improvement of Cryopreservation and Production of Attenuated Babesia Parasites to Prevent Bovine Babesiosis. Pathogens, 14(5), 498. https://doi.org/10.3390/pathogens14050498








