Abstract
Microsporidiosis is a zoonotic disease that derives from disparate sources. Most of the microsporidial agents are host-specific but some are capable of interspecies transmission, causing disease in various animals including humans. Human microsporidiosis may be caused by 17 species, with Encephalitozoon cuniculi, E. intestinalis and E. hellem mostly being responsible for human infections worldwide. Wildlife and migratory waterfowl can serve as reservoirs of these human-infectious agents and play a significant role in disseminating these pathogens into the environment. The aim of the study was to detect E. cuniculi, E. intestinalis and E. hellem in wild, migratory greater white-fronted geese (Anser albifrons) and other Anatidae members in feacal samples obtained in north-western Poland, using a molecular method. We collected 189 fecal droppings from Anatidae species (75 samples from greater white-fronted geese and 114 from other Anser spp.) during autumn migration. New species specific primers for PCR amplification were used to amplify a fragment of the small subunit ribosomal (SSU) rRNA of E. cuniculi, E. intestinalis and E. hellem. All fecal droppings were negative for E. intestinalis and E. hellem whereas E cuniculi was detected in 6 of 189 fecal samples (3.2%; 95% CI: 1.3–6.3%). In total, 1 of 75 tested fecal samples of greater white-fronted geese was positive (1.3%; 95% CI: 0.08–5.7%) while 5 of 114 (4.4%; 95% CI: 1.6–9.1%) tested fecal samples without exact species affiliation (only Anser sp.) were also positive. The phylogenetic analysis placed the sequences obtained from the birds’ droppings in the clade E. cuniculi from various rodents, wild carnivores and humans. Our results provide the first description of the occurrence and genotyping of the microsporidian E. cuniculi in greater white-fronted geese and in other members of the Anserinae Subfamily. Our findings support the results of other authors that E. cuniculi may originate from diverse sources, including common waterfowl. Our results are important in a One Health context, as wild migrating waterfowl may disseminate this zoonotic agent in remote regions through their migratory behaviour. These species should be considered significant sources of zoonotic pathogens, potentially hazardous to domestic and farmed animals as well as humans.
1. Introduction
Microsporidians (Microsporidia) are diverse, fungus-related obligate and intracellular pathogens. They have been reported from a wide range of host species belonging to protozoa and cold- and warm-blooded metazoa, including humans [1]. The spores of their infective stages have the ability to survive outside the host cell and have been found world wide in various habitats, including freshwater, brackish water, and marine and terrestrial environments [2]. Most of the 1700 described species are reported to infect only a single host, but those that are generalists can infect a large number of hosts and play a role as zoonotic sources of microsporidiosis [3]. In humans, the infection can be caused by 17 species from 220 genera including Encephalitozoon, Anncaliia, Pleistophora, Trachipleistophora, Vittaforma, Endoreticulatus, Microsporidium and Nosema. These microsporidians can cause a variety of clinical manifestations ranging from gastrointestinal disorders, such as enteritis, to diffuse systemic infections without specific manifestations in immunocompetent and immunocompromised individuals [4].
Currently, opportunistic Encephalitozoon cuniculi, E. intestinalis and E. hellem infections from disparate sources are mainly responsible for human microsporidiosis [5]. Since Encephalitozoon spillover is possible into unrelated and distant hosts, the role of such host is relevant in a One Health context [6,7]. Wild animals are relevant zoonotic pathogens disseminators, that transmit intestinal agents mainly via contaminated water sources to humans [8,9]. Based on recent data, the presence of the zoonotic E. bieneusi, E. hellem and E. cuniculi has been confirmed in many species of domestic, captive and wild populations of waterbirds worldwide [10,11,12]. One of the most important reservoirs for microsporidia is large migratory birds (e.g., the family Anatidae), which can spread infectious spores to remote regions, with Central Europe being a natural stopover habitat [8,13].
Reports of human microsporidiosis in Poland are rare. There are reports presenting microsporidia infections in patients with immunodeficiency. According to polymerase chain reaction (PCR) results, E. bieneusi and E. cuniculi were confirmed in these hospitalized individuals [14]. In other research, two species as E. cuniculi and E. intestinalis were confirmed in three patients using the fluorescence in situ hybridization (FISH) method in Poland [15].
Studies on microsporidiosis in wild animals in Poland are rare. So far, evidence of E. bieneusi has been reported in red foxes (Vulpes vulpes) and the two invasive species raccoon dog (Nyctereutes procyonoides) and raccoon (Procyon lotor) [16,17]. Another study in southwestern Poland confirmed the infection of various wild mouse species with E. bieneusi [18]. Epidemiological surveys on microsporidia also prove infections in various mammals from zoos. Captive animals from zoological gardens were positive for E. bieneusi [19]. Microsporidian DNA has also been confirmed in invertebrate hosts and one of the dominant kinds found in Culicidae hosts was E. hellem [1].
Birds have so far only been the subject of one study on microsporidia in Poland, where E. intestinalis and E. hellem were detected in wild and captive birds [8]. Evidence of E. cuniculi in wild animals, including geese, has so far been lacking in Poland.
Alongside environmental studies, modern surveillance of human-infectious microsporidia includes the examination of various wild and domestic animal species, including migratory waterfowl [20]. In terms of human microsporidia presence in wild birds associated with human-shared environments, wild waterfowl may become an important source of endemic microsporidiosis foci establishment. During migration, birds arrive in the open areas of national parks and stay there for extended periods of time. During this time, they are usually undisturbed by humans (e.g., by visitors, birdwatchers or farmers), which may lead to significant surface pollution where their feces accumulate [13]. The aim of the study was therefore to use molecular methods to detect microsporidia in wild, migratory greater white-fronted geese (Anser albifrons) and other members of the genus Anser in fecal samples obtained in north-western Poland
2. Material and Methods
2.1. Study Area
The Wielkopolski National Park (Polish: Wielkopolski Park Narodowy; WPN) is a lowland nature reserve in western Poland, located approximately 15 km southwest of the city of Poznan (Figure 1). The WPN is surrounded by several large (Luboń, Mosina, Puszczykowo, Stęszew) and many small villages. There are also several small settlements within the park itself. The WPN has a large number of lakes of various sizes as well as some periodic reservoirs [21]. The WPN was founded in 1957 and expanded in 1996 and currently covers an area of 7619.8 ha, whith a buffer zone of 7383.2 ha. The entire area of the WPN is part of the Ostoja Wielkopolska Natura 2000 nature reserve and serves as one of Poland’s most important resting places for migratory geese (>10,000). It is thus a bird sanctuary of international importance [22].
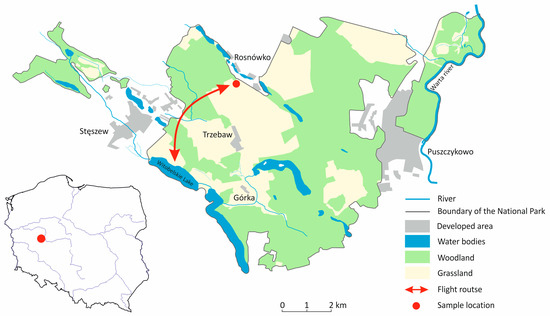
Figure 1.
Map of the Wielkopolska National Park (WNP) and its location in Poland. The map shows the activities of geese between Lake Witobelskie for resting and the agricultural areas for foraging within the WNP.
2.2. Sample Collection
During the 2020–2021 autumn migrations, 189 fresh fecal samples were collected in the WPN, in a field near Rosnówko (16°45′34″ E/52°17′27″ N) in the Greater Poland Voivodeship, Poland (Figure 1). The field was visited four times and fecal samples were collected. The greater white-fronted geese arrive in October and form a monospecific flock until early November. During this period, we visited the field twice to collect 75 (40%) fecal samples of this species. The classification of the samples was easy as only fresh droppings were collected and only this goose species used the field for foraging. The peak of the number of different Anserine species was recorded in December 2020 and January 2021. Besides greater white-fronted geese, the collected flock consisted of greylag geese (Anser anser) and tundra bean geese (Anser serrirostris) (Table 1). During this period, we collected a further 114 (60%) fecal samples. Flock size was estimated visually using binoculars. To avoid possible contamination, only fresh fecal samples were collected immediately after the geese had flown away. Furthermore, care was taken to ensure that no foreign bodies such as soil or plant parts contaminated the droppings samples. Bird activity covered the areas, including Lake Witobelskie and its surroundings as well as remote parts of farmland and farmland within the WNP, to which birds had unrestricted access (Figure 1).

Table 1.
The number of fecal samples collected from observable flocks of Anatidae during the autumn migration period in Wielkopolska National Park (Rosnówko), Poland.
2.3. Molecular Analysis
About 5–10 g of fecal material was collected from the ground, placed into plastic containers and stored at 4 °C in a portable fridge. Total DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, except for an overnight incubation with Proteinase K, and elution in 50 μL of elution buffer. DNA was stored at −20 °C.
We used Oligo software v. 7.60 (DBA Oligo, CA, USA) to design new Encephalitozoon specific PCR primers for the ampification of a small fragment of the small ribosomal subunit (SSU) rRNA of E. cuniculi, E. intestinalis and E. hellem (see Supplementary Materials Figure S1). The first set of primers MicunF (5′-ATAGTGGTCTGCCCCTGTG-3′) and MicunR (5′-GTCTTCGCATTTCACCTCTCG-3′) (amplicon size 439 bp) were designed by using a published GenBank sequences of E. cuniculi (Acc. no. L17072). The next set of primers MiinF (5′-GACGGCTCAGTGATAGTACG-3′) (amplicon size 420 bp) and MiinR (5′-AATCCCCCAAACAAAGACATACA-3′) (amplicon size 458 bp), was designed on the basis of a published GenBank sequences of E. intestinalis (Acc. no. U09929), whereas the pair of MihelF (5′-AGGTAAGTTCTGGGGGTGGT-3′) and MihelR (5′-CAGTCAGGGTCTTCGTATTTC-3′) (458 bp) was established on the basis of a published GenBank sequences of E. hellem (Acc. no. L19070). Polymerase chain reactions were performed in a total volume of 20 μL and included AmpliTaq Gold Fast PCR Master Mix UP, 0.6 μM of each primer and 2 μL of template DNA. Negative controls (master mix without template + water) were used in each reaction. DNA extracted from cultured microsporidian spores of the three commercial lines of microsporidian spores: E. cuniculi (P103C, 1 × 10/6), E. hellem (P103H, 1 × 10/6) and E. intestinalis (P103I, 1 × 10/6) (Waterborne Inc., New Orleans, LA, USA) were used as positive controls.
The specificity of the primers was verified by performing PCR using genomic DNA of the three commercial Encephalitozoon species (P103C E. cuniculi, P103H E. hellem and P103I E. intestinalis), G. duodenalis isolates belonging to assemblage A (Portland-1 isolate), assemblage B (HP-124 isolate) and Cryptosporidium parvum (cow isolate).
The PCR cycling conditions were as follows: 10 min at 94 °C, 45 cycles of 95 °C for 30 s, 56 °C (MicunF/R) or 55 °C (MihelF/R and MiinF/R) for 40 s, and 72 °C for 10 min. Amplicons were electrophoresed on 1% agarose gel and stained with Midori Green (EURx). Positive products were purified using the QIAquickPCR purification Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The amplicons were sequenced using the ABI Prism 3130 XL BigDye v3.1, Terminator Cycle (Applied Biosystems, CA, USA) in both directions with the same set of primers. Trace files were checked and edited using BioEdit version 7.0.5.3. (http://www.mbio.ncsu.edu/BioEdit/bioedit.html (10 May 2024)) software. Contigs were edited and manually assembled in GeneDoc v. 2.7.000 [23] and compared with deposited sequences in GenBank. A phylogenetic tree was constructed by the Maximum Likelihood algorithm and distance-based analyses were conducted using alignments obtained from ClustalW using MEGA version 11 [24]. Bootstrap proportions were calculated by analysis of the 1000 replicates of the phylogenetic tree.
2.4. Statistical Analysis
All principal statistical analyses were conducted following the guidelines outlined by Zar [25]. Prevalence, the percentage interpreted as the probability of finding positive samples in gathered probes and confidence intervals (95% CI) were calculated using R software R v.4.0.2 [26]. The graphics were prepared and provided with information from various publications and finalized with CorelDRAW 2021 (Corel, Ottawa, ON, Canada).
3. Results
Encephalitozoon cuniculi DNA was detected in six fecal samples (n = 189; 3.2%; 95% CI: 1.3–6.3%). One greater white-fronted goose samples was positive (n = 75; 1.3%; 95% CI: 0.08–5.7%), while five samples from the multispecies flock were also positive (n = 114; 4.4%; 95% CI: 1.6–9.1%) (Table 2). All faecal droppings were negative for E. intestinalis and E. hellem. The alignment of the five E. cuniculi sequences (isolates 165, 169, 171, 175, and 177) showed 100% identity with a sequence from a rabbit isolate (Acc. no. L17072). The sequence of E. cuniculi isolate 179 differed at two SNPs (Single Nucleotide Polymorphisms) compared to the same rabbit E. cuniculi isolate. Phylogenetic analysis of the SSU rRNA nucleotide sequences obtained from the microsporidia-positive isolates placed them in a clade alongside sequences obtained from rodents, wild carnivores and humans (Figure 2).

Table 2.
The number of positive samples obtained during autumn migration from monospecies flocks consisting only of geater white-fronted geese (Anser albifrons) and multi-species flocks, which included greylag geese (Anser anser) and tundra bean geese (Anser serrirostris) alongside greater white-fronted geese in Wielkopolska National Park (Rosnówko), Poland.
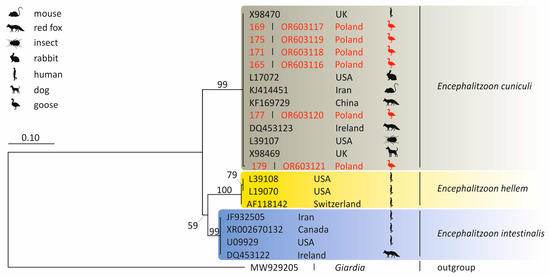
Figure 2.
Phylogenetic tree of the SSU rRNA gene region nucleotide sequences of Encephalitozoon cuniculi isolates obtained in Poland. The tree was constructed by using the Kimura2-parameter model and is in the units of the number of base substitutions per site. The Giardia sequence represents an outgroup.
Correct amplicons were obtained only when primer pairs designed to detect the species-specific DNA of Encephalitozoon species were used. The MicunF/MicunR, MihelF/MihelR and MiinF/MiinR primers were successfully used to amplify the DNA belonging to E. cuniculi (P103C), E. hellem (P103H), and E. intestinalis (P103I) isolates, respectively. The designed primers proved to be specific because they did not cross-amplify with the DNA of G. duodenalis belonging to assemblages A and B and C. parvum.
The sequences from this study have been submitted to GenBank under unique accession numbers: isolate 165 (Acc. no. OR603116), isolate 169 (Acc. no. OR603117), isolate 171 (Acc. no. OR603118), isolate 175 (Acc. no. OR603119), isolate 177 (Acc. no. OR603120) and isolate 179 (Acc. no. OR603121).
4. Discussion
To date, in addition to the main host of E. cuniculi (rabbit, Oryctolagus cuniculus), the presence of these microsporidia has been successfully demonstrated in many other reservoirs of rodents, birds, domestic, farm and wild animals, including humans, worldwide [27,28,29,30,31,32,33,34,35]. As the transmission of E. cuniculi to unrelated and distant hosts is possible, the role of such hosts is discussed in the context of One Health [6,7]. Wildlife, including waterfowl, are important spreaders of zoonotic agents that transmit enteric pathogens to humans mainly via contaminated water sources [8,9].
Currently, molecular approaches are the most efficient methods for detecting microsporidia in various samples. Like many other studies, we relied on molecular assays to detect and identify Encephalitozoon species [33,36,37,38]. Using species-specific primers for conventional PCR, this study demonstrated that greater white-fronted geese and other anserine species in Poland are infected with E. cuniculi. By targeting a fragment of the small subunit ribosomal (SSU) rDNA, we detected E. cuniculi DNA in a sample deposited by greater white-fronted geese on the ground during migration. This sampling approach allowed us to confidently attribute the detected DNA to greater white-fronted geese, as these birds formed flocks exclusively with members of their species before other Anserinae species appeared in the field. We also detected the genetic material of E. cuniculi in five other fecal samples, but could not associate the pathogen with a specific host species as the monitored waterfowl colony consisted of greylag geese, tundra bean geese and greater white-fronted geese.
The detection of E. cuniculi in any member of the Anserinae would be a first. Previously, E. cuniculi DNA and spores were detected in wild white storks (Ciconia ciconia), great cormorants (Phalacrocorax carbo) and great crested grebe (Podicepes cristatus) in Slovakia [39]. In Poland, only one species of domestic waterfowl, Anser anser domestica, has been reported as positive for E. intestinalis [40]. To our knowledge, the greater white-fronted goose is a newly identified host for E. cuniculi. The overall infection rate of E. cuniculi in the three waterfowl species in Slovakia was higher (40.4%) compared to our results (3.2%), which might be due to the higher sensitivity of the real-time PCR used by Malčeková et al. [39]. In addition, the broad host range of E. cuniculi makes the pathogen the most common microsporidian species in Slovakia, where it was detected in both animals and humans [29,41]. However, our results contrast with those of a study in Poland on free-ranging and domestic geese, in which E. hellem was primarily detected in waterfowl such as mallards (Anas platyrhynchos), greylag geese and mute swans (Cygnus olor) [8,40].
Encephalitozoon cuniculi has been classified into four genotypes (I–IV), identifiable by 5′-GTTT-3′ repeats within the internal transcribed spacer (ITS) molecular marker. Despite this genetic diversity, E. cuniculi exhibits low host specificity, underscoring its zoonotic potential [2,42,43,44]. Our phylogenetic analysis suggests that migrating geese may harbor and disseminate zoonotic microsporidia through their feces.
Encephalitozoon cuniculi spores can survive for up to six weeks or longer after being excreted into the environment, particularly under humid conditions and suitable temperatures [45]. The natural transmission route of E. cuniculi between individuals involves the ingestion of infective stages present in the environment, shed in feces—even from asymptomatic hosts [38,46,47,48]. Among Encephalitozoon species, E. cuniculi is notably prevalent in wild rodents, particularly within the murine and arvicoline subfamilies. In Japan, infection rates have reached 32% in Japanese field mice (Apodemus speciosus), 18.9% in Japanese grass mice [Microtus montebelli (=Alexandromys montebelli)], and 12.5% in field mice (Apodemus argenteus) [11]. Similar infection rates have been found in wild mice across three European countries, with the highest rates observed in yellow-necked mice (Apodemus flavicollis) at 21% and striped field mice (Apodemus agrarius) at 23.6%—in environments shared by humans, wild rodents, and waterfowl, including cities, reserves, and recreational areas [12,17,38,47].
Microsporidia excreted in feces can potentially be transmitted to new hosts. The likelihood of infection may be higher in areas where individuals use or share water resources. Lakes are commonly used by geese as resting places. They feed on land during the day and then return in flocks to rest on the water, often away from the shore, at night. One possible transmission route for microsporidia is water. Some studies have reported the presence of E. bieneusi and E. intestinalis in surface water and groundwater, respectively [49,50]. Although no reports of E. cuniculi in water bodies exist worldwide, our results suggest that waterfowl may contribute to water contamination, particularly during migration when they sometimes form large flocks. All goose species examined in our study utilized a shared water source, Lake Witobelskie, as a resting place.
As geese migrate in large flocks, their presence during the migration period can have a regional impact on the local environment. They leave large amounts of feces in the water and soil habitats they use [51], increasing the likelihood of fecal contamination in water bodies and fields during their migration [39,40,52]. Studies on the migration patterns of the Anser species we investigated show that the greylag goose is now primarily a short-distance migrant, and in some regions, a resident bird within Europe [53]. For the greylag goose, breeding and wintering areas often overlap under favorable climatic conditions. In contrast, the greater white-fronted goose and tundra bean goose are long-distance migrants, with their breeding areas in the Arctic clearly separated from their wintering grounds in Central Europe [54,55,56,57]. Based on findings from ringing and satellite telemetry studies, we have created a migration map for the greater white-fronted goose and the tundra bean goose (Figure 3) to illustrate how E. cuniculi could spread [54,57]. These migration routes of these two species once again illustrate the large catchment area during the migration period in fall and spring and the associated distribution of infected feces.
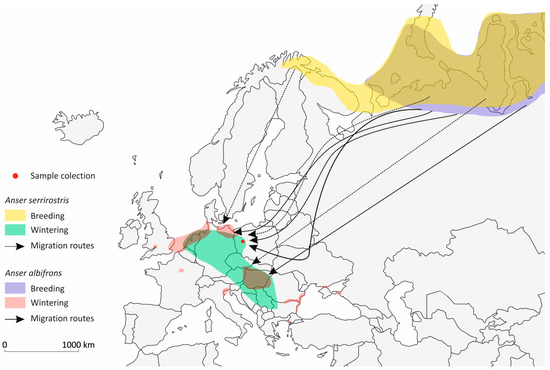
Figure 3.
Breeding and wintering areas of tundra bean goose (Anser serrirostris) and greater white-fronted goose (Anaser albifrons) including their main migration routes with in Europe (simplified after Kruckenberg et al. [54], Fox et al. [57]).
Interestingly, the agricultural land management in the study area within the WNP has remained unchanged in recent years, providing a favorable environment for migrating geese to utilize foraging habitats, which in turn has a significant impact on local ecosystems [58]. The presence of E. cuniculi DNA in goose fecal droppings found in arable fields used for crop production suggests a potential risk of infection for farm animals that are traditionally fed raw agricultural products. All goose species included in the study use remote areas for feeding during migration, so, aside from professional workers, there is a low probability of human infection through contact with bird feces in the national park. However, shared spaces, such as city parks or other suburban environments frequented by migrant birds, may pose a more obvious link to human health risks [9]. Although direct E. cuniculi transmission to humans from wild geese has yet to be proven, the main exposure of this micropathogen is via an indirect route through contaminated water during migration time. Lakes and ponds in sub-urban areas or urban public parks are the most direct links to the risk to human health.
5. Conclusions
Microsporidia are still an enigmatic and understudied group of eukaryotic pathogens with a global distribution. They appear to be more prevalent in free-ranging animals than previously thought. Although E. cuniculi infections can cause a range of systemic symptoms, particularly in immunocompromised individuals, this Encephalitozoon species should be considered a public health concern due to its significant adaptability to various animal hosts, which can spread it across broad geographical areas [59,60,61]. We designed new sets of primers to detect three Encephalitozoon species using PCR. The sets of primer pairs used in this study did not match the SSUr DNAsequence of isolates belonging to gastrointestinal protozoa from Giardia and Cryptosporidium. In conclusion, the use of Encephalitozoon species-specific primers with further sequencing of the amplification products designed in this study allows the quick and reliable identification of human-infectious microsporidia in goose droppings. This action allows us to detect three microsporidian species that differ in their epidemiology. Our findings support those of other researchers, confirming that E. cuniculi, with its broad host specificity, may originate from diverse sources, including common waterfowl. Given the zoonotic risk for individuals who may come into direct contact with birds (e.g., ornithologists, animal keepers, or farmers), personal hygiene practices should be emphasized. Our study fills a gap in the data by evaluating E. cuniculi in wild birds, which may disseminate this zoonotic agent to remote regions through their migratory behavior. In our opinion, targeted research is needed to determine the risk of environmental contamination by wild geese with E. cuniculi during migration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14050489/s1, Figure S1: Complete sequences of small subunit ribosomal RNA gene derived from E. hellem, E. intestinalis and E. cuniculi deposited in GenBank.
Author Contributions
P.S. conceptualized the project, collected the samples and wrote the manuscript; P.S., A.P.-M. and A.W.-G. carried out the research; A.P.-M. and M.H. created the graphics and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors also wish to thank Kamil Karaśkiewicz from the Wielkopolska National Park (WNP) for his excellent ornithological assistance and knowledge.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Trzebny, A.; Mizera, J.; Dabert, M. Microsporidians (Microsporidia) parasitic on mosquitoes (Culicidae) in central Europe are often multi-host species. J. Invertebr. Pathol. 2023, 197, 107873. [Google Scholar] [CrossRef] [PubMed]
- Didier, E.S.; Weiss, L.M. Microsporidiosis: Not just in AIDS patients. Curr. Opin. Infect. Dis. 2011, 24, 490–495. [Google Scholar] [CrossRef]
- Murareanu, B.M.; Sukhdeo, R.; Qu, R.; Jiang, J.; Reinke, A.W. Generation of a Microsporidia Species Attribute Database and Analysis of the Extensive Ecological and Phenotypic Diversity of Microsporidia. mBio 2021, 12, e0149021. [Google Scholar] [CrossRef] [PubMed]
- Anane, S.; Attouchi, H. Microsporidiosis: Epidemiology, clinical data and therapy. Gastroenterol. Clin. Biol. 2010, 34, 450–564. [Google Scholar] [CrossRef]
- Han, B.; Pan, G.; Weiss, L.M. Microsporidiosis in Humans. Clin. Microbiol. Rev. 2021, 34, e0001020. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Mathis, A.; Weber, R. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 2000, 6, 236–260. [Google Scholar] [CrossRef]
- Magalhães, T.R.; Pinto, F.F.; Queiroga, F.L. A multidisciplinary review about Encephalitozoon cuniculi in a One Health perspective. Parasitol. Res. 2022, 121, 2463–2479. [Google Scholar] [CrossRef]
- Solarczyk, P.; Wojtkowiak-Giera, A.; Heddergott, M. Migrating Anatidae as Sources of Environmental Contamination with Zoonotic Giardia, Cryptosporidium, Cyclospora and Microsporidia. Pathogens 2023, 12, 487. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Zhang, Y.; Wang, K.; Gazizova, A.; Wang, L.; Cao, L.; Zhang, Y.; Huang, J.; Cui, Y.; et al. First detection of Enterocytozoon bieneusi in whooper swans (Cygnus cygnus) in China. Parasit. Vectors 2020, 13, 5. [Google Scholar] [CrossRef]
- Tsukada, R.; Tsuchiyama, A.; Sasaki, M.; Park, C.H.; Fujii, Y.; Takesue, M.; Hatai, H.; Kudo, N.; Ikadai, H. Encephalitozoon infections in Rodentia and Soricomorpha in Japan. Vet. Parasitol. 2013, 198, 193–196. [Google Scholar] [CrossRef]
- Fuehrer, H.P.; Blöschl, I.; Siehs, C.; Hassl, A. Detection of Toxoplasma gondii, Neospora caninum, and Encephalitozoon cuniculi in the brains of common voles (Microtus arvalis) and water voles (Arvicola terrestris) by gene amplification techniques in western Austria (Vorarlberg). Parasitol. Res. 2010, 107, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Danišová, O.; Valenčáková, A.; Stanko, M.; Luptáková, L.; Hasajová, A. First report of Enterocytozoon bieneusi and Encephalitozoon intestinalis infection of wild mice in Slovakia. Ann. Agric. Environ. Med. 2015, 22, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Wuczyński, A.; Smyk, B.; Kołodziejczyk, P.; Lenkiewicz, W.; Orłowski, G.; Pola, A. Long-term changes in numbers of geese stopping over and wintering in south-western Poland. Cent. Eur. J. Biol. 2012, 7, 495–506. [Google Scholar] [CrossRef]
- Bednarska, M.; Bajer, A.; Graczyk, T.K.; Siński, E. Opportunistic parasites in immunocompetent and immunodeficient patients with diarrhea. In Proceedings of the 3rd International Giardia and Cryptosporidium Conference, Orvieto, Italy, 11–15 October 2009; Abstract P109. Local Organising Committee, Istituto Superiore di Sanita: Rome, Italy, 2009. [Google Scholar]
- Kicia, M.; Szydłowicz, M.; Cebulski, K.; Jakuszko, K.; Piesiak, P.; Kowal, A.; Sak, B.; Krajewska, M.; Hendrich, A.B.; Kváč, M.; et al. Symptomatic respiratory Encephalitozoon cuniculi infection in renal transplant recipients. Int. J. Infect. Dis. 2019, 79, 21–25. [Google Scholar] [CrossRef]
- Leśniańska, K.; Perec-Matysiak, A.; Hildebrand, J.; Buńkowska-Gawlik, K.; Piróg, A.; Popiołek, M. Cryptosporidium spp. and Enterocytozoon bieneusi in introduced raccoons (Procyon lotor)—First evidence from Poland and Germany. Parasitol. Res. 2016, 115, 4535–4541. [Google Scholar] [CrossRef]
- Perec-Matysiak, A.; Leśniańska, K.; Buńkowska-Gawlik, K.; Merta, D.; Popiołek, M.; Hildebrand, J. Zoonotic Genotypes of Enterocytozoon bieneusi in Wild Living Invasive and Native Carnivores in Poland. Pathogens 2021, 10, 1478. [Google Scholar] [CrossRef]
- Perec-Matysiak, A.; Buńkowska-Gawlik, K.; Kváč, M.; Sak, B.; Hildebrand, J.; Leśniańska, K. Diversity of Enterocytozoon bieneusi genotypes among small rodents in southwestern Poland. Vet. Parasitol. 2015, 214, 242–246. [Google Scholar] [CrossRef]
- Słodkowicz-Kowalska, A.; Graczyk, T.K.; Tamang, L.; Girouard, A.S. Asymptomatic Enterocytozoon bieneusi microsporidiosis in captive mammals. Parasitol. Res. 2007, 100, 505–509. [Google Scholar] [CrossRef]
- Ruan, Y.; Xu, X.; He, Q.; Li, L.; Guo, J.; Bao, J.; Pan, G.; Li, T.; Zhou, Z. The largest meta-analysis on the global prevalence of microsporidia in mammals, avian and water provides insights into the epidemic features of these ubiquitous pathogens. Parasit. Vectors 2021, 14, 186. [Google Scholar] [CrossRef]
- Solon, J.; Borzyszkowski, J.; Bidłasik, M.; Richling, A.; Badora, K.; Balon, J.; Brzezińska-Wójcik, T.; Chabudziński, Ł.; Dobrowolski, R.; Grzegorczyk, I.; et al. Physico-geographical mesoregions of Poland: Verification and adjustment of boundaries on the basis of contemporary spatial data. Geogr. Pol. 2018, 91, 143–170. [Google Scholar] [CrossRef]
- Ławicki, Ł.; Wylegała, P.; Wuczyński, A.; Smyk, B.; Lenkiewicz, W.; Polakowski, M.; Kruszyk, R.; Rubacha, S.; Janiszewski, T. Distribution, characteristics and conservation status of geese roosts in Poland. Ornis Pol. 2012, 53, 23–38. [Google Scholar]
- Nicholas, K.B.; Nicholas, H.B. GeneDoc: A tool for editing and annotating multiple sequence alignments. Comput. Sci. Biol. 2007, 8, 38. [Google Scholar]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 2010; ISBN 978-0-13-100846-5. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 2 February 2023).
- Visvesvara, G.S.; Leitch, G.J.; da Silva, A.J.; Croppo, G.P.; Moura, H.; Wallace, S.; Slemenda, S.B.; Schwartz, D.A.; Moss, D.; Bryan, R.T.; et al. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J. Clin. Microbiol. 1994, 32, 2760–2776. [Google Scholar] [CrossRef]
- Kasicková, D.; Sak, B.; Kvác, M.; Ditrich, O. Detection of Encephalitozoon cuniculi in a new host--cockateel (Nymphicus hollandicus) using molecular methods. Parasitol. Res. 2007, 101, 1685–1688. [Google Scholar] [CrossRef]
- Malčekova, B.; Valencakova, A.; Luptakova, L.; Molnar, L.; Ravaszova, P.; Novotny, F. First detection and genotyping of Encephalitozoon cuniculi in a new host species, gyrfalcon (Falco rusticolus). Parasitol. Res. 2011, 108, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zheng, J.; He, X.; Jia, H.; Zhang, Y. First characterization in China of Encephalitozoon cuniculi in the blue fox (Alopex lagopus). J. Eukaryot. Microbiol. 2014, 61, 580–585. [Google Scholar] [CrossRef]
- Reetz, J.; Nöckler, K.; Reckinger, S.; Vargas, M.M.; Weiske, W.; Broglia, A. Identification of Encephalitozoon cuniculi genotype III and two novel genotypes of Enterocytozoon bieneusi in swine. Parasitol. Int. 2009, 58, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wagnerová, P.; Sak, B.; Květoňová, D.; Buňatová, Z.; Civišová, H.; Maršálek, M.; Kváč, M. Enterocytozoon bieneusi and Encephalitozoon cuniculi in horses kept under different management systems in the Czech Republic. Vet. Parasitol. 2012, 190, 573–757. [Google Scholar] [CrossRef]
- Marková, J.; Machačová, T.; Bártová, E.; Sedlák, K.; Budíková, M.; Silvestre, P.; Laricchiuta, P.; Russo, M.; Veneziano, V. Toxoplasma gondii, Neospora caninum and Encephalitozoon cuniculi in Animals from Captivity (Zoo and Circus Animals). J. Eukaryot. Microbiol. 2019, 66, 442–446. [Google Scholar] [CrossRef]
- Doboși, A.A.; Bel, L.V.; Paștiu, A.I.; Pusta, D.L. A Review of Encephalitozoon cuniculi in Domestic Rabbits (Oryctolagus cuniculus)—Biology, Clinical Signs, Diagnostic Techniques, Treatment, and Prevention. Pathogens 2022, 11, 1486. [Google Scholar] [CrossRef] [PubMed]
- Kicia, M.; Zajączkowska, Ż.; Kváč, M.; Cebulski, K.; Holubová, N.; Wencel, P.; Mayer, L.; Wesołowska, M.; Sak, B. Encephalitozoon cuniculi and Extraintestinal Microsporidiosis in Bird Owners. Emerg. Infect. Dis. 2022, 28, 705–708. [Google Scholar] [CrossRef]
- Thurston-Enriquez, J.A.; Watt, P.; Dowd, S.E.; Enriquez, R.; Pepper, I.L.; Gerba, C.P. Detection of protozoan parasites and microsporidia in irrigation waters used for crop production. J. Food. Prot. 2002, 65, 378–382. [Google Scholar] [CrossRef]
- Sak, B.; Vecková, T.; Brdíčková, K.; Smetana, P.; Hlásková, L.; Kicia, M.; Holubová, N.; McEvoy, J.; Kváč, M. Experimental Encephalitozoon cuniculi Infection Acquired from Fermented Meat Products. Foodborne Pathog. Dis. 2019, 16, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Sak, B.; Kvác, M.; Petrzelková, K.; Kvetonová, D.; Pomajbíková, K.; Mulama, M.; Kiyang, J.; Modrý, D. Diversity of microsporidia (Fungi: Microsporidia) among captive great apes in European zoos and African sanctuaries: Evidence for zoonotic transmission? Folia Parasitol. 2011, 58, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Malčeková, B.; Valenčáková, A.; Molnár, L.; Kočišová, A. First detection and genotyping of human-associated microsporidia in wild waterfowl of Slovakia. Acta Parasitol. 2013, 58, 13–17. [Google Scholar] [CrossRef]
- Slodkowicz-Kowalska, A.; Graczyk, T.K.; Tamang, L.; Jedrzejewski, S.; Nowosad, A.; Zduniak, P.; Solarczyk, P.; Girouard, A.S.; Majewska, A.C. Microsporidian species known to infect humans are present in aquatic birds: Implications for transmission via water? Appl. Environ. Microbiol. 2006, 72, 4540–4544. [Google Scholar] [CrossRef]
- Malčeková, B.; Halánová, M.; Sulínová, Z.; Molnár, L.; Ravaszová, P.; Adam, J.; Halán, M.; Valocký, I.; Baranovič, M. Seroprevalence of antibodies to Encephalitozoon cuniculi and Encephalitozoon intestinalis in humans and animals. Res. Vet. Sci. 2010, 89, 358–361. [Google Scholar] [CrossRef]
- Didier, E.S. Microsporidiosis: An emerging and opportunistic infection in humans and animals. Acta Trop. 2005, 94, 61–76. [Google Scholar] [CrossRef]
- Mathis, A.; Weber, R.; Deplazes, P. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 2005, 18, 423–445. [Google Scholar] [CrossRef]
- Sokolova, O.I.; Demyanov, A.V.; Bowers, L.C.; Didier, E.S.; Yakovlev, A.V.; Skarlato, S.O.; Sokolova, Y.Y. Emerging microsporidian infections in Russian HIV-infected patients. J. Clin. Microbiol. 2011, 49, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Palmer, R.; Trout, J.M.; Fayer, R. Infectivity of microsporidia spores stored in water at environmental temperatures. J. Parasitol. 2003, 89, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.L.; Cleaveland, S.C.; Brown, J.; Mahajan, A.; Shaw, D.J. Seroprevalence of Encephalitozoon cuniculi in wild rodents, foxes and domestic cats in three sites in the United Kingdom. Transbound. Emerg. Dis. 2015, 62, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Perec-Matysiak, A.; Leśniańska, K.; Buńkowska-Gawlik, K.; Čondlová, Š.; Sak, B.; Kváč, M.; Rajský, D.; Hildebrand, J. The opportunistic pathogen Encephalitozoon cuniculi in wild living Murinae and Arvicolinae in Central Europe. Eur. J. Protistol. 2019, 69, 14–19. [Google Scholar] [CrossRef]
- Özkan, Ö.; Yücesan, B.; Babür, C.; Orkun, Ö. Seroprevalence of Encephalitozoon cuniculi, Francisella tularensis and Toxoplasma gondii in Zoonotic Diseases in European Hares (Lepus europaesus). Turkiye Parazitol. Derg. 2021, 45, 171–175. [Google Scholar] [CrossRef]
- Dowd, S.E.; Gerba, C.P.; Pepper, I.L. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl. Environ. Microbiol. 1998, 64, 3332–3335. [Google Scholar] [CrossRef]
- Fournier, S.; Liguory, O.; Santillana-Hayat, M.; Guillot, E.; Sarfati, C.; Dumoutier, N.; Molina, J.; Derouin, F. Detection of microsporidia in surface water: A one-year follow-up study. FEMS Immunol. Med. Microbiol. 2000, 29, 95–100. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Fayer, R.; Trout, J.M.; Lewis, E.J.; Farley, C.A.; Sulaiman, I.; Lal, A.A. Giardia sp. cysts and infectious Cryptosporidium parvum oocysts in the feces of migratory Canada geese (Branta canadensis). Appl. Environ. Microbiol. 1998, 64, 2736–2738. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Majewska, A.C.; Schwab, K.J. The role of birds in dissemination of human waterborne enteropathogens. Trends Parasitol. 2008, 24, 55–59. [Google Scholar] [CrossRef]
- Månsson, J.; Liljebäck, N.; Nilsson, L.; Olsson, C.; Kruckenberg, H.; Elmberg, J. Migration patterns of Swedish Greylag geese Anser anser—Implications for flyway management in a changing world. Eur. J. Wildl. Res. 2022, 68, 15. [Google Scholar] [CrossRef]
- Kruckenberg, H. Migratory behaviour of marked Greater White-fronted Geese Anser albifrons in western Europea—First results and outlook to future research. Charadius 2007, 43, 189–195. [Google Scholar]
- van Wijk, R.E.; Kölzsch, A.; Kruckenberg, H.; Ebbinge, B.S.; Müskens, G.J.D.M.; Nolet, B.A. Individually Tracked Geese Follow Peaks of Temperature Acceleration during Spring Migration. Oikos 2012, 121, 655–664. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Fox, A.D.; Fuller, R.; Griffin, L.; Mitchell, C.; Zhao, Y.; Moon, O.K.; Cabot, D.; Xu, Z.; et al. Stochastic simulations reveal few green wave surfing populations among spring migrating herbivorous waterfowl. Nat. Commun. 2019, 10, 2187. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.D.; Frederiksen, M.; Heinicke, T.; Clausen, K.K.; van der Jeugd, H.P. Annual survival estimates of Taiga Anser fabalis and Tundra Bean Geese A. serrirostris wintering in The Netherlands, 1967–1987. J. Ornithol. 2021, 162, 925–929. [Google Scholar] [CrossRef]
- Polakowski, M.; Broniszewska, M.; Kasprzykowski, Z. Differences in the time budget of the Greater White-fronted Goose (Anser albifrons) in grasslands and arable fields at an important spring stopover site in central Europe. Eur. Zool. J. 2021, 88, 549–555. [Google Scholar] [CrossRef]
- Abu-Akkada, S.S.; El Kerdany, E.D.; Mady, R.F.; Diab, R.G.; Khedr, G.A.; Ashmawy, K.I.; Lotfy, W.M. Encephalitozoon cuniculi infection among immunocompromised and immunocompetent humans in Egypt. Iran J. Parasitol. 2015, 10, 561–570. [Google Scholar]
- Brdíčková, K.; Sak, B.; Holubová, N.; Květoňová, D.; Hlásková, L.; Kicia, M.; Kopacz, Ż.; Kváč, M. Encephalitozoon cuniculi Genotype II Concentrates in Inflammation Foci. J. Inflamm. Res. 2020, 13, 583–593. [Google Scholar] [CrossRef]
- Ulusan Bagci, O.; Muftuoglu, C.; Guldaval, F.; Serce Unat, D.; Mert, U.; Polat, G.; Toz, S.O.; Moon, M.H.; Caner, A. Molecular Prevalence of Microsporidia Infection in Patients with Lung Cancer. Am. J. Trop. Med. Hyg. 2023, 108, 895–900. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).