Abstract
Fusarium head blight (FHB) is an important disease throughout the world due to its strong association with yield reduction, quality deterioration, and mycotoxin contamination in wheat. The use of FHB-resistant genotypes in wheat production can significantly reduce damage. The current study screened a panel of bread wheat from CIMMYT and South Asian countries for FHB resistance to identify promising genotypes useful for wheat breeding and to map the associated genomic regions and linked molecular markers through a genome-wide association study (GWAS). Spray-inoculated field experiments were conducted at CIMMYT, Mexico, over three years, and a wide range of phenotypic variations was observed. Four lines, CIM-39, CIM-29, CIM-9, and CIM-3, exhibited consistent resistance across experiments, with FHB indices ranging from 6.5 to 8.1. Genotyping was conducted using the Illumina Infinium 15 K Bead Chip, and 11,184 high-quality SNP markers were obtained and used for GWAS. Nineteen significant marker-trait associations (MTAs) were detected, among which MTAs at Ra_c58315_265 on 1A and Tdurum_contig102328_129 and Ku_c20136_198 on 7B showed reproducible results, with phenotypic effects on FHB resistance of 6.05%, 3.54%, and 3.92%, respectively. Several genes associated with disease resistance were found near the significant SNPs. The identified resistant genotypes and markers may be useful in future marker-assisted breeding in wheat.
1. Introduction
Wheat (Triticum aestivum L.) is a staple food crop for 35% of the global population, with an annual production of 783.43 million tonnes in 2023–2024 [1]. By 2050, with a projected global population of 9 billion, there is an urgent need to increase wheat production to meet the growing wheat demand [2]. Severe disease epidemics under climate change scenarios pose a high risk to wheat production [3,4]. Fusarium head blight (FHB), caused by Fusarium spp., is a fungal disease that hinders wheat production in warm and humid regions worldwide. In the 1990s, it led to significant outbreaks in the U.S. Midwest, and Canada, resulting in substantial financial losses due to reduced wheat yields amounting to billions of dollars [5,6]. FHB is the second most devastating disease affecting wheat production in the U.S. Midwest, and Canada [7]. Notable outbreaks have been reported in most wheat-producing countries, including the top producers like China, France, India, Russia, and the United States, which collectively contribute to 50% of global production [7]. Fusarium head blight produces a range of mycotoxins, especially deoxynivalenol (DON), and ingestion of mycotoxin-contaminated grains poses a threat to global food and feed safety, as it can lead to symptoms such as nausea, vomiting, diarrhoea, weight loss, and gastrointestinal abnormalities in both humans and livestock. The U.S. Food and Drug Administration sets guidelines on the maximum amounts of DON allowed in end-use products, and grains with high DON are often associated with significant price reductions and substantial economic damages [6].
FHB management requires an integrated approach, including host resistance, fungicide application, and agronomic practices [8]. Host resistance is widely accepted as a sustainable, economically efficient, and ecologically benign strategy for managing this disease. Resistance to FHB is inherited quantitatively and can be categorised into five distinct types, as described by Mesterházy (1997) and Mesterházy et al. (1999) [9,10]. Extensive research has been conducted on two types of resistance: type I, which confers resistance to initial infection based on FHB incidence and type II, which influences disease spread within spike tissues based on FHB severity. In the last two decades, resistance sources of type III, which reduce the accumulation of DON, and type IV, which reduce the occurrence of Fusarium-damaged kernels (FDK), have gained more attention from wheat breeders, due to the growing global concern about wheat grain quality and mycotoxin contamination [10,11,12]. Recent research advances have underscored the need for integrated multi-omics strategies to better understand the diverse molecular pathways involved in wheat FHB resistance [13].
Association mapping studies have been conducted on various wheat germplasms to investigate the genetics of resistance to FHB. Kollers et al., 2013 [14] utilised 732 microsatellite markers to conduct an association mapping study on 358 European winter wheat accessions and discovered genetic loci for FHB on all wheat chromosomes, except chromosome 6B. In another study, Arruda et al. (2016) [15] identified 10 significant marker-trait associations (MTAs) on chromosomes 4A, 6A, 7A, 1D, 4D, and 7D for various FHB resistance components. The availability of high-density markers has enabled genome-wide association studies (GWAS), which have become popular for identifying QTL that contribute to different types of FHB resistance. GWAS offers higher genetic resolution due to historical recombination events and the abundant genetic variation found across a wide range of accessions. Numerous studies using bi-parental mapping and GWAS have revealed over 500 QTL on all wheat chromosomes that are responsible for various types of FHB resistance [16,17,18,19,20,21,22]. Nevertheless, only a small number of QTL have undergone fine mapping, such as Fhb1 (Qfhs.ndsu-3BS) located on wheat chromosome 3BS [23], Fhb2 (Qfhs.nau-6B) on 6BS [24], and Qfhs.ifa-5A on 5A [25], all of which were derived from the FHB-resistant Chinese wheat cultivar Sumai#3. Additional Fhb genes include Fhb3 from Leymus racemosus, Fhb4 (Qfhi.nau-4B), Fhb5 (Qfhi.nau-5A), and Fhb8 (Qfdk.nau-7D) discovered in the Chinese landrace Wangshuibai, Fhb6 from Elymus tsukushiensis, Fhb7 from Thinopyrum elongatum, and Fhb9 from the Chinese variety Shi4185. Of these, only Fhb1 and Fhb7 have been successfully cloned in recent times [26,27,28]. Both genes have been utilised in wheat breeding programmes [29,30,31]. Nevertheless, it is more advantageous to incorporate resistance genes from indigenous sources through introgression, which minimises the potential introduction of unwanted genetic elements caused by linkage drag, which can have detrimental effects on crop output and end-use characteristics [30,32,33]. Furthermore, indigenous sources are better suited to local environmental circumstances than foreign genotypes.
Although South Asia is not a major FHB epidemic region, the disease incidence has been increasing in the last decades due to changes in climate and agricultural practices [34,35]. To prevent large-scale FHB outbreaks in the future, it is crucial to continue searching for and utilising sources of FHB resistance within the local gene pool, which, in combination with the introduction of major QTL through marker-assisted selection and genomic selection, will contribute to the improvement of FHB resistance in South Asia. The main goals of this study were to evaluate 174 bread wheat lines of CIMMYT and South Asian wheat germplasm (CSASWG) for FHB resistance under field conditions, as well as to identify and investigate genomic regions linked to FHB resistance.
2. Materials and Methodology
2.1. Plant Materials
In the present investigation, we utilised a panel of 174 CSASWG lines. Among them, 97 genotypes (CIM-1 to CIM-97) were obtained from CIMMYT-Mexico, 30 genotypes (IND-1 to IND-21, IND-28, IND-30 to IND-36, and IND-38) from India, 28 genotypes (NPL-1 to NPL-28) from Nepal, and 19 genotypes (BGD-1 to BGD-19) from Bangladesh (Figure 1). These genotypes are indicative of the current top-tier varieties and breeding lines within their respective organisations and countries (Supplementary Table S1).
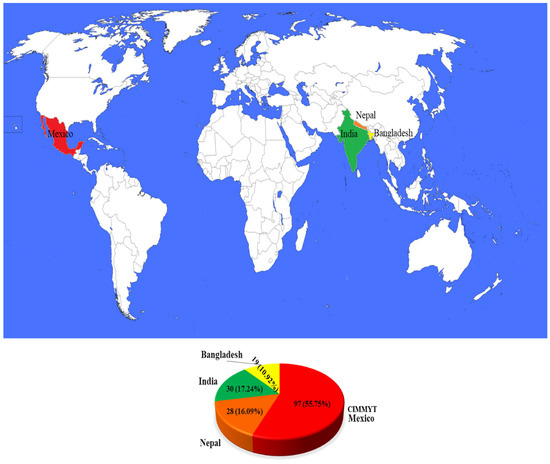
Figure 1.
Distribution of the studied germplasm among the four countries, represented by different colours. The pie chart shows the share of the CSASWG by country.
2.2. Field Trials and Disease Scoring
Field experiments for FHB were conducted at CIMMYT’s El Batán experimental station in Mexico in 2019, 2021, and 2023. The station is located at an altitude of 2240 m above sea level, with coordinates 19.5° North and 98.8° West. The average annual precipitation at the station was 625 mm. The experiments were conducted during the summer season, which spans from May to September and is characterised by concentrated rainfall [36]. CSASWG accessions were planted in double rows of 1 m in length, using a randomised complete block design with two replications. Four checks were included in the experiments: SUMAI #3 (resistant), HEILO (moderately resistant), OCORONI-F-86 (moderately susceptible), and GAMENYA (susceptible). Annually, five virulent F. graminearum isolates were collected, analysed, and employed in a mixture for field inoculation, adhering to the procedures outlined by [37]. Spray inoculation targeted the anthesis stage of each line using an inoculum concentration of 50,000 spores/mL at a rate of 60 mL/m2. This process was repeated two days after the initial spray. During the period from anthesis to early dough stages, the nursery was subjected to misting from 9:00 a.m. to 8:00 p.m. with 10 min of spraying every hour. This was done to establish a humid environment that would promote the development of FHB. In the nursery, wheat-maize rotation and conservation farming methods were implemented to improve the presence of natural inoculum (Figure 2). FHB symptoms were assessed 25 days after inoculation (dpi) on 10 spikes that were marked at flowering. The number of infected spikes and symptomatic spikelets on each spike was recorded in order to calculate the FHBindex using the formula FHBindex (%) = Severity × Incidence × 100 [38]. Severity was quantified as the mean percentage of spikelets affected by the disease, whereas incidence was defined as the percentage of spikes showing symptoms. Days to heading (DH) and plant height (PH) were also scored in all experiments.

Figure 2.
FHB disease screening at CIMMYT-Mexico. (A) Spray inoculation with F. graminearum inoculum; (B) Nozzle used for the automatic misting system; (C) Mist irrigation in the FHB screening field; (D) Laboratory culture of an F. graminearum isolate; (E) FHB on a wheat spike, showing symptoms of chlorosis, necrosis, and salmon-coloured mycelial mass; (F) A highly susceptible wheat line showing necrosis caused by FHB; (G) F. graminearum infected wheat grains.
2.3. Statistical Analysis
The adjusted FHBindex values were obtained by subtracting the residuals from the linear regression model with DH and PH as covariates, using the “resid” function of R software v 4.4.1, to reduce the influence of the two traits on FHB infection, and the adjusted values were used for all subsequent analyses. Analysis of variance for FHBindex was performed using the “agricolae” package, and genotype-by-year interaction was estimated with “metan” package in R. Broad-sense heritability was estimated for three years using the formula H2 = σ2g/((σ2g + σ2g-y/y) + σ2e/ry), where σ2g stands for genotypic variance, σ2e-y for genotype-by-year interactions, σ2e for error variance, y for the number of years, and r for the number of replications [39]. The raincloud plot was projected to show the distribution of FHBindex values across experiments using the “ggrain” package in R software v 4.4.1. Tukey’s HSD test was performed to determine the significant differences among genotypes using PAST v 4.03 software. All bar graphs were drawn using Microsoft Excel 2021.
2.4. Genotyping, Population Structure, and LD Analysis
Genomic DNA was isolated from seedling leaves three weeks old using the CTAB method. DNA quality and quantity were evaluated on a Thermo Scientific™ NanoDrop™ 2000 Spectrophotometer (Waltham, MA, USA), and then sent to Trait Genetics GmbH, Germany, for genotyping using Illumina Infinium 15 K Bead Chip. A total of 11,184 SNP markers were obtained after filtering (excluding SNPs with minor allele frequencies less than 5%, missing data points greater than 30%, and those with unknown chromosome positions). The optimum number of clusters PCA was calculated using GenAlex v 6.5 tools, followed by a phylogenetic tree, kinship analysis, and linkage disequilibrium were performed using Tassle v 5.0 and R software v 4.4.1 with the rMAV package.
2.5. Marker-Traits Association (MTA) for FHB
Multiple Loci Mixed Model (MLMM) was used to detect SNP-FHBindex associations with the “Gapit” package in R, and significant markers in all models were declared with a threshold of p < 0.01 [40]. FHB data for the three years were used individually for GWAS, followed by pooled FHBindex values.
3. Results
3.1. Reaction of Genotypes Against FHB and Identification of Resistant Lines
A raincloud plot (Figure 3A) depicts the average disease index of FHB throughout the three separate field trials in 2019, 2021, and 2023. The correlation coefficients of the FHBindex among the three field evaluations ranged from 0.41 to 0.67 (Figure 3B). The grand mean FHBindex for 2019, 2021, and 2023 were 22.91%, 15.10%, and 18.19%, respectively.
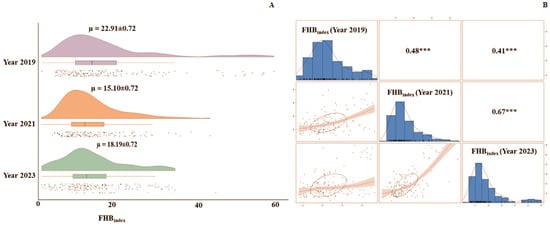
Figure 3.
FHB infection in the CSASWG panel. (A) Raincloud distribution plots for FHBindex in the three years, with mean FHBindex and S.E. values indicated at the top of each plot. (B) Histogram distribution of FHBindex in individual years and correlation plots and values for the three years. “***” indicates significance at the p < 0.0001 level.
The 174 wheat genotypes showed a wide range of variation, from resistant (0–9.0) to susceptible (>25). The majority of genotypes (>84.48%) had disease scores between 9.0 and 25.0, indicating a moderately resistant disease reaction. CIMMYT lines outperformed the other groups of lines, whereas the Indian varieties tested in this study exhibited generally high infection (Figure 4A). Only four genotypes had disease indices between 0 and 9.0, indicating a resistant disease reaction and 23 genotypes with FHBindex > 25.0 demonstrated a susceptible response (Figure 4B).
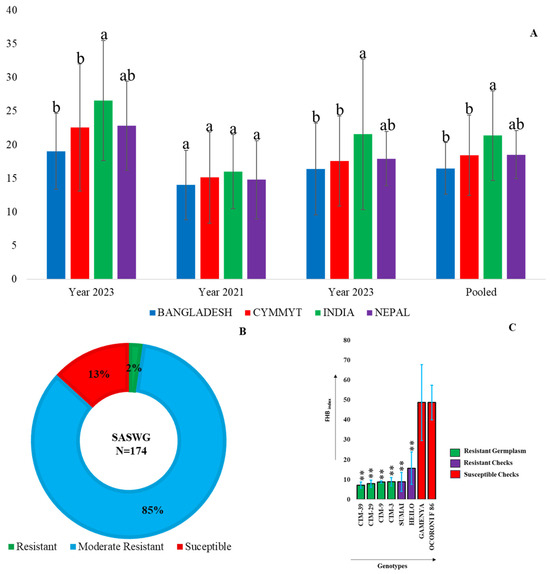
Figure 4.
(A) Mean FHB indices of different country groups in individual years. The letters above the bars indicate groups that are significantly different at the 5% significance level. (B) Distribution of 174 genotypes according to FHB resistance. (C) Differences between checks and identified resistant lines. “**” indicates significance at the 1% confidence level.
The resistant lines, such as CIM-39, CIM-29, CIM-9, and CIM-3, had a mean FHBindex ranging from 0.0 to 9.0 throughout all three years of field evaluation (Figure 4C). Significant effects were found for both “year” and “genotype”, whereas their interaction did not show significant effects (Table 1). The overall broad-sense heritability (H2) for the FHB index was 0.77.

Table 1.
Analysis of variance for FHBindex of the CSASWG panel across field experiments.
3.2. Genotyping, PCA, Kinship and Linkage Disequilibrium Analysis
A total of 16,028 SNP markers were generated using the Illumina Infinium 15 K Bead Chip platform, producing a total of 11,184 SNP markers after excluding markers with minor allele frequencies less than 0.05, unknown chromosome positions, or more than 10% missing SNP data. Before performing GWAS, the 11,184 SNP markers were used for PCA, which separated the 174 CSASWG lines into two clusters, that is, of CIMMYT origin and other than CIMMYT (Figure 5A), which agreed well with the dendrogram analysis using the same set of SNP markers (Figure 5B). Kinship analysis also revealed significant differences between the CIMMYT genotypes and those other than CIMMYT, as seen in Figure 5C. LD plot was generated using the same set of SNP markers based on the r2 values for the entire genome (Figure 5D).
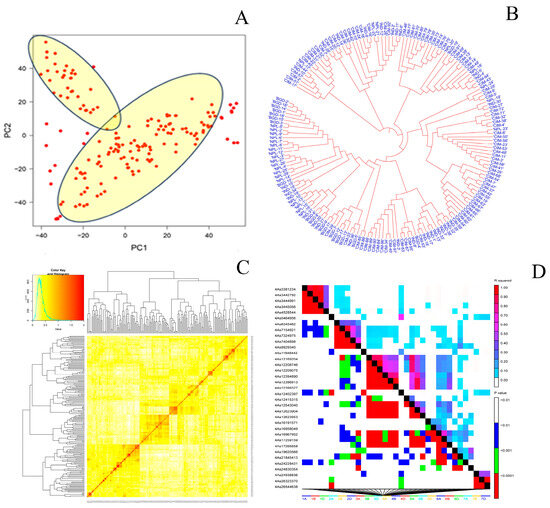
Figure 5.
(A) 2D-PCA plot and (B) phylogenetic tree explored diversity in the 174 wheat genotypes; (C) Heatmap and dendrogram of Kinship matrix estimated using Van Randen algorithm based on the 11,184 SNP markers; (D) LD plot of wheat genome based on 11,182 SNP markers. The red colour indicates the haploblocks.
3.3. Genome-Wide Association Study
Four different algorithms, namely GLM, MLM, MLMM, and FarmCPU, were initially compared to select the best algorithm for association analysis. MLMM better fits the FHB disease data and was used to identify significant MTAs with a significance threshold of p < 1 × 10−4. Nineteen significant markers were identified on eight chromosomes, of which three were repeatable across different experiments and were located on chromosomes 1A and 7B (Table 2 and Figure 6). Phenotypic variations explained (PVE) by the three significant SNPs, i.e., Ra_c58315_265 (on chromosome 1A), Tdurum_contig102328_129 (7B), and Ku_c20136_198 (7B), ranged from 3.34 to 6.05% (Table 2). PVEs of other significant SNPs were also low, ranging from 2.05 to 6.23% (Table 2).

Table 2.
Markers significantly associated with FHB resistance through MLMM.
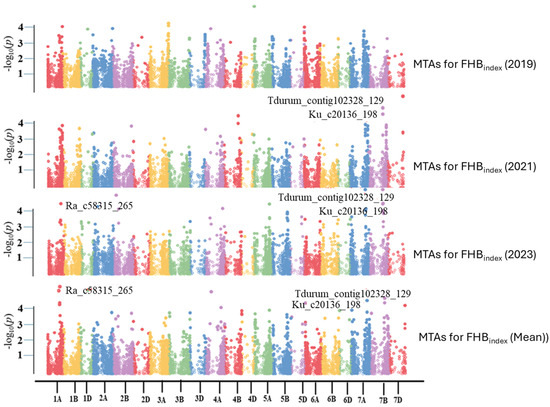
Figure 6.
Manhattan plots based on the Multiple Loci Mixed Model (MLMM) represent −log10 (p-value) for SNPs distributed across all chromosomes (x-axis).
Haplotype analysis revealed significant differences between the resistant and susceptible alleles for the three MTAs on 1A and 7B (Figure 7). Haplotype results also indicated that the allele “A” from SNP Ra_c58315_265, “C” allele from Ku_c20136_198, and “G” allele from Tdurum_contig102328_129 were associated with the FHB resistance mechanism in wheat. These alleles were present in all the most resistant genotypes (Table 3), suggesting an additive effect of these alleles on FHB resistance in wheat.
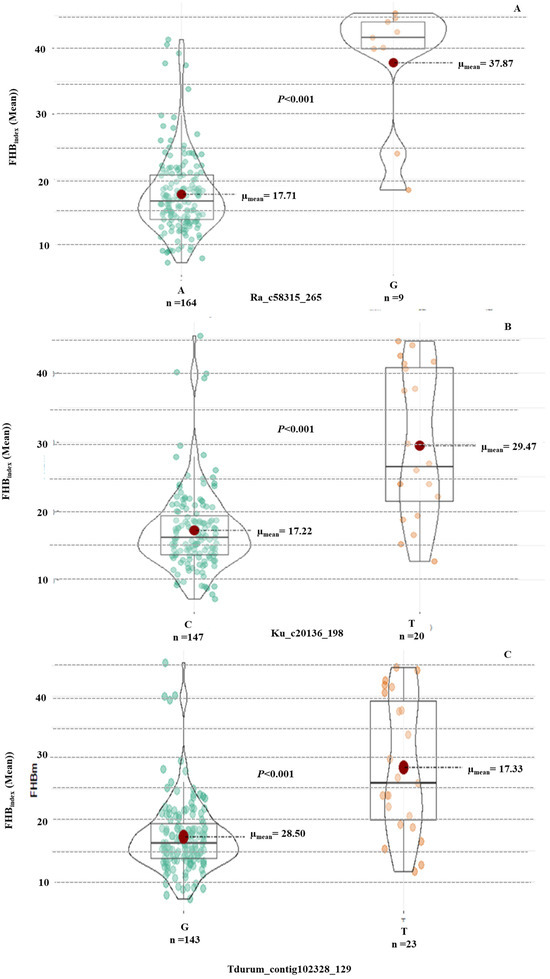
Figure 7.
Effect of different alleles using the Wilcoxon test for (A) MTA Ra_c58315_265 with FHBindex (mean), (B) MTA Ku_c20136_198 with FHBindex (mean), and (C) MTA Tdurum_contig102328_129 with FHBindex (mean).

Table 3.
The presence of different alleles associated with FHB disease resistance observed in the most resistant genotypes.
3.4. Candidate Genes for the Significant MTA
Sequences of the 19 significant SNP markers identified from GWAS were used in BLAST searches against the Triticum aestivum v2.2 reference genome available at Phytozome v 13 (accessed on 26.06.24) to identify candidate genes related to disease resistance mechanisms in plants. For each MTA, a two-Mb window was used to identify candidate genes, and a total of 12 genes were found (Table 4).

Table 4.
Functional annotation of candidate genes associated with disease resistance.
4. Discussion
FHB is a globally important wheat disease, and its incidence in South Asia has been increasing due to climate change [8,34]. Identification of genotypes with FHB resistance could be an important pre-emptive strategy for possible future epidemics in the region, and the significant markers identified through GWAS could be used in marker-assisted breeding programmes.
The observed variation in the responses of the wheat accessions over different years can be attributed to the fluctuating weather conditions, which were reflected in the significant “year” effect in ANOVA; however, no significant genotype-by-environment effect was observed for this panel. Admittedly, disease pressure in the three years was not very high, as reflected in the generally low FHB indices of the tested lines, as well as the susceptible checks that often reached 80–90% under high disease pressure. Therefore, lines with low infection in this study might be moderately resistant rather than fully resistant, as one would infer based on the FHB indices. Nevertheless, the results might still be highly relevant to the South Asian scenario with low FHB disease pressure. In a recent study on screening Indian wheat germplasm for FHB resistance, Kumar et al. (2021) evaluated 164 wheat genotypes and did not identify any resistant lines; only a few showed moderate resistance, while the rest were moderately susceptible or susceptible [35]. This is consistent with our results, although we used spray inoculation to assess a combination of type I and type II resistance, whereas they used the cotton web technique to evaluate type II resistance specifically. Heading date and plant height have long been associated with field FHB infection, which affects the precision evaluation of materials in field experiments [36]. In the current study, the two factors were used as covariates to correct the FHB index, which was used in all subsequent analyses, avoiding the confounding effects of heading and height, resulting in a better estimation of genetic resistance.
CIMMYT lines generally outperformed those from other South Asian countries in this study, and the best lines identified were all of CIMMYT origin, which was not unexpected because FHB resistance is an important target of wheat breeding at CIMMYT [37], but it has not become a major trait for South Asian wheat breeding programmes yet. Nevertheless, it is well known that the most effective FHB resistance genes/QTL like Fhb2, Fhb4, and Fhb5, are of very low frequencies, if not absent [41], whereas Fhb1 has been introduced into CIMMYT germplasm, but its frequency is still low and needs to be increased [42]. Significant breeding efforts are being conducted at CIMMYT to utilise these genes along with the recently cloned Fhb7 to improve FHB resistance, which may also contribute to South Asian wheat breeding, considering the frequent utilisation of CIMMYT germplasm as parents.
Nineteen MTAs were identified by the MLMM method in the present GWAS, which identified the highest number of MTAs on 7B chromosome, followed by 7A, 4A, and 1A. However, only three MTAs (Ra_c58315_265 on 1A, Tdurum_contig102328_129, and Ku_c20136_198 on 7B) These results revealed marker-trait relationships, indicating the presence of resistant alleles, with effects ranging from 3.54% to 6.05% for FHB resistance in the field. This resource plays a crucial role in the production of FHB resistance markers, thereby enhancing the efficacy of selecting FHB-resistant traits for variety improvement [43]. Consistent with a recent investigation, some QTLs were also reported by Shi et al., 2023 [44], on chromosome 1A (gFHB-1A.1, gFHB-1A.2, and gFHB-1A.3) and Chromosome 7B (gFHB-7B). According to meta-QTL analysis, five MQTL-related FHB indexes were reported on 1A chromosome [21].
The candidate genes linked to the identified MTAs included Glycosylase/Endonuclease III, Putative transmembrane protein cmp44E, and cysteine-rich repeat protein. Lu and Edwards (2016) and Guo et al. (2020) [45,46] reported that the cysteine-rich repeat protein has a significant role in the defence mechanism against both Rhizoctonia cerealis and Bipolaris sorokiniana, and Paudel et al. (2020) [47] reported that putative transmembrane protein is associated with Qfhb1 in wheat. While the precise molecular consequences of the associated polymorphisms remain to be elucidated, these loci may influence resistance through regulatory or structural changes in candidate genes. Further work is needed to confirm the roles of these candidate genes in FHB resistance.
FHB resistance is a complex quantitative trait and resistance in wheat is subject to environmental conditions. The current study identified 19 MTAs, of which only three were repeatable and can be useful in future marker-assisted breeding programmes. The identified resistant genotypes may be used as parents or released as varieties in the South Asian region, considering their good FHB resistance.
5. Conclusions
In conclusion, this study underscores the general susceptibility of South Asian wheat genotypes to Fusarium head blight (FHB) and emphasises the importance of pre-emptive strategies like identifying resistant wheat genotypes for their utilisation in breeding. Marker-assisted breeding programmes can greatly benefit from the findings of this GWAS study, particularly the three reproducible MTAs (Ra_c58315_265, Ku_c20136_198, and Tdurum_contig102328_129) identified on chromosomes 1A and 7B. While acknowledging the variable responses of wheat accessions across years, this study highlights CIMMYT lines as good performers in FHB resistance despite the challenges posed by the low frequencies of key resistance genes. These findings provide a foundation for improving FHB resistance in South Asian wheat varieties, thereby contributing to regional agricultural resilience.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14050490/s1, Supplementary Table S1: List of genotypes with their raw and adjusted FHBindex values.
Author Contributions
P.K.S. designed the research and supervised the experiments. G.P.S., A.K.J. and M.R.K. provided the plant materials. X.H. contributed to the genotyping work, and P.K.S. contributed to the phenotyping work. R.M. and X.H. analysed the data. R.M. wrote the first draft of the manuscript, and all authors contributed to and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the Indian Council of Agriculture Research (ICAR), India, USAID-Global Wheat Health Alliance, and One CGIAR Initiatives-ABI and PHI for conducting this research is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Phenotypic data are available in the Supplementary File, and the genotypic data are accessible at https://hdl.handle.net/11529/10548559 (accessed on 2 May 2025).
Acknowledgments
The first author is grateful for the financial support from the ICAR-NAHEB International Travel Grant. Technical support from Francisco Lopez, Nerida Lozano, Elizabeth Blanco Venegas, Mayela Flores Romero, and Mónica Preciado from Wheat Pathology, CIMMYT, is highly acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT. 2024. Available online: https://www.fao.org/faostat/en/#data (accessed on 2 May 2025).
- Khan, M.K.; Pandey, A.; Athar, T.; Choudhary, S.; Deval, R.; Gezgin, S.; Hamurcu, M.; Topal, A.; Atmaca, E.; Santos, P.A.; et al. Fusarium head blight in wheat: Contemporary status and molecular approaches. 3 Biotech 2020, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Friesen, T.L.; Faris, J.D.; Solomon, P.S.; Oliver, R.P. Host-specific toxins: Effectors of necrotrophic pathogenicity. Cell Microbiol. 2008, 10, 1421–1428. [Google Scholar] [CrossRef]
- Gurung, S.; Hansen, J.M.; Bonman, J.M.; Gironella, A.I.N.; Adhikari, T.B. Multiple disease resistance to four leaf spot diseases in winter wheat accessions from the USDA National Small Grains Collection. Crop Sci. 2012, 52, 1640–1650. [Google Scholar] [CrossRef]
- Nganje, W.E.; Kaitibie, S.; Wilson, W.W.; Leistritz, F.L.; Bangsund, D.A. Economic Impacts of Fusarium Head Blight in Wheat and Barley: 1993–2001 (Agribusiness and Applied Economics Report No 538); Department of Agribusiness and Applied Economics, Agricultural Experiment Station, North Dakota State University: Fargo, ND, USA, 2004. [Google Scholar]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Shude, S.P.N.; Yobo, K.S.; Mbili, N.C. Progress in the management of Fusarium head blight of wheat: An overview. S. Afr. J. Sci. 2020, 116, 11–12. [Google Scholar] [CrossRef]
- Mesterházy, Á. Methodology of resistance testing and breeding against Fusarium head blight in wheat and results of the selection. Cereal Res. Commun. 1997, 25, 631–637. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Bartók, T.; Mirocha, C.; Komoroczy, R. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- Gilbert, J.; Tekauz, A. Recent developments in research on Fusarium head blight of wheat in Canada. Can. J. Plant Pathol. 2000, 22, 1–8. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Bartok, T.; Lamper, C. Influence of wheat cultivar, species of Fusarium, and isolate aggressiveness on the efficacy of fungicides for control of Fusarium head blight. Plant Dis. 2003, 87, 1107–1115. [Google Scholar] [CrossRef]
- Sirangelo, T.M. Molecular investigations to improve Fusarium head blight resistance in wheat: An update focusing on multi-omics approaches. Plants 2024, 13, 2179. [Google Scholar] [CrossRef] [PubMed]
- Kollers, S.; Rodemann, B.; Ling, J.; Korzun, V.; Ebmeyer, E.; Argillier, O.; Hinze, M.; Plieske, J.; Kulosa, D.; Ganal, M.W.; et al. Whole genome association mapping of Fusarium head blight resistance in European winter wheat (Triticum aestivum L.). PLoS ONE 2013, 8, e57500. [Google Scholar] [CrossRef]
- Arruda, M.P.; Brown, P.; Brown-Guedira, G.; Krill, A.M.; Thurber, C.; Merrill, K.R.; Foresman, B.J.; Kolb, F.L. Genome-wide association mapping of Fusarium head blight resistance in wheat using genotyping-by-sequencing. Plant Genome 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H.; Léon, J. Breeding for Fusarium head blight resistance in wheat—Progress and challenges. Plant Breed. 2019, 139, 429–454. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.; Su, Z.; Li, T.; Zhang, X.; Bai, G. Meta-analysis of QTL for Fusarium head blight resistance in Chinese wheat landraces. Crop J. 2019, 7, 784–798. [Google Scholar] [CrossRef]
- Liu, S.; Hall, M.D.; Griffey, C.A.; McKendry, A.L. Meta-analysis of QTL associated with Fusarium head blight resistance in wheat. Crop Sci. 2009, 49, 1955–1968. [Google Scholar] [CrossRef]
- Löffler, M.; Schön, C.C.; Miedaner, T. Revealing the genetic architecture of FHB resistance in hexaploid wheat (Triticum aestivum L.) by QTL Meta-analysis. Mol. Breed. 2009, 23, 473–488. [Google Scholar] [CrossRef]
- Venske, E.; Dos Santos, R.S.; Farias, D.D.R.; Rother, V.; Da Maia, L.C.; Pegoraro, C.; Costa de Oliveira, A. Meta-analysis of the QTLome of Fusarium head blight resistance in bread wheat: Refining the current puzzle. Front. Plant Sci. 2019, 10, 727. [Google Scholar] [CrossRef]
- Zheng, T.; Hua, C.; Li, L.; Sun, Z.X.; Yuan, M.M.; Bai, G.H.; Humphreys, G.; Li, T. Integration of meta-QTL discovery with omics: Towards a molecular breeding platform for improving wheat resistance to Fusarium head blight. Crop J. 2021, 9, 739–749. [Google Scholar] [CrossRef]
- Cuthbert, P.A.; Somers, D.J.; Thomas, J.; Cloutier, S.; Brulé-Babel, A. Fine mapping Fhb1, a major gene controlling Fusarium head blight resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 112, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, P.A.; Somers, D.J.; Brulé-Babel, A. Mapping of Fhb2 on chromosome 6BS: A gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 114, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Buerstmayr, M.; Wagner, C.; Danler, A.; Eshonkulov, B.; Ehn, M.; Buerstmayr, H. Fine-mapping of the Fusarium head blight resistance QTL Qfhs.ifa-5A identifies two resistance QTL associated with anther extrusion. Theor. Appl. Genet. 2019, 132, 2039–2053. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, J.; Jia, H.; Gao, Z.; Fan, M.; Luo, Y.; Zhao, P.; Xue, S.; Li, N.; Yuan, Y.; et al. Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to Fusarium head blight. Nat. Genet. 2019, 51, 1106–1112. [Google Scholar] [CrossRef]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Ghimire, B.; Sapkota, S.; Bahri, B.A.; Martinez-Espinoza, A.D.; Buck, J.W.; Mergoum, M. Fusarium head blight and rust diseases in soft red winter wheat in the southeast United States: State of the art, challenges and future perspective for breeding. Front. Plant Sci. 2020, 11, 1080. [Google Scholar] [CrossRef]
- Zhu, Z.; Hao, Y.; Mergoum, M.; Bai, G.; Humphreys, G.; Cloutier, S.; Xia, X.; He, Z. Breeding wheat for resistance to Fusarium head blight in the Global North: China, USA, and Canada. Crop J. 2019, 7, 730–738. [Google Scholar] [CrossRef]
- Cai, X.; Danilova, T.; Charif, A.; Wang, F.; Zhang, W.; Zhang, M.; Ren, S.; Zhu, X.; Zhong, S.; Dykes, L.; et al. Registration of WGC002 spring wheat containing wild grass-derived Fusarium head blight resistance gene Fhb7The2. J. Plant Regist. 2024, 18, 179–186. [Google Scholar] [CrossRef]
- Brar, G.S.; Pozniak, C.J.; Kutcher, H.R.; Hucl, P.J. Evaluation of Fusarium head blight resistance genes Fhb1, Fhb2, and Fhb5 introgressed into elite Canadian hard red spring wheats: Effect on agronomic and end-use quality traits and implications for breeding. Mol. Breed. 2019, 39, 44. [Google Scholar] [CrossRef]
- Schweiger, W.; Steiner, B.; Vautrin, S.; Nussbaumer, T.; Siegwart, G.; Zamini, M.; Jungreithmeier, F.; Gratl, V.; Lemmens, M.; Mayer, K.F.X.; et al. Suppressed recombination and unique candidate genes in the divergent haplotype encoding Fhb1, a major Fusarium head blight resistance locus in wheat. Theor. Appl. Genet. 2016, 129, 1607–1623. [Google Scholar] [CrossRef]
- Saharan, M.S.; Kumar, H.M.A.; Gurjar, M.S.; Aggarwal, R. Fusarium head blight of wheat in India-variability in pathogens associated and sources of resistance: An overview. Indian Phytopathol. 2021, 74, 345–353. [Google Scholar] [CrossRef]
- Kumar, A.; Aggarwal, H.M.; Rashmi, G.M.S.; Prasad, V.L.; Saharan, M.S. Identification of Fusarium head blight resistant sources in wheat under artificially inoculated condition. Indian J. Agric. Sci. 2021, 91, 895–899. [Google Scholar] [CrossRef]
- He, X.; Singh, P.K.; Dreisigacker, S.; Singh, S.; Lillemo, M.; Duveiller, E. Dwarfing genes Rht-B1b and Rht-D1b are associated with both type I FHB susceptibility and low anther extrusion in two bread wheat populations. PLoS ONE 2016, 11, e0162499. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Singh, P.K.; Duveiller, E.; Schlang, N.; Dreisigacker, S.; Singh, R.P. Identification and characterization of international Fusarium head blight screening nurseries of wheat at CIMMYT, Mexico. Eur. J. Plant Pathol. 2013, 136, 123–134. [Google Scholar] [CrossRef]
- He, X.; Singh, P.K.; Duveiller, E.; Dreisigacker, S.; Singh, R.P. Development and characterization of International Maize and Wheat Improvement Center (CIMMYT) germplasm for Fusarium head blight resistance. In Fusarium Head Blight in Latin America; Alconada, T.M., Chulze, S.N., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 241–262. [Google Scholar]
- Zhang, J.; Gill, H.S.; Halder, J.; Brar, N.K.; Ali, S.; Bernardo, A.; Amand, P.S.; Bai, G.; Turnipseed, B.; Sehgal, S.K. Multi-locus genome-wide association studies to characterize Fusarium head blight (FHB) resistance in hard winter wheat. Front. Plant Sci. 2022, 13, 946700. [Google Scholar] [CrossRef] [PubMed]
- Navathe, S.; Pandey, A.K.; Sharma, S.; Chand, R.; Mishra, V.K.; Kumar, D.; Jaiswal, S.; Iquebal, M.A.; Govindan, V.; Joshi, A.K.; et al. New genomic regions identified for resistance to spot blotch and terminal heat stress in an interspecific population of Triticum aestivum and T. spelta. Plants 2022, 11, 2987. [Google Scholar] [CrossRef]
- Osman, M.; He, X.; Singh, R.P.; Duveiller, E.; Lillemo, M.; Pereyra, S.A.; Westerdijk-Hoks, I.; Kurushima, M.; Yau, S.-K.; Benedettelli, S.; et al. Phenotypic and genotypic characterization of CIMMYT’s 15th international Fusarium head blight screening nursery of wheat. Euphytica 2015, 205, 521–537. [Google Scholar]
- He, X.; Brar, G.S.; Bonnett, D.; Dreisigacker, S.; Hyles, J.; Spielmeyer, W.; Bhavani, S.; Singh, R.P.; Singh, P.K. Disease resistance evaluation of elite CIMMYT wheat lines containing the coupled Fhb1 and Sr2 genes. Plant Dis. 2020, 104, 2369–2376. [Google Scholar] [CrossRef]
- Bhatta, M.; Morgounov, A.; Belamkar, V.; Poland, J.; Baenziger, S.P. Unlocking the novel genetic diversity and population structure of synthetic hexaploid wheat. BMC Genom. 2018, 19, 591. [Google Scholar] [CrossRef]
- Shi, C.; Chao, H.; Sun, X.; Suo, Y.; Chen, Z.; Li, Z.; Ma, L.; Li, J.; Ren, Y.; Hua, W.; et al. Genome-wide association study for Fusarium head blight resistance in common wheat from China. Agronomy 2023, 13, 1712. [Google Scholar] [CrossRef]
- Lu, S.; Edwards, M.C. Genome-wide analysis of small secreted cysteine-rich proteins identifies candidate effector proteins potentially involved in Fusarium graminearum−wheat interactions. Phytopathology 2016, 106, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Shan, Z.; Yu, J.; Xu, G.; Zhang, Z. The cysteine-rich repeat protein TaCRR1 participates in defense against both Rhizoctonia cerealis and Bipolaris sorokiniana in wheat. Int. J. Mol. Sci. 2020, 21, 5698. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.; Zhuang, Y.; Galla, A.; Dahal, S.; Qiu, Y.; Ma, A.; Raihan, T.; Yen, Y. WFhb1-1 plays an important role in resistance against Fusarium head blight in wheat. Sci. Rep. 2020, 10, 7794. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).