Abstract
Background: This case is unique in demonstrating the reactivation of latent tuberculosis (TB) following co-infection with SARS-CoV-2 and Epstein–Barr virus (EBV) in an otherwise healthy young adult. It highlights a rare clinical scenario in which viral immune dysregulation likely facilitated TB progression. To date, few reports have explored the complex interplay between COVID-19, EBV reactivation, and TB in a single patient, particularly with isolated extrapulmonary involvement. Case Presentation: A 24-year-old woman presented with persistent low-grade fever, fatigue, night sweats, unintentional weight loss, and progressive cervical and supraclavicular lymphadenopathy. These symptoms emerged shortly after a moderate COVID-19 infection. Laboratory studies revealed elevated inflammatory markers and pronounced lymphopenia. EBV reactivation was confirmed via serology and PCR. Despite antiviral therapy, symptoms persisted, and imaging revealed necrotic lymphadenopathy. Tuberculous lymphadenitis was diagnosed through fine-needle aspiration cytology and PCR detection of Mycobacterium tuberculosis. The patient was treated with a standard anti-tuberculosis regimen, resulting in clinical, radiological, and immunological improvement. Conclusions: This case underscores the importance of considering latent TB reactivation in patients with persistent lymphadenopathy and recent viral infections, particularly in regions with high TB prevalence. It also emphasizes the need for thorough immunological and microbiological assessment in complex post-viral syndromes. The main clinical takeaway is that COVID-19 and EBV co-infection may create a permissive environment for TB reactivation through immune system compromise.
1. Introduction
Tuberculous lymphadenitis (scrofuloderma) is the most common form of extrapulmonary tuberculosis, presenting as chronic lymphadenopathy that can mimic a wide range of infectious, autoimmune, and neoplastic diseases [1,2]. The diagnostic challenge is further compounded by overlapping clinical manifestations with systemic illnesses, particularly viral infections such as COVID-19 and Epstein–Barr virus (EBV) [3,4,5]. Both viruses can cause prolonged lymphadenopathy, immune dysfunction, and lymphopenia, complicating the determination of the underlying etiology [6,7].
EBV is a well-known trigger of lymphoproliferative disorders and can cause infectious mononucleosis, characterized by generalized lymphadenopathy, hepatosplenomegaly, and immune disturbances [8,9]. At the same time, COVID-19, beyond its respiratory manifestations, has been associated with persistent immune alterations, including lymphocyte depletion, which may increase susceptibility to secondary bacterial and mycobacterial infections [10,11,12,13].
Given the epidemiological significance of tuberculosis and the growing role of viral infections in immune system modulation, maintaining a high level of clinical suspicion is crucial when evaluating patients with persistent lymphadenopathy [14,15]. In this context, timely differential diagnosis between tuberculous lymphadenitis, viral infections, and other systemic diseases is essential for appropriate treatment and improved patient outcomes [16,17]. Other causes of lymphadenopathy include bacterial infections such as Bartonella henselae or Brucella spp., parasitic diseases like toxoplasmosis, autoimmune conditions including systemic lupus erythematosus and sarcoidosis, and malignancies such as lymphoma and leukemia [17,18,19]. Differentiating among these conditions requires a systematic approach combining clinical examination with imaging, laboratory tests, and histopathological evaluation of lymph node tissue [20,21]. Awareness of the full spectrum of potential etiologies is critical to avoid diagnostic delays and ensure timely, targeted management [22].
2. Case Description
2.1. Patient Information
A 24-year-old female presented with a subacute onset of systemic and localized symptoms following a documented episode of moderate COVID-19, which occurred three months prior to consultation. Her primary complaints included persistent low-grade fever, fatigue, generalized lymphadenopathy, unintentional weight loss, and night sweats, with a particularly concerning development of a painful, bluish supraclavicular mass two days before presentation. The patient had no prior history of tuberculosis, immunodeficiency, or chronic illness, and no relevant family history of infectious or autoimmune diseases was reported. She did not use immunosuppressive therapy prior to her COVID-19 treatment and had no significant psychosocial or environmental risk factors for tuberculosis. Immunological evaluation revealed substantial lymphopenia, including reductions in CD4+ and CD8+ T cells, B cells, and NK cells, alongside low levels of complement C4 and immunoglobulins, indicating notable immune dysregulation.
2.2. Clinical Findings
On presentation, physical examination revealed a raised, bluish mass over the left supraclavicular region, accompanied by localized skin erythema, tension, and mild tenderness. The lesion demonstrated inflammatory features including edema and a dome-shaped protrusion. Palpation identified multiple firm, mobile, non-tender lymph nodes in both cervical and supraclavicular areas, with some forming clusters. No hepatosplenomegaly or signs of respiratory involvement were observed. Vital signs were stable, although the patient continued to report fever and a marked decline in physical stamina. General examination was otherwise unremarkable, aside from signs of anemia (Figure 1).
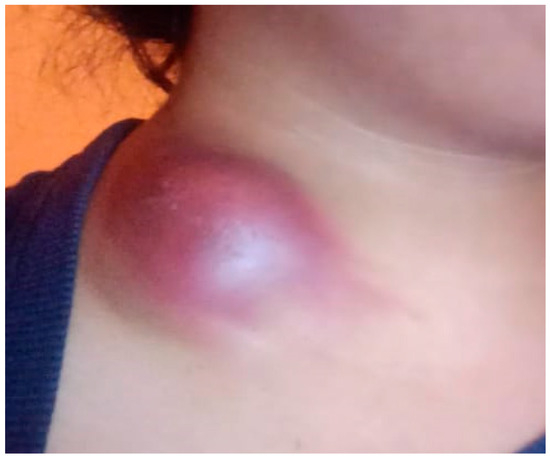
Figure 1.
Acute subcutaneous inflammatory mass in the neck region. The image shows the clinical presentation observed in the patient, which includes a pathological mass on the anterolateral surface of the neck with marked localized hyperemia, edema, and a dome-shaped tissue protrusion. The skin in this area appears taut and glossy, indicating increased intratissue pressure.
2.3. Timeline
The patient developed moderate COVID-19 three months prior to evaluation and received a 10-day course of dexamethasone, combined with nirmatrelvir/ritonavir and azithromycin. Although she initially improved, constitutional symptoms and cervical lymphadenopathy developed shortly thereafter. EBV reactivation was diagnosed one month later, and antiviral therapy with acyclovir was initiated. Despite partial symptom relief, progressive lymphadenopathy and the emergence of a supraclavicular mass led to a renewed diagnostic workup. Two months after symptom onset, tuberculosis was diagnosed, and anti-tubercular therapy began. By the end of the second treatment month, substantial clinical improvement was noted, with resolution of inflammatory markers and radiographic regression of lymphadenopathy (Figure 2).
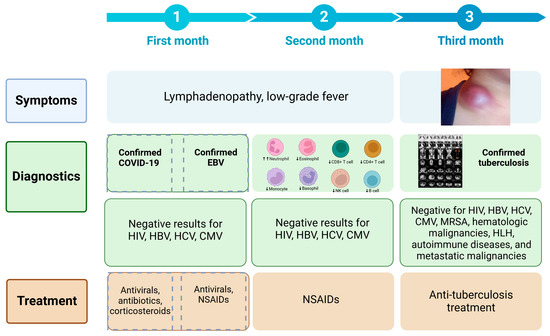
Figure 2.
Timeline of symptom progression, diagnostics, and treatment over three months.
2.4. Diagnostic Assessment
Initial laboratory evaluation showed leukocytosis with neutrophilia, anemia, and lymphopenia. Inflammatory markers including ESR, CRP, ferritin, procalcitonin, and D-dimer were consistently elevated, aligning with ongoing systemic inflammation. EBV reactivation was confirmed via serology (positive IgM and IgG for EBV VCA, elevated IgG to EBV EA) and PCR detection of EBV DNA (Table 1 and Table 2). Subsequent EBV testing indicated a transition to a latent phase, with IgM decline and negative EBV DNA. The PCR assays for EBV were conducted using the QuantStudio™ 5 Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA), targeting the BamHI-W region for EBV DNA detection. Serological testing for EBV was performed using the Vidas® EBV IgM/IgG enzyme-linked fluorescent assay (BioMérieux, Marcy-l’Étoile, France), while inflammatory markers were assessed using standard laboratory platforms such as the Cobas 6000 analyzer (Roche Diagnostics, Basel, Switzerland) for CRP and ESR, and the Siemens ADVIA® 2120i Hematology System for complete blood count and D-dimer levels.

Table 1.
Dynamics of laboratory parameters in the patient.

Table 2.
Immunological profile changes in the patient.
Neck ultrasound demonstrated bilateral hypoechoic lymphadenopathy with increased vascularity. CT imaging revealed a large, encapsulated fluid collection in the left supraclavicular area, alongside enlarged paratracheal and subcarinal lymph nodes with necrotic changes (Figure 3). No pulmonary lesions or abdominal pathology were noted. Imaging was performed using a Philips iU22 ultrasound system with a high-frequency linear probe (12–15 MHz) for detailed soft tissue imaging. CT scanning was carried out on a Siemens Somatom Definition AS 64-slice CT scanner (Siemens Healthineers, Forchheim, Germany), with contrast enhancement for better visualization of the fluid collection and lymph node enlargement.
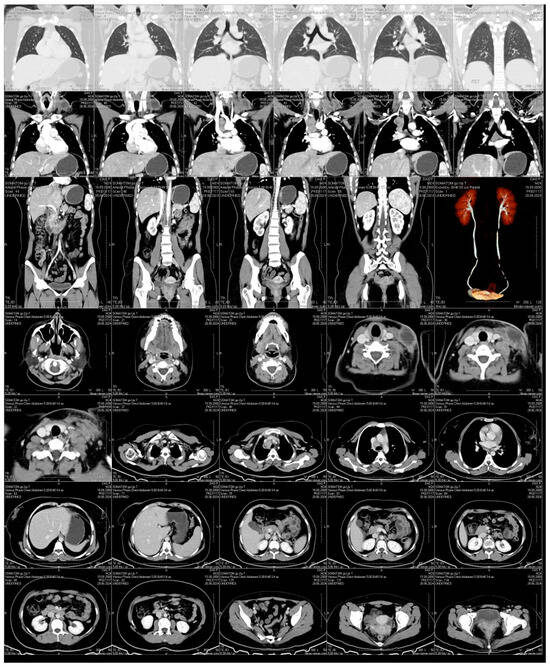
Figure 3.
Contrast-Enhanced CT Scan. An encapsulated fluid collection with well-defined margins is visualized in the left supraclavicular region, consistent with an abscess or a necrotically transformed lymph node. Prominent lymphadenopathy is also observed in the paratracheal and subcarinal regions, with evidence of central necrosis. No signs of pulmonary involvement or abdominal pathology are detected.
It is essential to emphasize the need for cytological and histopathological examinations, as only through these investigations was the case accurately elucidated. An extensive differential diagnosis excluded bacterial abscesses, hematologic malignancies, autoimmune disorders, and metastatic cancer. Diagnostic confirmation of tuberculous lymphadenitis was achieved through fine-needle aspiration cytology (FNAC), which showed caseating granulomatous inflammation, and PCR detection of Mycobacterium tuberculosis. The PCR assay targeted the IS6110 and MPB64 genes, both of which are specific for the M. tuberculosis complex, thereby enhancing the diagnostic accuracy. A positive IGRA, elevated ADA levels in aspirate, and positive TST further substantiated the diagnosis. Chest imaging ruled out pulmonary tuberculosis, supporting an extrapulmonary presentation. The PCR assay was conducted using a Roche LightCycler® 480 System, with primers and probes specific for the IS6110 and MPB64 genes, ensuring high sensitivity and specificity. The IGRA test was performed using the QuantiFERON®-TB Gold Plus assay (QIAGEN, Hilden, Germany), and ADA levels were measured using the Cobas 6000 analyzer (Roche Diagnostics).
2.5. Therapeutic Intervention
The initial antiviral regimen for COVID-19 consisted of nirmatrelvir (300 mg) plus ritonavir (100 mg) twice daily for five days, alongside azithromycin (500 mg/day for three days) and dexamethasone (6 mg/day for ten days), following the recommendations for managing moderate COVID-19 with suspected bacterial co-infection and inflammatory complications. Following EBV confirmation, acyclovir (400 mg five times daily for ten days) was administered, consistent with CDC-recommended antiviral therapy for immunocompetent individuals with severe or prolonged symptoms of Epstein–Barr virus. Symptomatic therapy with paracetamol and ibuprofen was administered as needed.
Upon confirmation of tuberculous lymphadenitis, the patient commenced standard first-line anti-tuberculosis therapy. The intensive phase included daily isoniazid (300 mg), rifampicin (600 mg), pyrazinamide (1500 mg), and ethambutol (1200 mg) for two months, with pyridoxine supplementation (25–50 mg/day) for neuroprotection. The continuation phase (currently ongoing) consists of isoniazid and rifampicin at the same dosages for at least four additional months. Treatment adherence has been consistent, with no modifications required due to side effects. This regimen corresponds to the standardized 2HRZE/4HR protocol. Treatment adherence has remained consistent, and no modifications have been necessary due to adverse drug reactions.
2.6. Follow-Up and Outcomes
After two months of anti-tuberculosis therapy, the patient demonstrated substantial clinical improvement. Fatigue, fever, and night sweats resolved, and the supraclavicular mass markedly regressed. Repeat CT imaging showed reduction in lymph node size and resolution of necrotic changes. Laboratory assessments revealed normalization of inflammatory markers, restoration of hematologic values, and improved immunological parameters. Microbiological testing of aspirated lymph node material was negative for M. tuberculosis, indicating microbiological clearance. No adverse drug reactions were reported. Treatment tolerance was excellent, with adherence assessed through regular clinical evaluations and patient self-report.
3. Discussion
The co-infection of SARS-CoV-2 and Epstein–Barr virus (EBV) presents a considerable immunological challenge, particularly due to its association with lymphopenia and broader implications for immune dysregulation (Figure 4) [23,24].
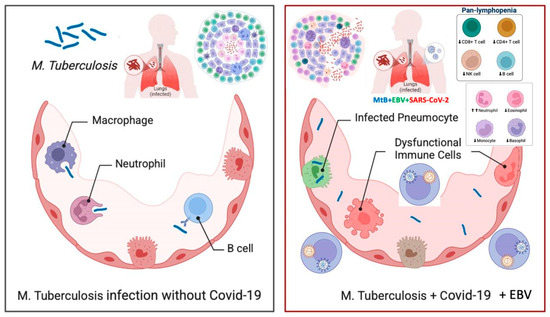
Figure 4.
The impact of SARS-CoV-2 and Epstein–Barr Virus (EBV) co-infection on tuberculosis progression. The figure compares tuberculosis infection in the absence (left) and presence (right) of SARS-CoV-2 and EBV co-infection. The left panel illustrates an effective immune response against Mycobacterium tuberculosis (M. tuberculosis), where macrophages, neutrophils, and B cells help control the infection. In contrast, the right panel shows how co-infection with SARS-CoV-2 and EBV leads to immune dysfunction, including infected pneumocytes, dysregulated immune cells, and pan-lymphopenia (reduced CD4+ T cells, CD8+ T cells, NK cells, and B cells). This weakened immune defense increases the risk of tuberculosis reactivation and progression, underscoring the need for close monitoring and early intervention in affected patients.
Lymphopenia is a well-documented consequence of both SARS-CoV-2 and EBV infections [25,26,27]. Notably, the reduction in CD4+ and CD8+ T lymphocytes impairs the host’s capacity to mount effective cellular immune responses, increasing the risk of reactivation of latent infections, including TB [28,29,30]. Host genetic predisposition may further influence immune resilience, severity of viral illness, and therapeutic outcomes. Genetic factors have been shown to modulate the likelihood of TB reactivation and determine the trajectory of post-viral immune recovery [31,32,33].
SARS-CoV-2 has been widely recognized for inducing a dysregulated immune response, often culminating in hyperinflammation or “cytokine storm” syndromes [34,35]. Elevated levels of interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) have been implicated in such states, contributing to systemic inflammation and subsequent immune suppression [36,37,38]. EBV, a ubiquitous herpesvirus capable of establishing latency in B cells, can similarly undermine immune homeostasis during periods of immune suppression or reactivation [39,40,41]. The synergistic effect of both viruses may extend the duration of lymphopenia and further diminish immune surveillance mechanisms, thereby promoting the progression of latent infections into active disease [42,43].
Latent tuberculosis remains a global health concern, with approximately one-quarter of the world’s population harboring dormant M. tuberculosis [44,45,46]. Normally, this latent state is contained through T-cell-mediated immunity and granuloma formation [47,48]. However, SARS-CoV-2 and EBV co-infection may disrupt this equilibrium [49,50,51]. Emerging evidence, including the present case, supports the hypothesis that viral infections can act as triggers for TB reactivation [52,53].
The depletion of CD4+ T cells, which play a pivotal role in granuloma formation and TB containment, further impairs the body’s ability to suppress active infection [54,55]. The compounded effects of SARS-CoV-2 and EBV on immune regulation may accelerate granuloma breakdown, progressing latent TB to an active disease state [50,56]. This highlights the need for proactive TB screening in patients with prolonged post-viral symptoms, especially in high-prevalence settings [57,58].
Given the overlapping symptomatology of COVID-19, EBV, and TB—such as fever, lymphadenopathy, and fatigue—accurate and timely differential diagnosis is essential [59,60]. Comprehensive diagnostics, including serological, molecular, and histopathological evaluation, are necessary to identify the causative agent [61,62,63]. Co-infections may obscure the clinical picture and delay treatment initiation [64,65,66], particularly in patients with comorbidities that further compromise immunity [67,68].
Identifying pharmacologic strategies to mitigate co-infection-related immune dysfunction remains a critical research priority [69,70,71,72]. Additionally, the immunosuppressive effects of corticosteroids or biologics—often used to manage severe COVID-19 or post-viral syndromes—may increase TB reactivation risk [52,73]. Optimal dosing strategies and treatment duration must be carefully evaluated, especially in individuals with prior TB exposure or sustained lymphopenia [74,75]. Alterations in gut microbiota (dysbiosis) may also influence immune regulation and disease severity, contributing to viral persistence and impaired containment of TB [76,77,78]. Comorbid conditions such as diabetes mellitus are known to worsen outcomes in both TB and COVID-19, complicating disease management [79,80,81].
The immunological interplay between SARS-CoV-2, EBV, and M. tuberculosis demands further investigation, particularly regarding long-term immune consequences and susceptibility to latent infection reactivation [49,82]. From a public health perspective, screening for latent TB infection in individuals recovering from severe viral illnesses may be warranted, especially in TB-endemic regions [83,84]. Early surveillance and consideration of preventive TB therapy could reduce the burden of reactivation [85,86,87]. Despite accumulating evidence, gaps remain in understanding the long-term immunologic consequences of SARS-CoV-2 and EBV co-infection. These areas require further research and integration into public health policy.
This case underscores the complexity of diagnosing and managing TB in the context of viral co-infections [60,88]. Unraveling the mechanisms of immune suppression and reactivation is essential for improving patient outcomes and reducing post-viral complications [89,90,91].
While no prior cases describing triple co-infection with COVID-19, EBV, and TB have been formally documented, existing studies support the pathophysiological plausibility and highlight the need for vigilance. EBV and COVID-19 co-infection has been linked to heightened inflammatory responses [92,93,94,95], while multiple reports have detailed the clinical consequences of TB-COVID-19 co-infection [96,97,98,99]. A systematic review of viral reactivations in COVID-19, including EBV, has further illustrated the relevance of these interactions [100].
Additional support for the possible causal relationship between COVID-19 and TB reactivation comes from previously published case reports and systematic reviews. Rawaa S. Al-kayali et al. described two patients who developed active TB after recovering from COVID-19, both presenting with persistent cough and fever—symptoms aligning with our case [101]. Moreover, a systematic review by Ayinalem Alemu et al. analyzed 33 cases from 21 studies across 13 countries, all documenting TB reactivation in COVID-19 survivors [102]. The median age of patients was 44 years (range: 13.5–80), and more than half were male. Notably, 62.5% had received corticosteroids for COVID-19 treatment. TB manifestations included pulmonary (60.6%), extrapulmonary (33.3%), and disseminated forms (6.1%). The review also found that TB onset could occur up to seven months post-COVID-19 recovery. These findings reinforce the hypothesis that immune suppression following SARS-CoV-2 infection may facilitate TB reactivation in predisposed individuals, as observed in our patient.
This case highlights how post-viral immune dysregulation may contribute to the reactivation of latent TB. Persistent lymphadenopathy and systemic inflammation, in the context of recent SARS-CoV-2 and EBV infections, required comprehensive diagnostic evaluation. Ultimately, biopsy and molecular diagnostics were key to confirming the diagnosis. Clinically, this case emphasizes the need for a high index of suspicion for TB in post-COVID-19 patients with persistent or unexplained symptoms. Future clinical care should integrate multidisciplinary collaboration to facilitate timely diagnosis and individualized management.
A limitation of this report is its single-case design. Although it offers insight into a potentially emerging clinical phenomenon, broader studies are needed to confirm these observations and deepen understanding of the underlying immune mechanisms.
Author Contributions
Conceptualization and writing—original draft preparation, I.H. and O.K.; writing—review and editing, I.H., P.P., V.O. and O.K.; supervision, O.K. and V.O.; project administration, V.O. and O.K.; visualization, I.H., P.P. and O.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol met the requirements for biomedical research and was approved by the Local Ethics Committee of the I. Horbachevsky Ternopil National Medical University as protocol No. 78, 18 August 2024.
Informed Consent Statement
The patient signed an informed consent for the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deveci, H.S.; Kule, M.; Kule, Z.A.; Habesoglu, T.E. Diagnostic challenges in cervical tuberculous lymphadenitis: A review. North. Clin. Istanb. 2016, 3, 150–155. [Google Scholar] [CrossRef]
- Gopalaswamy, R.; Dusthackeer, V.N.A.; Kannayan, S.; Subbian, S. Extrapulmonary Tuberculosis—An Update on the Diagnosis, Treatment and Drug Resistance. J. Respir. 2021, 1, 141–164. [Google Scholar] [CrossRef]
- Odumade, O.A.; Hogquist, K.A.; Balfour, H.H., Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin. Microbiol. Rev. 2011, 24, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Bernal, K.D.E.; Whitehurst, C.B. Incidence of Epstein-Barr virus reactivation is elevated in COVID-19 patients. Virus Res. 2023, 334, 199157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Q.; Zhang, B.; Dai, Y.; Gao, Y.; Li, C.; Yu, Y.; Li, C. Epstein–Barr Viruses: Their Immune Evasion Strategies and Implications for Autoimmune Diseases. Int. J. Mol. Sci. 2024, 25, 8160. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Z.; Prajapati, M.; Li, Y. Lymphopenia Caused by Virus Infections and the Mechanisms Beyond. Viruses 2021, 13, 1876. [Google Scholar] [CrossRef] [PubMed]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the Ageing Immune System. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef]
- Geng, L.; Wang, X. Epstein-Barr Virus-associated lymphoproliferative disorders: Experimental and clinical developments. Int. J. Clin. Exp. Med. 2015, 8, 14656–14671. [Google Scholar]
- Quintanilla-Martinez, L.; Swerdlow, S.H.; Tousseyn, T.; Barrionuevo, C.; Nakamura, S.; Jaffe, E.S. New concepts in EBV-associated B, T, and NK cell lymphoproliferative disorders. Virchows Arch. Int. J. Pathol. 2023, 482, 227–244. [Google Scholar] [CrossRef]
- Marrella, V.; Nicchiotti, F.; Cassani, B. Microbiota and Immunity during Respiratory Infections: Lung and Gut Affair. Int. J. Mol. Sci. 2024, 25, 4051. [Google Scholar] [CrossRef]
- Sankar, P.; Mishra, B.B. Early innate cell interactions with Mycobacterium tuberculosis in protection and pathology of tuberculosis. Front. Immunol. 2023, 14, 1260859. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Najafi-Fard, S.; Goletti, D. Initial immune response after exposure to Mycobacterium tuberculosis or to SARS-COV-2: Similarities and differences. Front. Immunol. 2023, 14, 1244556. [Google Scholar] [CrossRef] [PubMed]
- Petakh, P.; Kamyshna, I.; Oksenych, V.; Kamyshnyi, O. Metformin Alters mRNA Expression of FOXP3, RORC, and TBX21 and Modulates Gut Microbiota in COVID-19 Patients with Type 2 Diabetes. Viruses 2024, 16, 281. [Google Scholar] [CrossRef]
- Carabalí-Isajar, M.L.; Rodríguez-Bejarano, O.H.; Amado, T.; Patarroyo, M.A.; Izquierdo, M.A.; Lutz, J.R.; Ocampo, M. Clinical manifestations and immune response to tuberculosis. World J. Microbiol. Biotechnol. 2023, 39, 206. [Google Scholar] [CrossRef] [PubMed]
- Sia, J.K.; Rengarajan, J. Immunology of Mycobacterium tuberculosis Infections. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Thakkar, K.; Ghaisas, S.M.; Singh, M. Lymphadenopathy: Differentiation between Tuberculosis and Other Non-Tuberculosis Causes like Follicular Lymphoma. Front. Public Health 2016, 4, 31. [Google Scholar] [CrossRef]
- Cataño, J.C.; Robledo, J. Tuberculous Lymphadenitis and Parotitis. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Trapani, S.; Fiordelisi, A.; Stinco, M.; Resti, M. Update on Fever of Unknown Origin in Children: Focus on Etiologies and Clinical Approach. Children 2024, 11, 20. [Google Scholar] [CrossRef]
- Karaosmanoglu, A.D.; Uysal, A.; Onder, O.; Hahn, P.F.; Akata, D.; Ozmen, M.N.; Karcaaltıncaba, M. Cross-sectional imaging findings of splenic infections: Is differential diagnosis possible? Abdom. Radiol. 2021, 46, 4828–4852. [Google Scholar] [CrossRef]
- Khan, R. Lymph Node Disease and Advanced Head and Neck Imaging: A Review of the 2013 Literature. Curr. Radiol. Rep. 2014, 2, 58. [Google Scholar] [CrossRef]
- Chowdhury, R.; Turkdogan, S.; Alsayegh, R.; Almhanedi, H.; Al Majid, D.; Le Blanc, G.; Gerardis, G.; Himdi, L. Comprehensive Diagnostic Approach to Head and Neck Masses. J. Otorhinolaryngol. Hear. Balance Med. 2024, 5, 17. [Google Scholar] [CrossRef]
- Lyratzopoulos, G.; Vedsted, P.; Singh, H. Understanding missed opportunities for more timely diagnosis of cancer in symptomatic patients after presentation. Br. J. Cancer 2015, 112, S84–S91. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rasizadeh, R.; Sharaflou, S.; Aghbash, P.S.; Shamekh, A.; Jafari-Sales, A.; Bannazadeh Baghi, H. Coinfection of EBV with other pathogens: A narrative review. Front. Virol. 2024, 4, 1482329. [Google Scholar] [CrossRef]
- Sausen, D.G.; Poirier, M.C.; Spiers, L.M.; Smith, E.N. Mechanisms of T cell evasion by Epstein-Barr virus and implications for tumor survival. Front. Immunol. 2023, 14, 1289313. [Google Scholar] [CrossRef]
- Paolucci, S.; Cassaniti, I.; Novazzi, F.; Fiorina, L.; Piralla, A.; Comolli, G.; Bruno, R.; Maserati, R.; Gulminetti, R.; Novati, S.; et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int. J. Infect. Dis. 2021, 104, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.; Wolf, J.; Kalbitz, S.; Kellner, N.; Lübbert, C.; Borte, S. Comparative Analysis of Lymphocyte Populations in Post-COVID-19 Condition and COVID-19 Convalescent Individuals. Diagnostics 2024, 14, 1286. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; He, G. Hematological findings in coronavirus disease 2019: Indications of progression of disease. Ann. Hematol. 2020, 99, 1421–1428. [Google Scholar] [CrossRef]
- Hosseinian, K.; Gerami, A.; Bral, M.; Venketaraman, V. Mycobacterium tuberculosis-Human Immunodeficiency Virus Infection and the Role of T Cells in Protection. Vaccines 2024, 12, 730. [Google Scholar] [CrossRef]
- Morgan, J.; Muskat, K.; Tippalagama, R.; Sette, A.; Burel, J.; Lindestam Arlehamn, C.S. Classical CD4 T cells as the cornerstone of antimycobacterial immunity. Immunol. Rev. 2021, 301, 10–29. [Google Scholar] [CrossRef]
- McLaughlin, T.A.; Khayumbi, J.; Ongalo, J.; Tonui, J.; Campbell, A.; Allana, S.; Gurrion Ouma, S.; Odhiambo, F.H.; Gandhi, N.R.; Day, C.L. CD4 T Cells in Mycobacterium tuberculosis and Schistosoma mansoni Co-infected Individuals Maintain Functional TH1 Responses. Front. Immunol. 2020, 11, 127. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Budarna, O.; Halabitska, I.; Petakh, P.; Kamyshnyi, O. Genomic insight into COVID-19 severity in MAFLD patients: A single-center prospective cohort study. Front. Genet. 2024, 15, 1460318. [Google Scholar] [CrossRef] [PubMed]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Vorobets, I.; Halabitska, I.; Kamyshnyi, O. Modulatory Roles of AHR, FFAR2, FXR, and TGR5 Gene Expression in Metabolic-Associated Fatty Liver Disease and COVID-19 Outcomes. Viruses 2024, 16, 985. [Google Scholar] [CrossRef]
- Britton, W.J.; Fernando, S.L.; Saunders, B.M.; Sluyter, R.; Wiley, J.S. The genetic control of susceptibility to Mycobacterium tuberculosis. In Homology (Novartis Foundation Symposia); Wiley: Hoboken, NJ, USA, 2007; Volume 281, pp. 79–89. [Google Scholar]
- Weatherhead, J.E.; Clark, E.; Vogel, T.P.; Atmar, R.L.; Kulkarni, P.A. Inflammatory syndromes associated with SARS-CoV-2 infection: Dysregulation of the immune response across the age spectrum. J. Clin. Investig. 2020, 130, 6194–6197. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Que, Y.; Hu, C.; Wan, K.; Hu, P.; Wang, R.; Luo, J.; Li, T.; Ping, R.; Hu, Q.; Sun, Y.; et al. Cytokine release syndrome in COVID-19: A major mechanism of morbidity and mortality. Int. Rev. Immunol. 2022, 41, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, Y.; Ghasemi, M.; Yassaghi, Y.; Nazerian, A.; Hashemi, S.M. Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options. Int. Immunopharmacol. 2022, 113 Pt B, 109428. [Google Scholar] [CrossRef]
- Müller-Durovic, B.; Jäger, J.; Bantug, G.R.; Hess, C. Epstein-Barr virus hijacks B cell metabolism to establish persistent infection and drive pathogenesis. Trends Immunol. 2025, 46, 7–16. [Google Scholar] [CrossRef]
- Weidner-Glunde, M.; Kruminis-Kaszkiel, E.; Savanagouder, M. Herpesviral Latency—Common Themes. Pathogens 2020, 9, 125. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, M. EBV-induced T-cell responses in EBV-specific and nonspecific cancers. Front. Immunol. 2023, 14, 1250946. [Google Scholar] [CrossRef]
- Dropulic, L.K.; Lederman, H.M. Overview of Infections in the Immunocompromised Host. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Baral, B.; Saini, V.; Kandpal, M.; Kundu, P.; Dixit, A.K.; Parmar, H.S.; Meena, A.K.; Trivedi, P.; Jha, H.C. The interplay of co-infections in shaping COVID-19 severity: Expanding the scope beyond SARS-CoV-2. J. Infect. Public Health 2024, 17, 102486. [Google Scholar] [CrossRef]
- Kiazyk, S.; Ball, T.B. Latent tuberculosis infection: An overview. Can. Commun. Dis. Rep. 2017, 43, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.K.; Karakousis, P.C. Latent tuberculosis infection: Myths, models, and molecular mechanisms. Microbiol. Mol. Biol. Rev. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, J.; Sounderrajan, V.; Thangam, T.; Rao, S.; Harshavardhan, S.; Parthasarathy, K. Latent Tuberculosis: Challenges in Diagnosis and Treatment, Perspectives, and the Crucial Role of Biomarkers. Curr. Microbiol. 2023, 80, 392. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Flynn, J.L. Understanding latent tuberculosis: A moving target. J. Immunol. 2010, 185, 15–22. [Google Scholar] [CrossRef]
- Lin, P.L.; Flynn, J.L. CD8 T cells and Mycobacterium tuberculosis infection. Semin. Immunopathol. 2015, 37, 239–249. [Google Scholar] [CrossRef]
- Chinna, P.; Bratl, K.; Lambarey, H.; Blumenthal, M.J.; Schäfer, G. The Impact of Co-Infections for Human Gammaherpesvirus Infection and Associated Pathologies. Int. J. Mol. Sci. 2023, 24, 13066. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Saidara, E.; Maes, M. Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID. Viruses 2023, 15, 400. [Google Scholar] [CrossRef]
- Naidu, A.S.; Wang, C.K.; Rao, P.; Mancini, F.; Clemens, R.A.; Wirakartakusumah, A.; Chiu, H.F.; Yen, C.H.; Porretta, S.; Mathai, I.; et al. Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID. npj Sci. Food 2024, 8, 19. [Google Scholar] [CrossRef]
- Dormans, T.; Zandijk, E.; Stals, F. Late Tuberculosis Reactivation After Severe COVID-19. Eur. J. Case Rep. Intern. Med. 2024, 11, 004406. [Google Scholar] [CrossRef] [PubMed]
- Alene, K.A.; Wangdi, K.; Clements, A.C.A. Impact of the COVID-19 Pandemic on Tuberculosis Control: An Overview. Trop. Med. Infect. Dis. 2020, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Hoerter, A.; Arnett, E.; Schlesinger, L.S.; Pienaar, E. Systems biology approaches to investigate the role of granulomas in TB-HIV coinfection. Front. Immunol. 2022, 13, 1014515. [Google Scholar] [CrossRef]
- Yang, H.; Lei, X.; Chai, S.; Su, G.; Du, L. From pathogenesis to antigens: The key to shaping the future of TB vaccines. Front. Immunol. 2024, 15, 1440935. [Google Scholar] [CrossRef] [PubMed]
- Booysen, P.; Wilkinson, K.A.; Sheerin, D.; Waters, R.; Coussens, A.K.; Wilkinson, R.J. Immune interaction between SARS-CoV-2 and Mycobacterium tuberculosis. Front. Immunol. 2023, 14, 1254206. [Google Scholar] [CrossRef]
- Tan, M.; Menzies, D.; Schwartzman, K. Tuberculosis screening of travelers to higher-incidence countries: A cost-effectiveness analysis. BMC Public Health 2008, 8, 201. [Google Scholar] [CrossRef]
- Bhatt, A.; Quazi Syed, Z.; Singh, H. Converging Epidemics: A Narrative Review of Tuberculosis (TB) and Human Immunodeficiency Virus (HIV) Coinfection. Cureus 2023, 15, e47624. [Google Scholar] [CrossRef]
- Cioboata, R.; Biciusca, V.; Olteanu, M.; Vasile, C.M. COVID-19 and Tuberculosis: Unveiling the Dual Threat and Shared Solutions Perspective. J. Clin. Med. 2023, 12, 4784. [Google Scholar] [CrossRef]
- Whittaker, E.; López-Varela, E.; Broderick, C.; Seddon, J.A. Examining the Complex Relationship Between Tuberculosis and Other Infectious Diseases in Children. Front. Pediatr. 2019, 7, 233. [Google Scholar] [CrossRef]
- Ali, M.; Elhatw, A.; Hegazy, M.; Albeyoumi, H.; Sakr, N.; Deyab, A.M.; Soliman, A.Y.; Said, E.; Samir Elbehwashy, A.; Nassar, M.; et al. The Evaluation of Lymphadenopathy in a Resource-Limited Setting. Cureus 2022, 14, e30623. [Google Scholar] [CrossRef]
- Mohseni, S.; Shojaiefard, A.; Khorgami, Z.; Alinejad, S.; Ghorbani, A.; Ghafouri, A. Peripheral lymphadenopathy: Approach and diagnostic tools. Iran. J. Med. Sci. 2014, 39 (Suppl. 2), 158–170. [Google Scholar] [PubMed]
- Petakh, P.; Kobyliak, N.; Kamyshnyi, A. Gut microbiota in patients with COVID-19 and type 2 diabetes: A culture-based method. Front. Cell. Infect. Microbiol. 2023, 13, 1142578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, J.; Gao, Q.; Zhao, Y.; Lai, Y. Clinical and immunologic features of co-infection in COVID-19 patients, along with potential traditional Chinese medicine treatments. Front. Immunol. 2024, 15, 1357638. [Google Scholar] [CrossRef]
- Mohan, S.M.K.; Vijayaraghavan, A.; Sundaram, S.; Nair, S.; Sukumaran, S. Post-infectious Neurological Complications of COVID-19—A tertiary care centre experience. J. Clin. Virol. Plus 2023, 3, 100165. [Google Scholar] [CrossRef]
- Petakh, P.; Oksenych, V.; Kamyshnyi, A. The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin. Biomed. Pharmacother. 2023, 163, 114892. [Google Scholar] [CrossRef]
- Halabitska, I.; Babinets, L.; Oksenych, V.; Kamyshnyi, O. Diabetes and Osteoarthritis: Exploring the Interactions and Therapeutic Implications of Insulin, Metformin, and GLP-1-Based Interventions. Biomedicines 2024, 12, 1630. [Google Scholar] [CrossRef]
- Callender, L.A.; Curran, M.; Bates, S.M.; Mairesse, M.; Weigandt, J.; Betts, C.J. The Impact of Pre-existing Comorbidities and Therapeutic Interventions on COVID-19. Front. Immunol. 2020, 11, 1991. [Google Scholar] [CrossRef]
- Lebu, S.; Kibone, W.; Muoghalu, C.C.; Ochaya, S.; Salzberg, A.; Bongomin, F.; Manga, M. Soil-transmitted helminths: A critical review of the impact of co-infections and implications for control and elimination. PLoS Neglected Trop. Dis. 2023, 17, e0011496. [Google Scholar] [CrossRef] [PubMed]
- Halabitska, I.; Oksenych, V.; Kamyshnyi, O. Exploring the Efficacy of Alpha-Lipoic Acid in Comorbid Osteoarthritis and Type 2 Diabetes Mellitus. Nutrients 2024, 16, 3349. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Kamyshnyi, A. Gene expression of protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), solute carrier family 2 member 1 (SLC2A1) and mechanistic target of rapamycin (MTOR) in metformin-treated type 2 diabetes patients with COVID-19: Impact on inflammation markers. Inflammopharmacology 2024, 32, 885–891. [Google Scholar]
- Buchynskyi, M.; Kamyshna, I.; Lyubomirskaya, K.; Moshynets, O.; Kobyliak, N.; Oksenych, V.; Kamyshnyi, A. Efficacy of interferon alpha for the treatment of hospitalized patients with COVID-19: A meta-analysis. Front. Immunol. 2023, 14, 1069894. [Google Scholar] [CrossRef]
- Picchianti Diamanti, A.; Rosado, M.M.; Pioli, C.; Sesti, G.; Laganà, B. Cytokine Release Syndrome in COVID-19 Patients, A New Scenario for an Old Concern: The Fragile Balance between Infections and Autoimmunity. Int. J. Mol. Sci. 2020, 21, 3330. [Google Scholar] [CrossRef]
- Nahid, P.; Dorman, S.E.; Alipanah, N.; Barry, P.M.; Brozek, J.L.; Cattamanchi, A.; Chaisson, L.H.; Chaisson, R.E.; Daley, C.L.; Grzemska, M.; et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin. Infect. Dis. 2016, 63, e147–e195. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, D.; Zeng, Q.; Du, Y. Possible Mechanisms of Lymphopenia in Severe Tuberculosis. Microorganisms 2023, 11, 2640. [Google Scholar] [CrossRef] [PubMed]
- Petakh, P.; Kamyshna, I.; Nykyforuk, A.; Yao, R.; Imbery, J.F.; Oksenych, V.; Korda, M.; Kamyshnyi, A. Immunoregulatory Intestinal Microbiota and COVID-19 in Patients with Type Two Diabetes: A Double-Edged Sword. Viruses 2022, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Petakh, P.; Griga, V.; Mohammed, I.B.; Loshak, K.; Poliak, I.; Kamyshnyiy, A. Effects of Metformin, Insulin on Hematological Parameters of COVID-19 Patients with Type 2 Diabetes. Med. Arch. 2022, 76, 329–332. [Google Scholar] [CrossRef]
- Comberiati, P.; Di Cicco, M.; Paravati, F.; Pelosi, U.; Di Gangi, A.; Arasi, S.; Barni, S.; Caimmi, D.; Mastrorilli, C.; Licari, A.; et al. The Role of Gut and Lung Microbiota in Susceptibility to Tuberculosis. Int. J. Environ. Res. Public Health 2021, 18, 12220. [Google Scholar] [CrossRef]
- Halabitska, I.; Petakh, P.; Kamyshna, I.; Oksenych, V.; Kainov, D.E.; Kamyshnyi, O. The interplay of gut microbiota, obesity, and depression: Insights and interventions. Cell. Mol. Life Sci. 2024, 81, 443. [Google Scholar] [CrossRef]
- Gęca, T.; Wojtowicz, K.; Guzik, P.; Góra, T. Increased Risk of COVID-19 in Patients with Diabetes Mellitus-Current Challenges in Pathophysiology, Treatment and Prevention. Int. J. Environ. Res. Public Health 2022, 19, 6555. [Google Scholar] [CrossRef]
- Halabitska, I.; Petakh, P.; Lushchak, O.; Kamyshna, I.; Oksenych, V.; Kamyshnyi, O. Metformin in Antiviral Therapy: Evidence and Perspectives. Viruses 2024, 16, 1938. [Google Scholar] [CrossRef]
- Dass, S.A.; Balakrishnan, V.; Arifin, N.; Lim, C.S.Y.; Nordin, F.; Tye, G.J. The COVID-19/Tuberculosis Syndemic and Potential Antibody Therapy for TB Based on the Lessons Learnt from the Pandemic. Front. Immunol. 2022, 13, 833715. [Google Scholar] [CrossRef] [PubMed]
- Fehily, S.R.; Al-Ani, A.H.; Abdelmalak, J.; Rentch, C.; Zhang, E.; Denholm, J.T.; Johnson, D.; Ng, S.C.; Sharma, V.; Rubin, D.T.; et al. Review article: Latent tuberculosis in patients with inflammatory bowel diseases receiving immunosuppression-risks, screening, diagnosis and management. Aliment. Pharmacol. Ther. 2022, 56, 6–27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Goletti, D.; Pisapia, R.; Fusco, F.M.; Aiello, A.; Van Crevel, R. Epidemiology, pathogenesis, clinical presentation and management of TB in patients with HIV and diabetes. Int. J. Tuberc. Lung Dis. 2023, 27, 284–290. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef]
- Zhuang, L.; Yang, L.; Li, L.; Ye, Z.; Gong, W. Mycobacterium tuberculosis: Immune response, biomarkers, and therapeutic intervention. MedComm 2024, 5, e419. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Paladin, F.; Mangini, G.; Tiso, D.; Gangemi, S. TBC and COVID: An interplay between two infections. Expert Opin. Drug Saf. 2023, 22, 303–311. [Google Scholar] [CrossRef]
- Sharan, R.; Bucşan, A.N.; Ganatra, S.; Paiardini, M.; Mohan, M.; Mehra, S.; Khader, S.A.; Kaushal, D. Chronic Immune Activation in TB/HIV Co-infection. Trends Microbiol. 2020, 28, 619–632. [Google Scholar] [CrossRef]
- Olivier, C.; Luies, L. Metabolic insights into HIV/TB co-infection: An untargeted urinary metabolomics approach. Metabolomics 2024, 20, 78. [Google Scholar] [CrossRef]
- Herbert, C.; Luies, L.; Loots, D.T.; Williams, A.A. The metabolic consequences of HIV/TB co-infection. BMC Infect. Dis. 2023, 23, 536. [Google Scholar] [CrossRef]
- Nadeem, A.; Suresh, K.; Awais, H.; Waseem, S. Epstein-Barr Virus Coinfection in COVID-19. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211040626. [Google Scholar] [CrossRef]
- Shafiee, A.; Aghajanian, S.; Athar, M.M.T.; Gargari, O.K. Epstein-Barr virus and COVID-19. J. Med. Virol. 2022, 94, 4040–4042. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, L.; Qu, J.; Wang, C.; Shi, X.; Lei, Y. Chronic active Epstein-Barr virus infection with reinfection of SARS-CoV-2: A case report. Virol. J. 2024, 21, 142. [Google Scholar] [CrossRef]
- Rousseau, B.A.; Bhaduri-McIntosh, S. Inflammation and Epstein–Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection. Viruses 2023, 15, 949. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liang, M.; Wang, L.; Zhang, M.; Liu, R.; Liang, H.; Wang, C.; Jia, L.; Zeng, Q.; Zhu, P. Clinical characteristics and outcomes of patients with COVID-19 and tuberculosis coinfection. Infect. Dis. 2023, 55, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Mahmood, K.; Ahmad, L.; Gul, H.; Hayat, A.; Ur Rehman, M. Clinical manifestations of active tuberculosis patients coinfected with severe acute respiratory syndrome coronavirus-2. J. Clin. Tuberc. Other Mycobact. Dis. 2023, 31, 100359. [Google Scholar] [CrossRef]
- Jhaveri, T.A.; Fung, C.; LaHood, A.N.; Lindeborg, A.; Zeng, C.; Rahman, R.; Bain, P.A.; Velásquez, G.E.; Mitnick, C.D. Clinical Outcomes of Individuals with COVID-19 and Tuberculosis during the Pre-Vaccination Period of the Pandemic: A Systematic Review. J. Clin. Med. 2022, 11, 5656. [Google Scholar] [CrossRef]
- Song, W.M.; Zhao, J.Y.; Zhang, Q.Y.; Liu, S.Q.; Zhu, X.H.; An, Q.Q.; Xu, T.T.; Li, S.J.; Liu, J.Y.; Tao, N.N.; et al. COVID-19 and Tuberculosis Coinfection: An Overview of Case Reports/Case Series and Meta-Analysis. Front. Med. 2021, 8, 657006. [Google Scholar] [CrossRef]
- Kim, J.Y.H.; Ragusa, M.; Tortosa, F.; Torres, A.; Gresh, L.; Méndez-Rico, J.A.; Alvarez-Moreno, C.A.; Lisboa, T.C.; Valderrama-Beltrán, S.L.; Aldighieri, S.; et al. Viral reactivations and co-infections in COVID-19 patients: A systematic review. BMC Infect. Dis. 2023, 23, 259. [Google Scholar] [CrossRef]
- Al-Kayali, R.S.; Kashkash, M.F.; Alhussein Alhajji, A.H.; Khouri, A. Activation of tuberculosis in recovered COVID-19 patients: A case report. Ann. Med. Surg. 2023, 85, 280–283. [Google Scholar] [CrossRef]
- Alemu, A.; Bitew, Z.W.; Seid, G.; Diriba, G.; Gashu, E.; Berhe, N.; Mariam, S.H.; Gumi, B. Tuberculosis in individuals who recovered from COVID-19: A systematic review of case reports. PLoS ONE 2022, 17, e0277807. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).