Molecular Detection of Encephalitozoon cuniculi in Migratory Waterfowl of the Genus Anser (Anseriformes: Anatidae) in Poland
Abstract
1. Introduction
2. Material and Methods
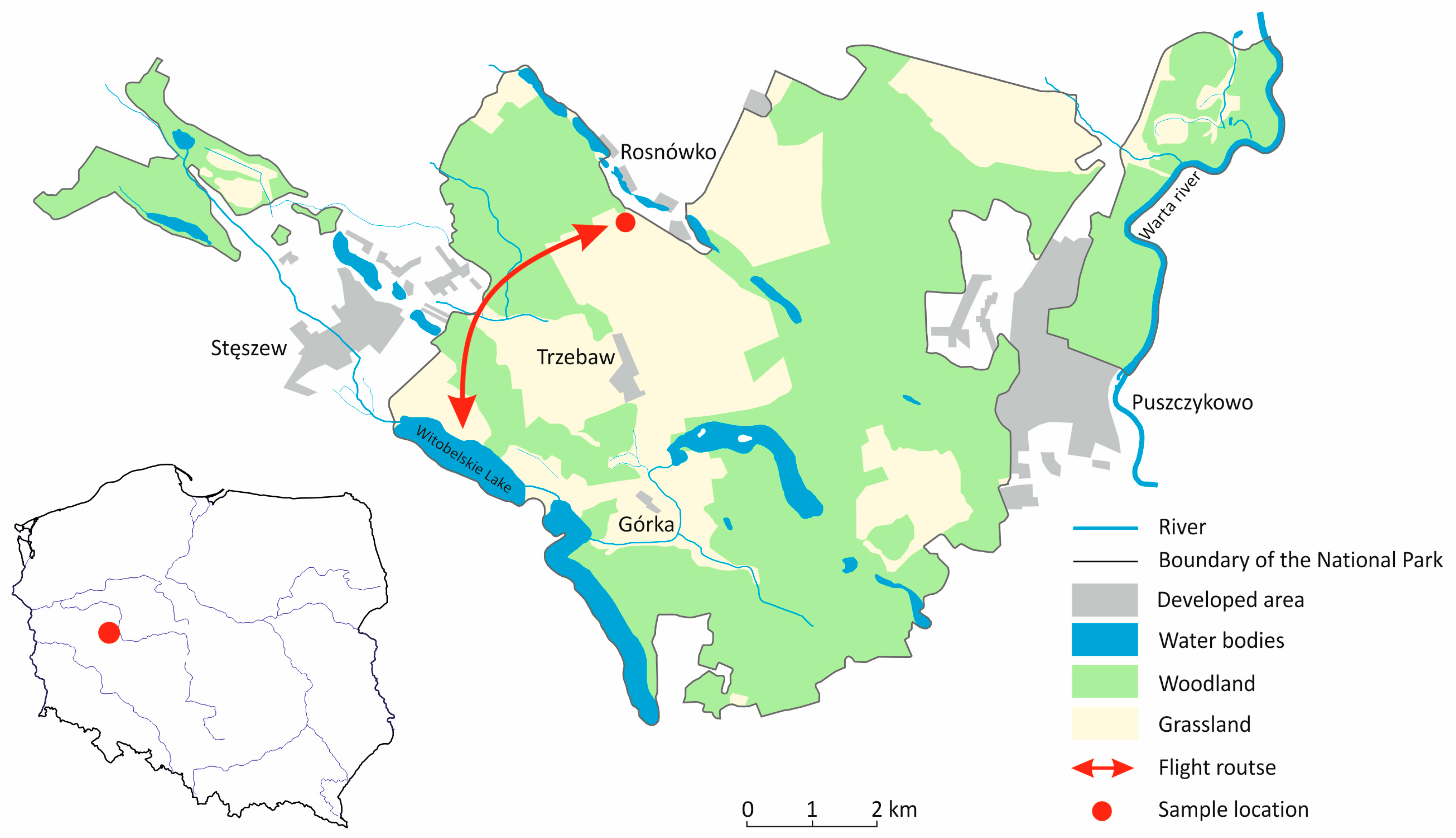
2.1. Study Area
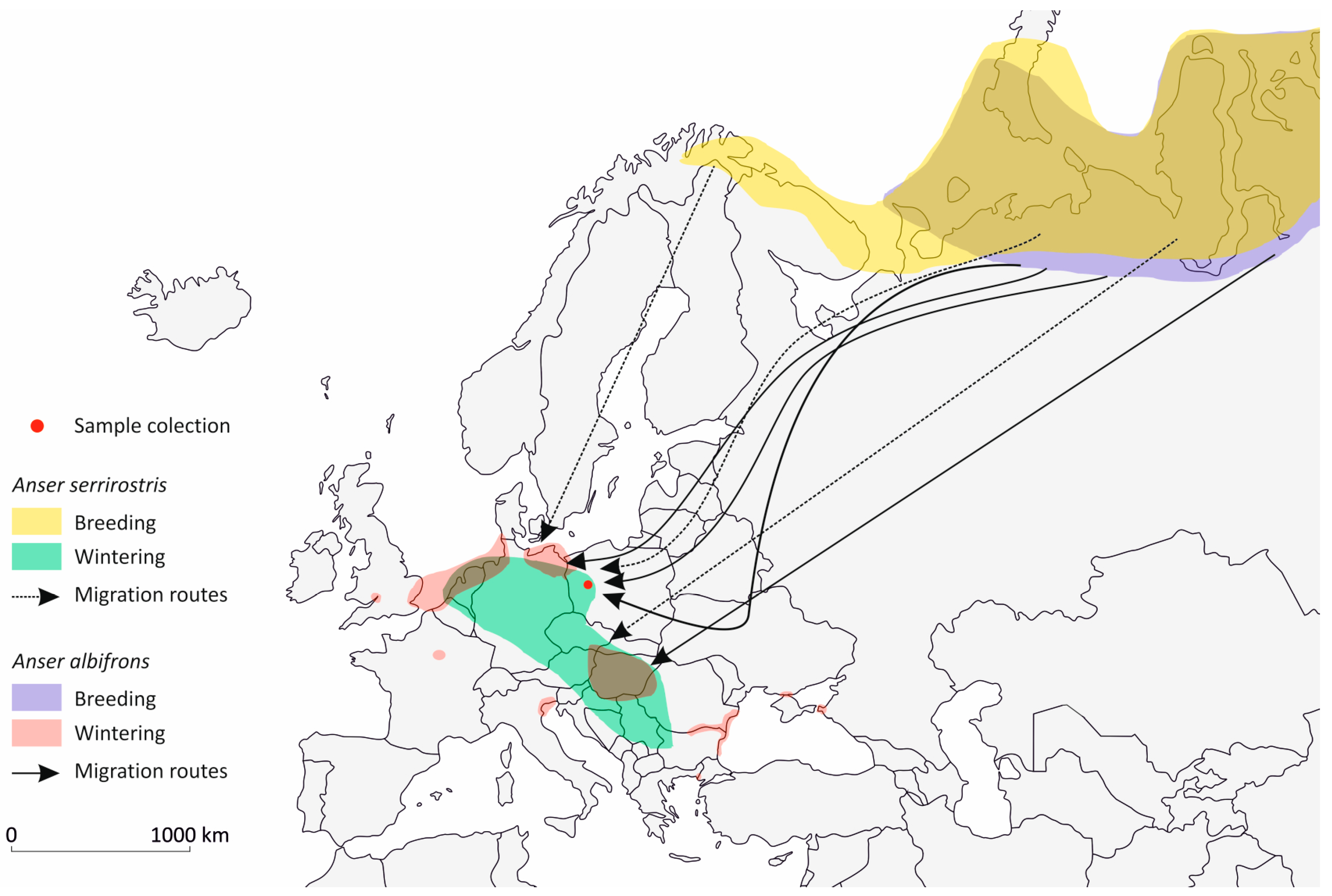
2.2. Sample Collection
2.3. Molecular Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trzebny, A.; Mizera, J.; Dabert, M. Microsporidians (Microsporidia) parasitic on mosquitoes (Culicidae) in central Europe are often multi-host species. J. Invertebr. Pathol. 2023, 197, 107873. [Google Scholar] [CrossRef] [PubMed]
- Didier, E.S.; Weiss, L.M. Microsporidiosis: Not just in AIDS patients. Curr. Opin. Infect. Dis. 2011, 24, 490–495. [Google Scholar] [CrossRef]
- Murareanu, B.M.; Sukhdeo, R.; Qu, R.; Jiang, J.; Reinke, A.W. Generation of a Microsporidia Species Attribute Database and Analysis of the Extensive Ecological and Phenotypic Diversity of Microsporidia. mBio 2021, 12, e0149021. [Google Scholar] [CrossRef] [PubMed]
- Anane, S.; Attouchi, H. Microsporidiosis: Epidemiology, clinical data and therapy. Gastroenterol. Clin. Biol. 2010, 34, 450–564. [Google Scholar] [CrossRef]
- Han, B.; Pan, G.; Weiss, L.M. Microsporidiosis in Humans. Clin. Microbiol. Rev. 2021, 34, e0001020. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Mathis, A.; Weber, R. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 2000, 6, 236–260. [Google Scholar] [CrossRef]
- Magalhães, T.R.; Pinto, F.F.; Queiroga, F.L. A multidisciplinary review about Encephalitozoon cuniculi in a One Health perspective. Parasitol. Res. 2022, 121, 2463–2479. [Google Scholar] [CrossRef]
- Solarczyk, P.; Wojtkowiak-Giera, A.; Heddergott, M. Migrating Anatidae as Sources of Environmental Contamination with Zoonotic Giardia, Cryptosporidium, Cyclospora and Microsporidia. Pathogens 2023, 12, 487. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Zhang, Y.; Wang, K.; Gazizova, A.; Wang, L.; Cao, L.; Zhang, Y.; Huang, J.; Cui, Y.; et al. First detection of Enterocytozoon bieneusi in whooper swans (Cygnus cygnus) in China. Parasit. Vectors 2020, 13, 5. [Google Scholar] [CrossRef]
- Tsukada, R.; Tsuchiyama, A.; Sasaki, M.; Park, C.H.; Fujii, Y.; Takesue, M.; Hatai, H.; Kudo, N.; Ikadai, H. Encephalitozoon infections in Rodentia and Soricomorpha in Japan. Vet. Parasitol. 2013, 198, 193–196. [Google Scholar] [CrossRef]
- Fuehrer, H.P.; Blöschl, I.; Siehs, C.; Hassl, A. Detection of Toxoplasma gondii, Neospora caninum, and Encephalitozoon cuniculi in the brains of common voles (Microtus arvalis) and water voles (Arvicola terrestris) by gene amplification techniques in western Austria (Vorarlberg). Parasitol. Res. 2010, 107, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Danišová, O.; Valenčáková, A.; Stanko, M.; Luptáková, L.; Hasajová, A. First report of Enterocytozoon bieneusi and Encephalitozoon intestinalis infection of wild mice in Slovakia. Ann. Agric. Environ. Med. 2015, 22, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Wuczyński, A.; Smyk, B.; Kołodziejczyk, P.; Lenkiewicz, W.; Orłowski, G.; Pola, A. Long-term changes in numbers of geese stopping over and wintering in south-western Poland. Cent. Eur. J. Biol. 2012, 7, 495–506. [Google Scholar] [CrossRef]
- Bednarska, M.; Bajer, A.; Graczyk, T.K.; Siński, E. Opportunistic parasites in immunocompetent and immunodeficient patients with diarrhea. In Proceedings of the 3rd International Giardia and Cryptosporidium Conference, Orvieto, Italy, 11–15 October 2009; Abstract P109. Local Organising Committee, Istituto Superiore di Sanita: Rome, Italy, 2009. [Google Scholar]
- Kicia, M.; Szydłowicz, M.; Cebulski, K.; Jakuszko, K.; Piesiak, P.; Kowal, A.; Sak, B.; Krajewska, M.; Hendrich, A.B.; Kváč, M.; et al. Symptomatic respiratory Encephalitozoon cuniculi infection in renal transplant recipients. Int. J. Infect. Dis. 2019, 79, 21–25. [Google Scholar] [CrossRef]
- Leśniańska, K.; Perec-Matysiak, A.; Hildebrand, J.; Buńkowska-Gawlik, K.; Piróg, A.; Popiołek, M. Cryptosporidium spp. and Enterocytozoon bieneusi in introduced raccoons (Procyon lotor)—First evidence from Poland and Germany. Parasitol. Res. 2016, 115, 4535–4541. [Google Scholar] [CrossRef]
- Perec-Matysiak, A.; Leśniańska, K.; Buńkowska-Gawlik, K.; Merta, D.; Popiołek, M.; Hildebrand, J. Zoonotic Genotypes of Enterocytozoon bieneusi in Wild Living Invasive and Native Carnivores in Poland. Pathogens 2021, 10, 1478. [Google Scholar] [CrossRef]
- Perec-Matysiak, A.; Buńkowska-Gawlik, K.; Kváč, M.; Sak, B.; Hildebrand, J.; Leśniańska, K. Diversity of Enterocytozoon bieneusi genotypes among small rodents in southwestern Poland. Vet. Parasitol. 2015, 214, 242–246. [Google Scholar] [CrossRef]
- Słodkowicz-Kowalska, A.; Graczyk, T.K.; Tamang, L.; Girouard, A.S. Asymptomatic Enterocytozoon bieneusi microsporidiosis in captive mammals. Parasitol. Res. 2007, 100, 505–509. [Google Scholar] [CrossRef]
- Ruan, Y.; Xu, X.; He, Q.; Li, L.; Guo, J.; Bao, J.; Pan, G.; Li, T.; Zhou, Z. The largest meta-analysis on the global prevalence of microsporidia in mammals, avian and water provides insights into the epidemic features of these ubiquitous pathogens. Parasit. Vectors 2021, 14, 186. [Google Scholar] [CrossRef]
- Solon, J.; Borzyszkowski, J.; Bidłasik, M.; Richling, A.; Badora, K.; Balon, J.; Brzezińska-Wójcik, T.; Chabudziński, Ł.; Dobrowolski, R.; Grzegorczyk, I.; et al. Physico-geographical mesoregions of Poland: Verification and adjustment of boundaries on the basis of contemporary spatial data. Geogr. Pol. 2018, 91, 143–170. [Google Scholar] [CrossRef]
- Ławicki, Ł.; Wylegała, P.; Wuczyński, A.; Smyk, B.; Lenkiewicz, W.; Polakowski, M.; Kruszyk, R.; Rubacha, S.; Janiszewski, T. Distribution, characteristics and conservation status of geese roosts in Poland. Ornis Pol. 2012, 53, 23–38. [Google Scholar]
- Nicholas, K.B.; Nicholas, H.B. GeneDoc: A tool for editing and annotating multiple sequence alignments. Comput. Sci. Biol. 2007, 8, 38. [Google Scholar]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 2010; ISBN 978-0-13-100846-5. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 2 February 2023).
- Visvesvara, G.S.; Leitch, G.J.; da Silva, A.J.; Croppo, G.P.; Moura, H.; Wallace, S.; Slemenda, S.B.; Schwartz, D.A.; Moss, D.; Bryan, R.T.; et al. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J. Clin. Microbiol. 1994, 32, 2760–2776. [Google Scholar] [CrossRef]
- Kasicková, D.; Sak, B.; Kvác, M.; Ditrich, O. Detection of Encephalitozoon cuniculi in a new host--cockateel (Nymphicus hollandicus) using molecular methods. Parasitol. Res. 2007, 101, 1685–1688. [Google Scholar] [CrossRef]
- Malčekova, B.; Valencakova, A.; Luptakova, L.; Molnar, L.; Ravaszova, P.; Novotny, F. First detection and genotyping of Encephalitozoon cuniculi in a new host species, gyrfalcon (Falco rusticolus). Parasitol. Res. 2011, 108, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zheng, J.; He, X.; Jia, H.; Zhang, Y. First characterization in China of Encephalitozoon cuniculi in the blue fox (Alopex lagopus). J. Eukaryot. Microbiol. 2014, 61, 580–585. [Google Scholar] [CrossRef]
- Reetz, J.; Nöckler, K.; Reckinger, S.; Vargas, M.M.; Weiske, W.; Broglia, A. Identification of Encephalitozoon cuniculi genotype III and two novel genotypes of Enterocytozoon bieneusi in swine. Parasitol. Int. 2009, 58, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wagnerová, P.; Sak, B.; Květoňová, D.; Buňatová, Z.; Civišová, H.; Maršálek, M.; Kváč, M. Enterocytozoon bieneusi and Encephalitozoon cuniculi in horses kept under different management systems in the Czech Republic. Vet. Parasitol. 2012, 190, 573–757. [Google Scholar] [CrossRef]
- Marková, J.; Machačová, T.; Bártová, E.; Sedlák, K.; Budíková, M.; Silvestre, P.; Laricchiuta, P.; Russo, M.; Veneziano, V. Toxoplasma gondii, Neospora caninum and Encephalitozoon cuniculi in Animals from Captivity (Zoo and Circus Animals). J. Eukaryot. Microbiol. 2019, 66, 442–446. [Google Scholar] [CrossRef]
- Doboși, A.A.; Bel, L.V.; Paștiu, A.I.; Pusta, D.L. A Review of Encephalitozoon cuniculi in Domestic Rabbits (Oryctolagus cuniculus)—Biology, Clinical Signs, Diagnostic Techniques, Treatment, and Prevention. Pathogens 2022, 11, 1486. [Google Scholar] [CrossRef] [PubMed]
- Kicia, M.; Zajączkowska, Ż.; Kváč, M.; Cebulski, K.; Holubová, N.; Wencel, P.; Mayer, L.; Wesołowska, M.; Sak, B. Encephalitozoon cuniculi and Extraintestinal Microsporidiosis in Bird Owners. Emerg. Infect. Dis. 2022, 28, 705–708. [Google Scholar] [CrossRef]
- Thurston-Enriquez, J.A.; Watt, P.; Dowd, S.E.; Enriquez, R.; Pepper, I.L.; Gerba, C.P. Detection of protozoan parasites and microsporidia in irrigation waters used for crop production. J. Food. Prot. 2002, 65, 378–382. [Google Scholar] [CrossRef]
- Sak, B.; Vecková, T.; Brdíčková, K.; Smetana, P.; Hlásková, L.; Kicia, M.; Holubová, N.; McEvoy, J.; Kváč, M. Experimental Encephalitozoon cuniculi Infection Acquired from Fermented Meat Products. Foodborne Pathog. Dis. 2019, 16, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Sak, B.; Kvác, M.; Petrzelková, K.; Kvetonová, D.; Pomajbíková, K.; Mulama, M.; Kiyang, J.; Modrý, D. Diversity of microsporidia (Fungi: Microsporidia) among captive great apes in European zoos and African sanctuaries: Evidence for zoonotic transmission? Folia Parasitol. 2011, 58, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Malčeková, B.; Valenčáková, A.; Molnár, L.; Kočišová, A. First detection and genotyping of human-associated microsporidia in wild waterfowl of Slovakia. Acta Parasitol. 2013, 58, 13–17. [Google Scholar] [CrossRef]
- Slodkowicz-Kowalska, A.; Graczyk, T.K.; Tamang, L.; Jedrzejewski, S.; Nowosad, A.; Zduniak, P.; Solarczyk, P.; Girouard, A.S.; Majewska, A.C. Microsporidian species known to infect humans are present in aquatic birds: Implications for transmission via water? Appl. Environ. Microbiol. 2006, 72, 4540–4544. [Google Scholar] [CrossRef]
- Malčeková, B.; Halánová, M.; Sulínová, Z.; Molnár, L.; Ravaszová, P.; Adam, J.; Halán, M.; Valocký, I.; Baranovič, M. Seroprevalence of antibodies to Encephalitozoon cuniculi and Encephalitozoon intestinalis in humans and animals. Res. Vet. Sci. 2010, 89, 358–361. [Google Scholar] [CrossRef]
- Didier, E.S. Microsporidiosis: An emerging and opportunistic infection in humans and animals. Acta Trop. 2005, 94, 61–76. [Google Scholar] [CrossRef]
- Mathis, A.; Weber, R.; Deplazes, P. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 2005, 18, 423–445. [Google Scholar] [CrossRef]
- Sokolova, O.I.; Demyanov, A.V.; Bowers, L.C.; Didier, E.S.; Yakovlev, A.V.; Skarlato, S.O.; Sokolova, Y.Y. Emerging microsporidian infections in Russian HIV-infected patients. J. Clin. Microbiol. 2011, 49, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Palmer, R.; Trout, J.M.; Fayer, R. Infectivity of microsporidia spores stored in water at environmental temperatures. J. Parasitol. 2003, 89, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.L.; Cleaveland, S.C.; Brown, J.; Mahajan, A.; Shaw, D.J. Seroprevalence of Encephalitozoon cuniculi in wild rodents, foxes and domestic cats in three sites in the United Kingdom. Transbound. Emerg. Dis. 2015, 62, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Perec-Matysiak, A.; Leśniańska, K.; Buńkowska-Gawlik, K.; Čondlová, Š.; Sak, B.; Kváč, M.; Rajský, D.; Hildebrand, J. The opportunistic pathogen Encephalitozoon cuniculi in wild living Murinae and Arvicolinae in Central Europe. Eur. J. Protistol. 2019, 69, 14–19. [Google Scholar] [CrossRef]
- Özkan, Ö.; Yücesan, B.; Babür, C.; Orkun, Ö. Seroprevalence of Encephalitozoon cuniculi, Francisella tularensis and Toxoplasma gondii in Zoonotic Diseases in European Hares (Lepus europaesus). Turkiye Parazitol. Derg. 2021, 45, 171–175. [Google Scholar] [CrossRef]
- Dowd, S.E.; Gerba, C.P.; Pepper, I.L. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl. Environ. Microbiol. 1998, 64, 3332–3335. [Google Scholar] [CrossRef]
- Fournier, S.; Liguory, O.; Santillana-Hayat, M.; Guillot, E.; Sarfati, C.; Dumoutier, N.; Molina, J.; Derouin, F. Detection of microsporidia in surface water: A one-year follow-up study. FEMS Immunol. Med. Microbiol. 2000, 29, 95–100. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Fayer, R.; Trout, J.M.; Lewis, E.J.; Farley, C.A.; Sulaiman, I.; Lal, A.A. Giardia sp. cysts and infectious Cryptosporidium parvum oocysts in the feces of migratory Canada geese (Branta canadensis). Appl. Environ. Microbiol. 1998, 64, 2736–2738. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Majewska, A.C.; Schwab, K.J. The role of birds in dissemination of human waterborne enteropathogens. Trends Parasitol. 2008, 24, 55–59. [Google Scholar] [CrossRef]
- Månsson, J.; Liljebäck, N.; Nilsson, L.; Olsson, C.; Kruckenberg, H.; Elmberg, J. Migration patterns of Swedish Greylag geese Anser anser—Implications for flyway management in a changing world. Eur. J. Wildl. Res. 2022, 68, 15. [Google Scholar] [CrossRef]
- Kruckenberg, H. Migratory behaviour of marked Greater White-fronted Geese Anser albifrons in western Europea—First results and outlook to future research. Charadius 2007, 43, 189–195. [Google Scholar]
- van Wijk, R.E.; Kölzsch, A.; Kruckenberg, H.; Ebbinge, B.S.; Müskens, G.J.D.M.; Nolet, B.A. Individually Tracked Geese Follow Peaks of Temperature Acceleration during Spring Migration. Oikos 2012, 121, 655–664. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Fox, A.D.; Fuller, R.; Griffin, L.; Mitchell, C.; Zhao, Y.; Moon, O.K.; Cabot, D.; Xu, Z.; et al. Stochastic simulations reveal few green wave surfing populations among spring migrating herbivorous waterfowl. Nat. Commun. 2019, 10, 2187. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.D.; Frederiksen, M.; Heinicke, T.; Clausen, K.K.; van der Jeugd, H.P. Annual survival estimates of Taiga Anser fabalis and Tundra Bean Geese A. serrirostris wintering in The Netherlands, 1967–1987. J. Ornithol. 2021, 162, 925–929. [Google Scholar] [CrossRef]
- Polakowski, M.; Broniszewska, M.; Kasprzykowski, Z. Differences in the time budget of the Greater White-fronted Goose (Anser albifrons) in grasslands and arable fields at an important spring stopover site in central Europe. Eur. Zool. J. 2021, 88, 549–555. [Google Scholar] [CrossRef]
- Abu-Akkada, S.S.; El Kerdany, E.D.; Mady, R.F.; Diab, R.G.; Khedr, G.A.; Ashmawy, K.I.; Lotfy, W.M. Encephalitozoon cuniculi infection among immunocompromised and immunocompetent humans in Egypt. Iran J. Parasitol. 2015, 10, 561–570. [Google Scholar]
- Brdíčková, K.; Sak, B.; Holubová, N.; Květoňová, D.; Hlásková, L.; Kicia, M.; Kopacz, Ż.; Kváč, M. Encephalitozoon cuniculi Genotype II Concentrates in Inflammation Foci. J. Inflamm. Res. 2020, 13, 583–593. [Google Scholar] [CrossRef]
- Ulusan Bagci, O.; Muftuoglu, C.; Guldaval, F.; Serce Unat, D.; Mert, U.; Polat, G.; Toz, S.O.; Moon, M.H.; Caner, A. Molecular Prevalence of Microsporidia Infection in Patients with Lung Cancer. Am. J. Trop. Med. Hyg. 2023, 108, 895–900. [Google Scholar] [CrossRef]

| Year | Month | No. of Samples Instead | No. of Samples Instead | Flock Size (Number of Spotted Birds) |
|---|---|---|---|---|
| 2020 | October (31.10.2020) | 35 | 39 A.albifrons | |
| November (06.11.2020) | 40 | 47 A. albifrons | ||
| December (25.12.2020) | 20 | 298 A. anser 97 A. serrirostris 91 A. albifrons 9 Anser spp. | ||
| 2021 | January (17.01.2021) | 94 | 510 A. serrirostris 370 A. albifrons 198 A. anser 93 Anser spp. | |
| Total | 189 | |||
| Year | Month | No. of Samples/Positive in Monospecies Flock | No. of Samples/Positive in Multi-Species Flock | Isolate No. |
|---|---|---|---|---|
| 2020 | October | 35/0 | ||
| November | 40/1 | 165 | ||
| December | 20 | |||
| 2021 | January | 94/5 | 169, 171, 175, 177, 179 | |
| Negative/positive | 75/1 | 114/5 | ||
| Total negative/positive | 189/6 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solarczyk, P.; Perec-Matysiak, A.; Wojtkowiak-Giera, A.; Heddergott, M. Molecular Detection of Encephalitozoon cuniculi in Migratory Waterfowl of the Genus Anser (Anseriformes: Anatidae) in Poland. Pathogens 2025, 14, 489. https://doi.org/10.3390/pathogens14050489
Solarczyk P, Perec-Matysiak A, Wojtkowiak-Giera A, Heddergott M. Molecular Detection of Encephalitozoon cuniculi in Migratory Waterfowl of the Genus Anser (Anseriformes: Anatidae) in Poland. Pathogens. 2025; 14(5):489. https://doi.org/10.3390/pathogens14050489
Chicago/Turabian StyleSolarczyk, Piotr, Agnieszka Perec-Matysiak, Agnieszka Wojtkowiak-Giera, and Mike Heddergott. 2025. "Molecular Detection of Encephalitozoon cuniculi in Migratory Waterfowl of the Genus Anser (Anseriformes: Anatidae) in Poland" Pathogens 14, no. 5: 489. https://doi.org/10.3390/pathogens14050489
APA StyleSolarczyk, P., Perec-Matysiak, A., Wojtkowiak-Giera, A., & Heddergott, M. (2025). Molecular Detection of Encephalitozoon cuniculi in Migratory Waterfowl of the Genus Anser (Anseriformes: Anatidae) in Poland. Pathogens, 14(5), 489. https://doi.org/10.3390/pathogens14050489





