From the Gut to the Brain: Transcriptomic Insights into Neonatal Meningitis Escherichia coli Across Diverse Host Niches
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Cell Culture and Media
2.3. Incubation of NMEC Strain RS218 in Host-Stimulating Environments
2.4. Total RNA Extraction, Microbial RNA Enrichment, and mRNA Purification
2.5. Transcript Library Construction and Sequencing
2.6. Sequence Analysis
2.7. RNA Isolation, cDNA Synthesis, and Quantitative RT-PCR (qRT-PCR) Validation of RNA-seq Data
2.8. Statistical Analysis, Software, and Data Preparation
2.9. Data Availability
3. Results
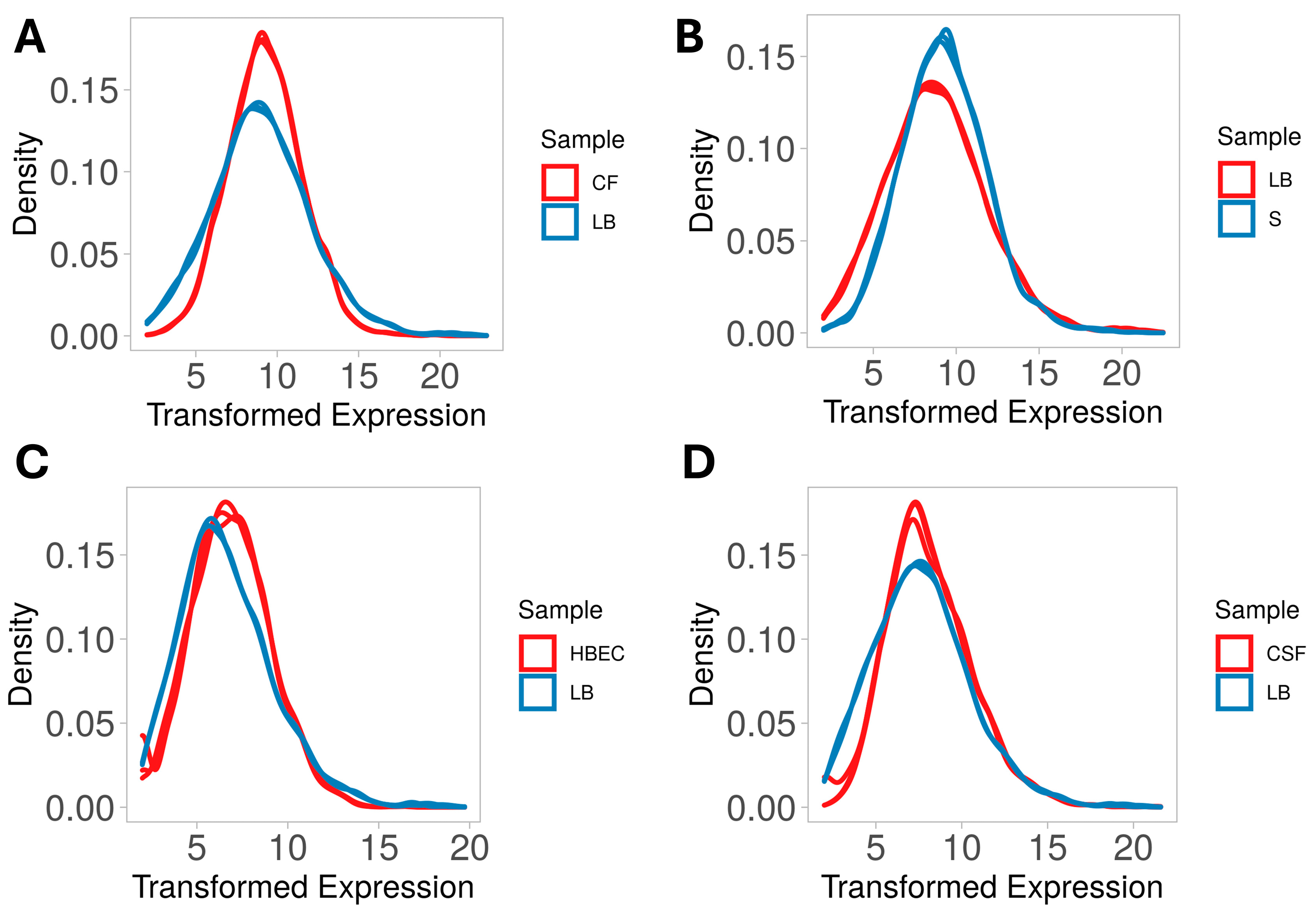
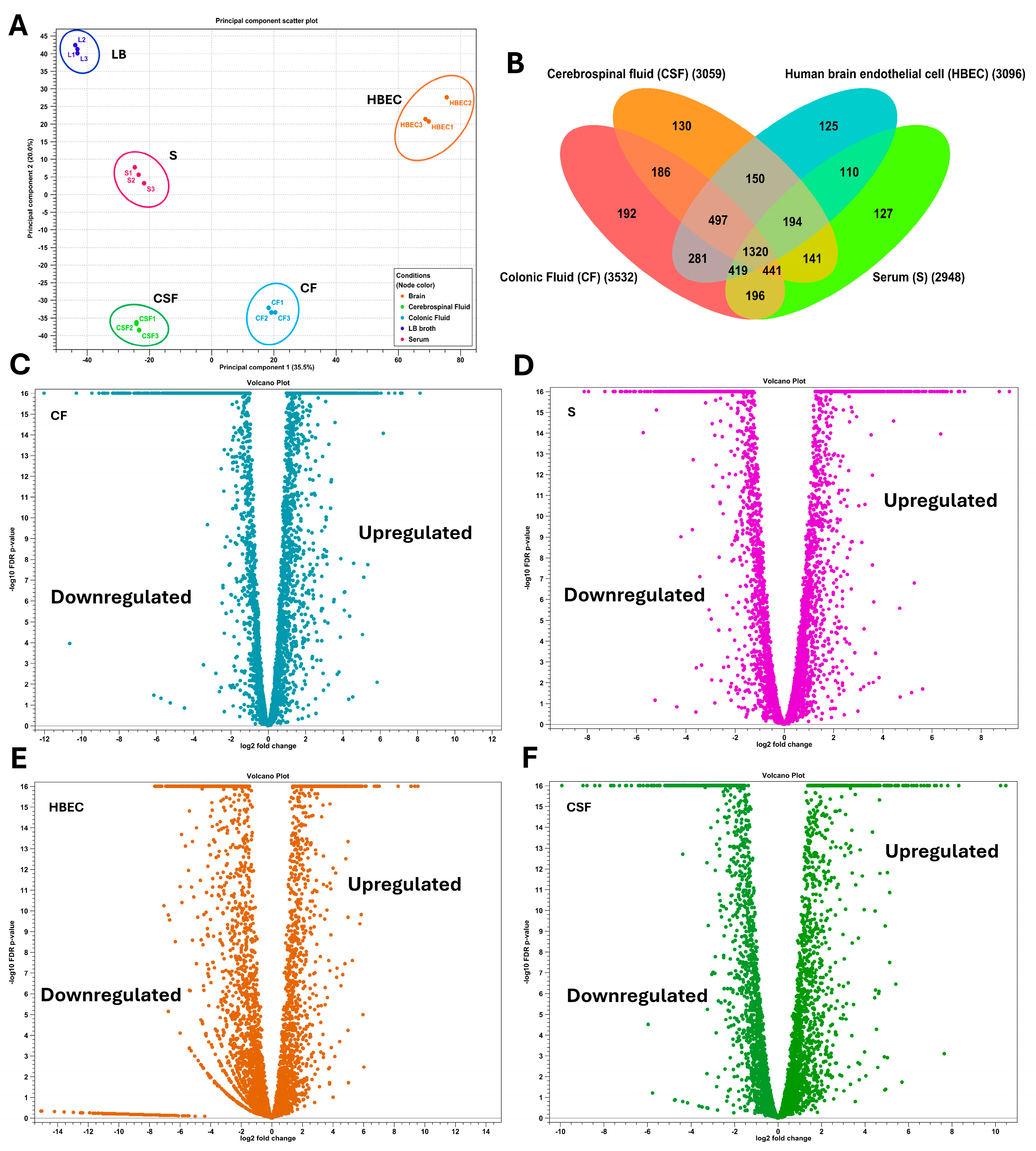
3.1. Overall Transcriptome Profiling
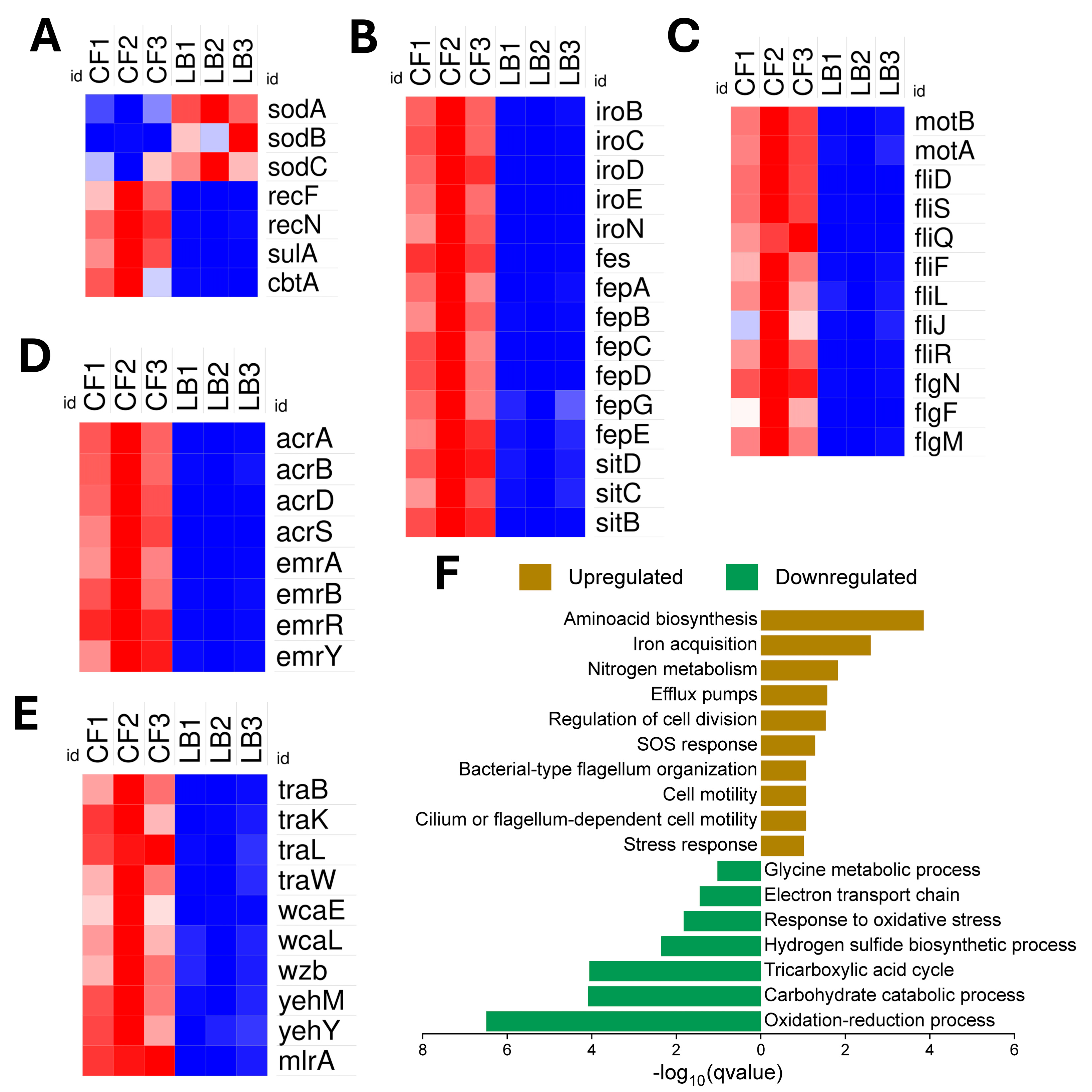
3.2. NMEC’s Transcriptomic Adaptations for Thriving in the Competitive and Hypoxic Colonic Environment
3.3. Overcoming Nutrient Limitations in the Serum
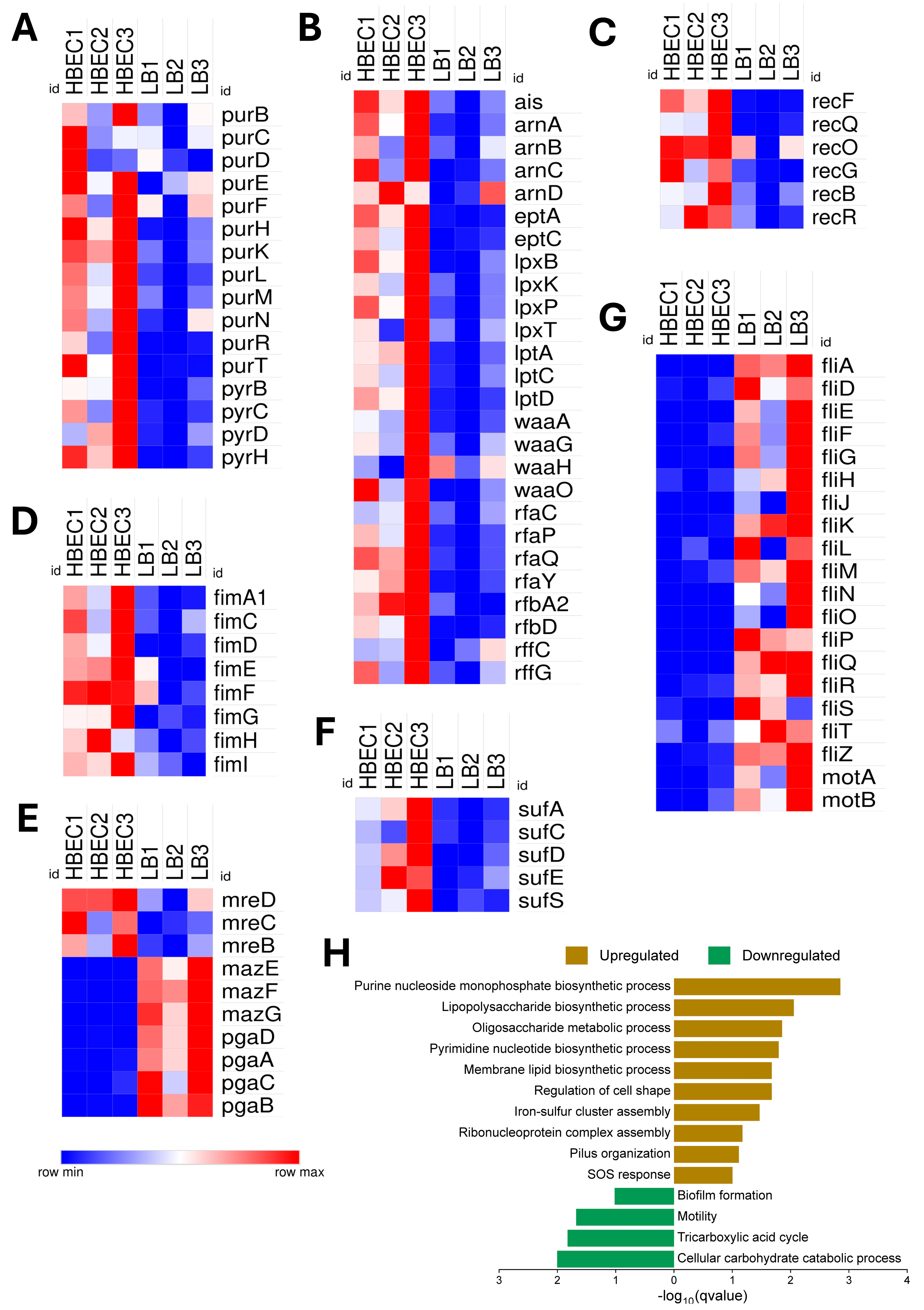
3.4. Gene Expression Associated with Blood–Brain Barrier (BBB) Interaction in Human Brain Endothelial Cells (HBECs)
3.5. Adapting to the Cerebrospinal Fluid (CSF)
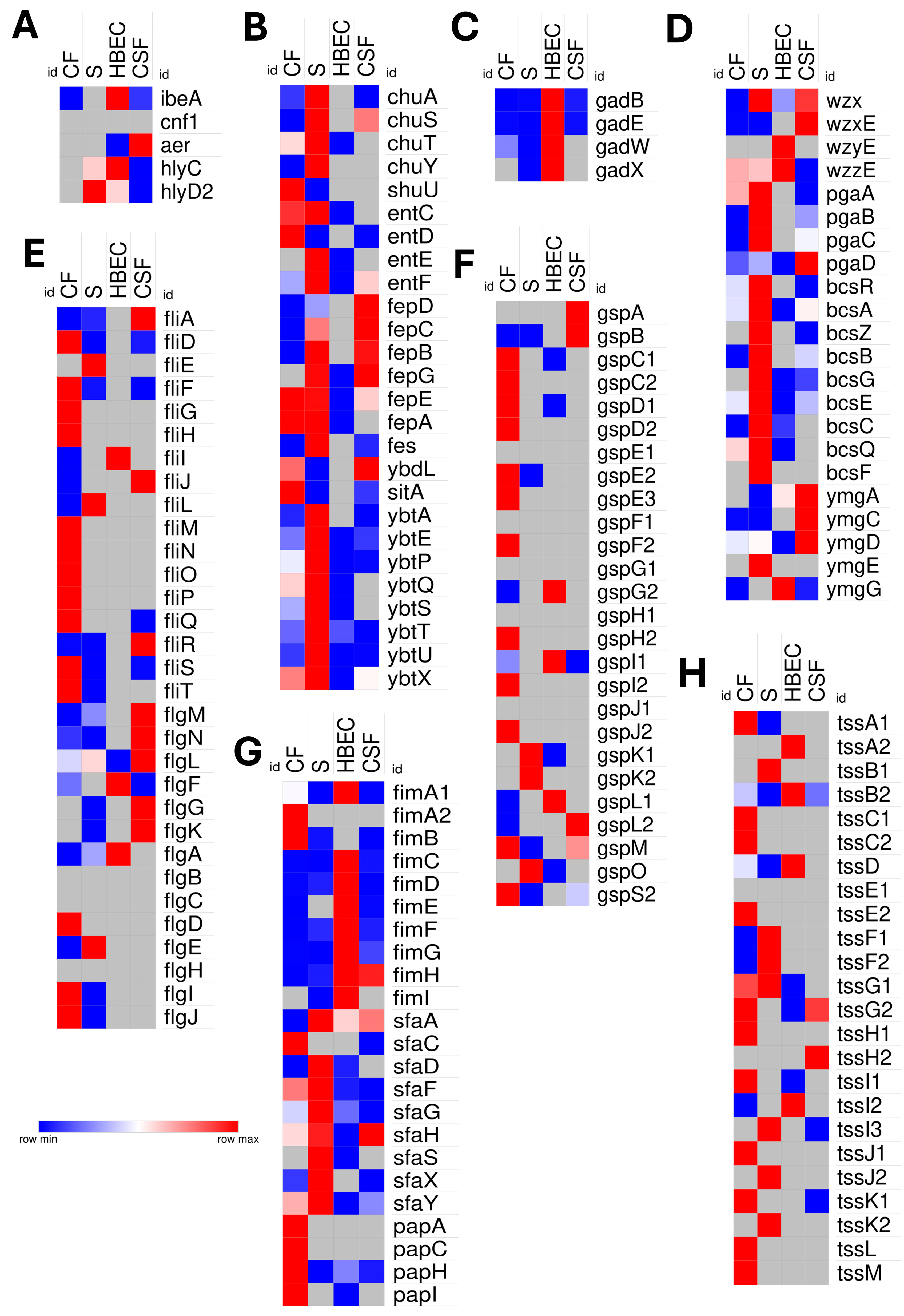
3.6. Comparative Virulence Strategies into Niche-Specific Adaptations
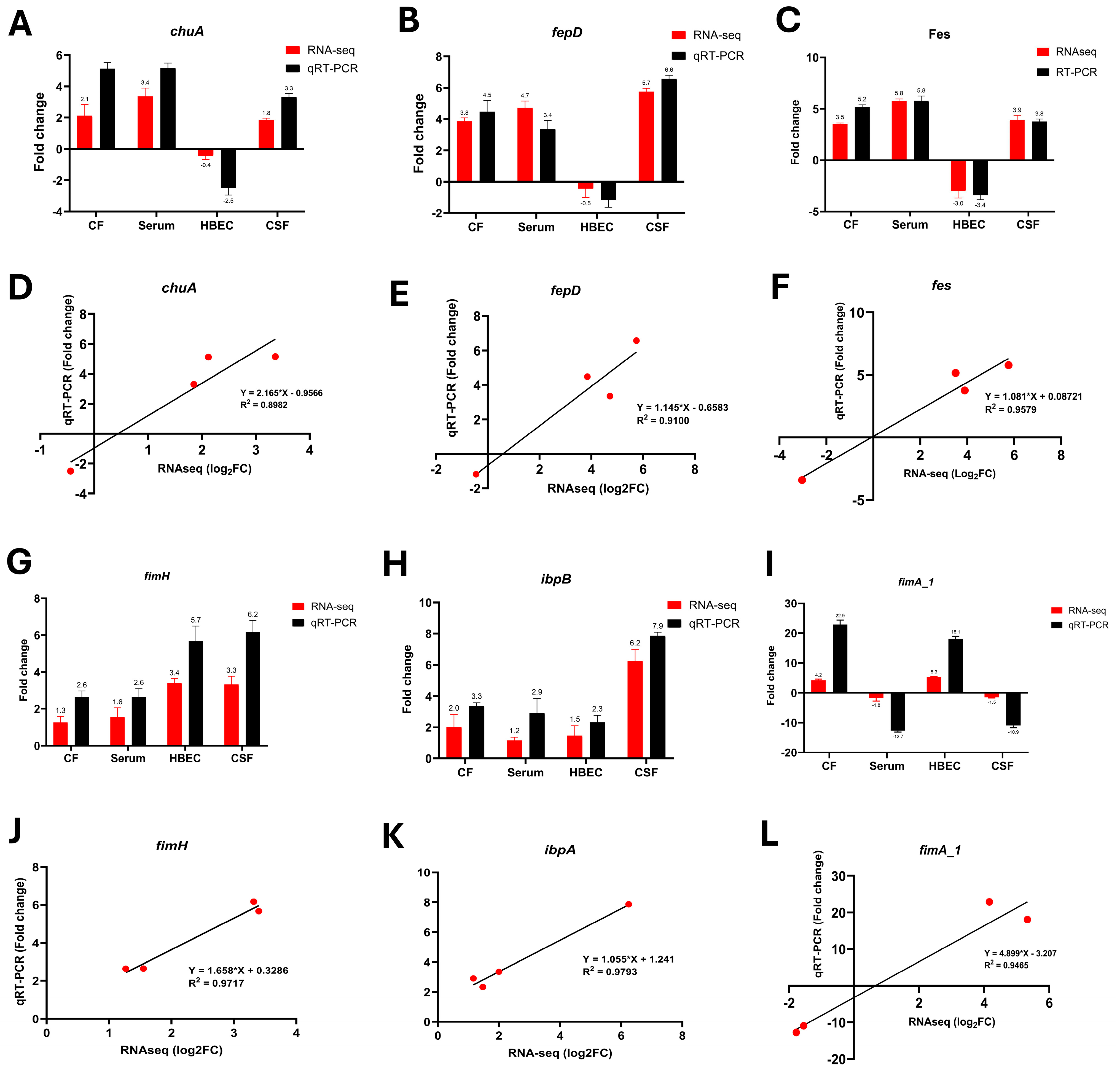
3.7. qRT-PCR Validation of Key Transcriptional Changes
4. Discussion
4.1. Adaptive Strategies of NMEC for Survival and Persistence in the Colonic Environment
4.2. NMEC’s Metabolic Adaptations to Overcome Nutrient Limitations in the Bloodstream
4.3. Metabolic and Virulence Adaptations of NMEC During Blood–Brain Barrier Invasion
4.4. Survival and Persistence Strategies of NMEC in Cerebrospinal Fluid
4.5. Niche-Specific Virulence Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMEC | Neonatal Meningitis Escherichia coli |
| CF | Colonic fluid |
| S | Serum |
| HBEC | Human brain endothelial cells |
| CSF | Cerebrospinal fluid |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| LB | Luria–Bertani broth |
| DGE | Differentially expressed genes |
| TCA | Tricarboxylic acid |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
References
- Liu, T.; Xu, Y.; Gong, Y.; Zheng, J.; Chen, Z. The Global Burden of Disease Attributable to Preterm Birth and Low Birth Weight in 204 Countries and Territories from 1990 to 2019: An Analysis of the Global Burden of Disease Study. J. Glob. Health 2024, 14, 04109. [Google Scholar] [CrossRef]
- Su, Y.; Ma, G.; Zheng, Y.; Qin, J.; Li, X.; Ge, Q.; Sun, H.; Liu, B. Neonatal Meningitis-Causing Escherichia coli Induces Microglia Activation Which Acts as a Double-Edged Sword in Bacterial Meningitis. Int. J. Mol. Sci. 2023, 24, 9915. [Google Scholar] [CrossRef] [PubMed]
- Nhu, N.T.K.; Phan, M.D.; Hancock, S.J.; Peters, K.M.; Alvarez-Fraga, L.; Forde, B.M.; Andersen, S.B.; Miliya, T.; Harris, P.N.A.; Beatson, S.A.; et al. High-Risk Escherichia coli Clones That Cause Neonatal Meningitis and Association with Recrudescent Infection. Elife 2023, 12, RP91853. [Google Scholar] [CrossRef]
- Banks, W.A. The Blood-Brain Barrier: Connecting the Gut and the Brain. Regul. Pept. 2008, 149, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.A.; West, A.C.; Mangiamele, P.; Barbieri, N.; Wannemuehler, Y.; Nolan, L.K.; Logue, C.M.; Li, G. Genetic Characterization of ExPEC-like Virulence Plasmids among a Subset of NMEC. PLoS ONE 2016, 11, e0147757. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, V.; King, O.G.; Yip, A.Y.G.; Marques, I.M.; McDonald, J.A.K. Role of the Gut Microbiota in Nutrient Competition and Protection against Intestinal Pathogen Colonization. Microbiology 2023, 169, 001377. [Google Scholar] [CrossRef]
- Paydarnia, P.; Mayeli, M.; Shafie, M.; Agah, E.; Hasani, S.A.; Jazani, M.R.; Sarraf, P. Alterations of the Serum and CSF Ferritin Levels and the Diagnosis and Prognosis of Amyotrophic Lateral Sclerosis. eNeurologicalSci 2021, 25, 100379. [Google Scholar] [CrossRef]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; St John, J.A.; Ekberg, J.A.K.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens Penetrating the Central Nervous System: Infection Pathways and the Cellular and Molecular Mechanisms of Invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef]
- Wang, S.; Niu, C.; Shi, Z.; Xia, Y.; Yaqoob, M.; Dai, J.; Lu, C. Effects of IbeA Deletion on Virulence and Biofilm Formation of Avian Pathogenic Escherichia coli. Infect. Immun. 2011, 79, 279–287. [Google Scholar] [CrossRef]
- Sora, V.M.; Meroni, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Extraintestinal Pathogenic Escherichia coli: Virulence Factors and Antibiotic Resistance. Pathogens 2021, 10, 1355. [Google Scholar] [CrossRef]
- Krishnan, S.; Chang, A.C.; Hodges, J.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Nicholson, B.A.; Nolan, L.K.; Prasadarao, N.V. Serotype O18 Avian Pathogenic and Neonatal Meningitis Escherichia coli Strains Employ Similar Pathogenic Strategies for the Onset of Meningitis. Virulence 2015, 6, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Human Meningitis-Associated Escherichia coli. EcoSal Plus 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, V. Pathogens’ Adaptation to the Human Host. Proc. Natl. Acad. Sci. USA 2018, 115, 9342–9343. [Google Scholar] [CrossRef]
- Zlatkov, N.; Uhlin, B.E. Absence of Global Stress Regulation in Escherichia coli Promotes Pathoadaptation and Novel C-Di-GMP-Dependent Metabolic Capability. Sci. Rep. 2019, 9, 2600. [Google Scholar] [CrossRef]
- Singhal, R.; Shah, Y.M. Oxygen Battle in the Gut: Hypoxia and Hypoxia-Inducible Factors in Metabolic and Inflammatory Responses in the Intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef]
- Jones, S.A.; Gibson, T.; Maltby, R.C.; Chowdhury, F.Z.; Stewart, V.; Cohen, P.S.; Conway, T. Anaerobic Respiration of Escherichia coli in the Mouse Intestine. Infect. Immun. 2011, 79, 4218–4226. [Google Scholar] [CrossRef]
- Fee, J.A. Regulation of Sod Genes in Escherichia coli: Relevance to Superoxide Dismutase Function. Mol. Microbiol. 1991, 5, 2599–2610. [Google Scholar] [CrossRef]
- Wijetunge, D.S.S.; Karunathilake, K.H.E.M.; Chaudhari, A.; Katani, R.; Dudley, E.G.; Kapur, V.; Debroy, C.; Kariyawasam, S. Complete Nucleotide Sequence of PRS218, a Large Virulence Plasmid, That Augments Pathogenic Potential of Meningitis-Associated Escherichia coli Strain RS218. BMC Microbiol. 2014, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Jellen-Ritter, A.S.; Kern, W.V. Enhanced Expression of the Multidrug Efflux Pumps AcrAB and AcrEF Associated with Insertion Element Transposition in Escherichia coli Mutants Selected with a Fluoroquinolone. Antimicrob. Agents Chemother. 2001, 45, 1467–1472. [Google Scholar] [CrossRef]
- Samuels, A.N.; Roggiani, M.; Zhu, J.; Goulian, M.; Kohli, R.M. The SOS Response Mediates Sustained Colonization of the Mammalian Gut. Infect. Immun. 2019, 87, 1–13. [Google Scholar] [CrossRef]
- Cordell, S.C.; Robinson, E.J.H.; Lö, J. Crystal Structure of the SOS Cell Division Inhibitor SulA and in Complex with FtsZ. Proc. Natl. Acad. Sci. USA 2003, 100, 7889–7894. [Google Scholar] [CrossRef]
- Horvath, D.J.; Li, B.; Casper, T.; Partida-Sanchez, S.; Hunstad, D.A.; Hultgren, S.J.; Justice, S.S. Morphological Plasticity Promotes Resistance to Phagocyte Killing of Uropathogenic Escherichia coli. Microbes Infect. 2011, 13, 426–437. [Google Scholar] [CrossRef]
- Gauger, E.J.; Leatham, M.P.; Mercado-Lubo, R.; Laux, D.C.; Conway, T.; Cohen, P.S. Role of Motility and the FlhDC Operon in Escherichia coli MG1655 Colonization of the Mouse Intestine. Infect. Immun. 2007, 75, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of Antimicrobial Resistance in Biofilms. Npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.; Mobley, H.L.T. Virulence and Fitness Determinants of Uropathogenic Escherichia coli. Microbiol. Spectr. 2015, 3, 235–261. [Google Scholar] [CrossRef]
- Huja, S.; Oren, Y.; Biran, D.; Meyer, S.; Dobrindt, U.; Bernhard, J.; Becher, D.; Hecker, M.; Sorek, R.; Ron, E.Z. Fur Is the Master Regulator of the Extraintestinal Pathogenic Escherichia coli Response to Serum. mBio 2014, 5, e01460-14. [Google Scholar] [CrossRef]
- Edison, L.K.; Kudva, I.T.; Kariyawasam, S. Comparative Transcriptome Analysis of Shiga Toxin-Producing Escherichia coli O157:H7 on Bovine Rectoanal Junction Cells and Human Colonic Epithelial Cells during Initial Adherence. Microorganisms 2023, 11, 2562. [Google Scholar] [CrossRef]
- Richard, K.L.; Kelley, B.R.; Johnson, J.G. Heme Uptake and Utilization by Gram-Negative Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Pelton, J.G.; Inwood, W.B.; Andersen, U.; Kustu, S.; Wemmer, D.E. The Rut Pathway for Pyrimidine Degradation: Novel Chemistry and Toxicity Problems. J. Bacteriol. 2010, 192, 4089–4102. [Google Scholar] [CrossRef]
- Nanduri, B.; Swiatlo, E. The Expansive Effects of Polyamines on the Metabolism and Virulence of Streptococcus pneumoniae. Pneumonia 2021, 13, 4. [Google Scholar] [CrossRef]
- Xie, Y.; Parthasarathy, G.; Di Cello, F.; Teng, C.H.; Paul-Satyaseela, M.; Kim, K.S. Transcriptome of Escherichia coli K1 Bound to Human Brain Microvascular Endothelial Cells. Biochem. Biophys. Res. Commun. 2008, 365, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Thai, V.C.; Stubbs, K.A.; Sarkar-Tyson, M.; Kahler, C.M. Phosphoethanolamine Transferases as Drug Discovery Targets for Therapeutic Treatment of Multi-Drug Resistant Pathogenic Gram-Negative Bacteria. Antibiotics 2023, 12, 1382. [Google Scholar] [CrossRef]
- Jang, S.; Imlay, J.A. Hydrogen Peroxide Inactivates the Escherichia coli Isc Iron-Sulphur Assembly System, and OxyR Induces the Suf System to Compensate. Mol. Microbiol. 2010, 78, 1448–1467. [Google Scholar] [CrossRef]
- Henry, C.; Loiseau, L.; Vergnes, A.; Vertommen, D.; Mérida-Floriano, A.; Chitteni-Pattu, S.; Wood, E.A.; Casadesús, J.; Cox, M.M.; Barras, F.; et al. Redox Controls Reca Protein Activity via Reversible Oxidation of Its Methionine Residues. Elife 2021, 10, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.H.; Cai, M.; Shin, S.; Xie, Y.; Kim, K.J.; Naveed, A.K.; Di Cello, F.; Kwang, S.K. Escherichia coli K1 RS218 Interacts with Human Brain Microvascular Endothelial Cells via Type 1 Fimbria Bacteria in the Fimbriated State. Infect. Immun. 2005, 73, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.; Christopher, R.; Furniss, D.; Fecht, S.; Leung, R.C.Y.; Spiga, L.; Mavridou, D.A.I.; Filloux, A. A Novel Stabilization Mechanism for the Type VI Secretion System Sheath. Proc. Natl. Acad. Sci. USA 2021, 118, e2008500118. [Google Scholar] [CrossRef]
- Navarro-Garcia, F.; Ruiz-Perez, F.; Cataldi, Á.; Larzábal, M. Type VI Secretion System in Pathogenic Escherichia coli: Structure, Role in Virulence, and Acquisition. Front. Microbiol. 2019, 10, 1965. [Google Scholar] [CrossRef] [PubMed]
- Monjarás Feria, J.; Valvano, M.A. An Overview of Anti-Eukaryotic T6SS Effectors. Front. Cell. Infect. Microbiol. 2020, 10, 584751. [Google Scholar] [CrossRef]
- Sauvonnet, N.; Gounon, P.; Pugsley, A.P. PpdD Type IV Pilin of Escherichia coli K-12 Can Be Assembled into Pili in Pseudomonas aeruginosa. J. Bacteriol. 2000, 182, 848–854. [Google Scholar] [CrossRef]
- Roth, M.; Jaquet, V.; Lemeille, S.; Bonetti, E.J.; Cambet, Y.; François, P.; Krause, K.H. Transcriptomic Analysis of E. coli after Exposure to a Sublethal Concentration of Hydrogen Peroxide Revealed a Coordinated Up-Regulation of the Cysteine Biosynthesis Pathway. Antioxidants 2022, 11, 655. [Google Scholar] [CrossRef]
- Podlesek, Z.; Žgur Bertok, D. The DNA Damage Inducible SOS Response Is a Key Player in the Generation of Bacterial Persister Cells and Population Wide Tolerance. Front. Microbiol. 2020, 11, 1785. [Google Scholar] [CrossRef]
- Wang, X.; Preston, J.F.; Romeo, T. The PgaABCD Locus of Escherichia coli Promotes the Synthesis of a Polysaccharide Adhesin Required for Biofilm Formation. J. Bacteriol. 2004, 186, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K. Insights on Escherichia coli Biofilm Formation and Inhibition from Whole-Transcriptome Profiling. Environ. Microbiol. 2009, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Reading, N.C.; Rasko, D.A.; Torres, A.G.; Sperandio, V. The Two-Component System QseEF and the Membrane Protein QseG Link Adrenergic and Stress Sensing to Bacterial Pathogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 5889–5894. [Google Scholar] [CrossRef]
- Koczula, A.; Jarek, M.; Visscher, C.; Valentin-Weigand, P.; Goethe, R.; Willenborg, J. Transcriptomic Analysis Reveals Selective Metabolic Adaptation of Streptococcus suis to Porcine Blood and Cerebrospinal Fluid. Pathogens 2017, 6, 7. [Google Scholar] [CrossRef]
- Rokney, A.; Kobiler, O.; Amir, A.; Court, D.L.; Stavans, J.; Adhya, S.; Oppenheim, A.B. Host Responses Influence on the Induction of Lambda Prophage. Mol. Microbiol. 2008, 68, 29–36. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, E.; Almendros, C.; Mojica, F.J.M.; Guzmán, N.M.; García-Martínez, J. CRISPR Content Correlates with the Pathogenic Potential of Escherichia coli. PLoS ONE 2015, 10, e0131935. [Google Scholar] [CrossRef]
- Rowley, C.A.; Anderson, C.J.; Kendall, M.M. Ethanolamine Influences Human Commensal Escherichia coli Growth, Gene Expression, and Competition with Enterohemorrhagic E. coli O157:H7. MBio 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Akouris, P.P.; Stuivenberg, G.A.; Chmiel, J.A.; Kiattiburut, W.; Poon, A.; Reid, G.; Burton, J.P. Ethanolamine Enhances Adhesion, Promotes Microcompartment Formation, and Modulates Gene Expression in Levilactobacillus brevis ATCC 14869. Gut Microbes 2024, 16, 2350778. [Google Scholar] [CrossRef]
- Sande, C.; Whitfield, C. Capsules and Extracellular Polysaccharides in Escherichia coli and Salmonella. EcoSal Plus 2021, 9, eESP-0033-2020. [Google Scholar] [CrossRef]
- Che, X.; Chi, F.; Wang, L.; Jong, T.D.; Wu, C.H.; Wang, X.; Huang, S.H. Involvement of IbeA in Meningitic Escherichia Coli K1-Induced Polymorphonuclear Leukocyte Transmigration across Brain Endothelial Cells. Brain Pathol. 2011, 21, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Breland, E.J.; Eberly, A.R.; Hadjifrangiskou, M. An Overview of Two-Component Signal Transduction Systems Implicated in Extra-Intestinal Pathogenic E. coli Infections. Front Cell Infect Microbiol 2017, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Wijetunge, D.S.S.; Gongati, S.; Debroy, C.; Kim, K.S.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Kariyawasam, S. Characterizing the Pathotype of Neonatal Meningitis Causing Escherichia coli (NMEC) Applied Microbiology. BMC Microbiol. 2015, 15, 211. [Google Scholar] [CrossRef]
- Kouokam, J.C.; Wai, S.N.; Fällman, M.; Dobrindt, U.; Hacker, J.; Uhlin, B.E. Active Cytotoxic Necrotizing Factor 1 Associated with Outer Membrane Vesicles from Uropathogenic Escherichia coli. Infect. Immun. 2006, 74, 2022–2030. [Google Scholar] [CrossRef]
- Campbell, A.J.; Watts, K.J.; Johnson, M.S.; Taylor, B.L. Role of the F1 Region in the Escherichia coli Aerotaxis Receptor Aer. J. Bacteriol. 2011, 193, 358–366. [Google Scholar] [CrossRef]
- Payne, S.M. Genetics and Regulation of Heme Iron Transport in Shigella dysenteriae and Detection of an Analogous System in Escherichia coli O157:H7. J. Bacteriol. 1995, 177, 3004–3009. [Google Scholar]
- Mey, A.R.; Gómez-Garzón, C.; Payne, S.M. Iron Transport and Metabolism in Escherichia, Shigella, and Salmonella. EcoSal Plus 2021, 9, eESP-0034. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Zhou, Y.; Imlay, J.A. During Oxidative Stress the Clp Proteins of Escherichia Coli Ensure That Iron Pools Remain Sufficient To Reactivate Oxidized Metalloenzymes. J. Bacteriol. 2020, 202, e00235-20. [Google Scholar] [CrossRef]
- Rouquette, C.; De Chastellier, C.; Nair, S.; Berche, P. The ClpC ATPase of Listeria Monocytogenes Is a General Stress Protein Required for Virulence and Promoting Early Bacterial Escape from the Phagosome of Macrophages. Mol. Microbiol. 1998, 27, 1235–1245. [Google Scholar] [CrossRef]
- Tramonti, A.; De Canio, M.; Delany, I.; Scarlato, V.; De Biase, D. Mechanisms of Transcription Activation Exerted by GadX and GadW at the GadA and GadBC Gene Promoters of the Glutamate-Based Acid Resistance System in Escherichia coli. J. Bacteriol. 2006, 188, 8118–8127. [Google Scholar] [CrossRef]
- Seo, S.W.; Kim, D.; O’Brien, E.J.; Szubin, R.; Palsson, B.O. Decoding Genome-Wide GadEWX-Transcriptional Regulatory Networks Reveals Multifaceted Cellular Responses to Acid Stress in Escherichia coli. Nat. Commun. 2015, 6, 7970. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Clark, J.R.; Chang, J.D.; Zulk, J.J.; Chirman, D.M.; Piedra, F.A.; Vaughan, E.E.; Hernandez Santos, H.J.; Patras, K.A.; Maresso, A.W. Progress toward a Vaccine for Extraintestinal Pathogenic E. coli (ExPEC) II: Efficacy of a Toxin-Autotransporter Dual Antigen Approach. Infect. Immun. 2024, 92, e0044023. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Davidson, B.A.; Genagon, S.A.; Warholic, N.M.; MacDonald, U.; Pawlicki, P.D.; Beanan, J.M.; Olson, R.; Holm, B.A.; Knight, P.R., III. E. coli Virulence Factor Hemolysin Induces Neutrophil Apoptosis and Necrosis/Lysis in Vitro and Necrosis/Lysis and Lung Injury in a Rat Pneumonia Model. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, J.; Yu, H.; Ni, J.; Zeng, L.; Teng, Q.; Kim, K.S.; Zhao, G.P.; Guo, X.; Yao, Y. Hcp Family Proteins Secreted via the Type VI Secretion System Coordinately Regulate Escherichia coli K1 Interaction with Human Brain Microvascular Endothelial Cells. Infect. Immun. 2012, 80, 1243–1251. [Google Scholar] [CrossRef]
- Ho, T.D.; Davis, B.M.; Ritchie, J.M.; Waldor, M.K. Type 2 Secretion Promotes Enterohemorrhagic Escherichia coli Adherence and Intestinal Colonization. Infect. Immun. 2008, 76, 1858–1865. [Google Scholar] [CrossRef]
- Verger, D.; Miller, E.; Remaut, H.; Waksman, G.; Hultgren, S. Molecular Mechanism of P Pilus Termination in Uropathogenic Escherichia coli. EMBO Rep. 2006, 7, 1228–1232. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edison, L.K.; Kariyawasam, S. From the Gut to the Brain: Transcriptomic Insights into Neonatal Meningitis Escherichia coli Across Diverse Host Niches. Pathogens 2025, 14, 485. https://doi.org/10.3390/pathogens14050485
Edison LK, Kariyawasam S. From the Gut to the Brain: Transcriptomic Insights into Neonatal Meningitis Escherichia coli Across Diverse Host Niches. Pathogens. 2025; 14(5):485. https://doi.org/10.3390/pathogens14050485
Chicago/Turabian StyleEdison, Lekshmi K., and Subhashinie Kariyawasam. 2025. "From the Gut to the Brain: Transcriptomic Insights into Neonatal Meningitis Escherichia coli Across Diverse Host Niches" Pathogens 14, no. 5: 485. https://doi.org/10.3390/pathogens14050485
APA StyleEdison, L. K., & Kariyawasam, S. (2025). From the Gut to the Brain: Transcriptomic Insights into Neonatal Meningitis Escherichia coli Across Diverse Host Niches. Pathogens, 14(5), 485. https://doi.org/10.3390/pathogens14050485





