Is Pulmonary Mycoses Shadowed by Tuberculosis? Mandate to Hit the Bull’s Eye—An Indian Perspective
Abstract
1. Introduction
2. Pulmonary Fungal Infections
2.1. Pulmonary Aspergillosis
- Allergic Bronchopulmonary Aspergillosis;
- Invasive Pulmonary Aspergillosis;
- Chronic Pulmonary Aspergillosis.
2.1.1. Allergic Bronchopulmonary Aspergillosis (ABPA)
2.1.2. Invasive Pulmonary Aspergillosis (IPA)
2.1.3. Chronic Pulmonary Aspergillosis (CPA)
- Aspergillus nodules;
- Simple Aspergilloma;
- Chronic Cavitary Pulmonary Aspergillosis;
- Subacute Invasive Pulmonary Aspergillosis;
- Chronic Fibrosing Pulmonary Aspergillosis.
Aspergillus Nodules
Aspergilloma
Chronic Cavitary Pulmonary Aspergillosis (CCPA)
Subacute Invasive Pulmonary Aspergillosis (SAIA)
Chronic Fibrosing Pulmonary Aspergillosis (CFPA)
2.2. Pulmonary Cryptococcosis
2.3. Pneumocystis Pneumonia
2.4. Pulmonary Blastomycosis
2.5. Pulmonary Coccidioidomycosis
2.6. Pulmonary Histoplasmosis
2.7. Pulmonary Candidiasis
2.8. Pulmonary Mucormycosis
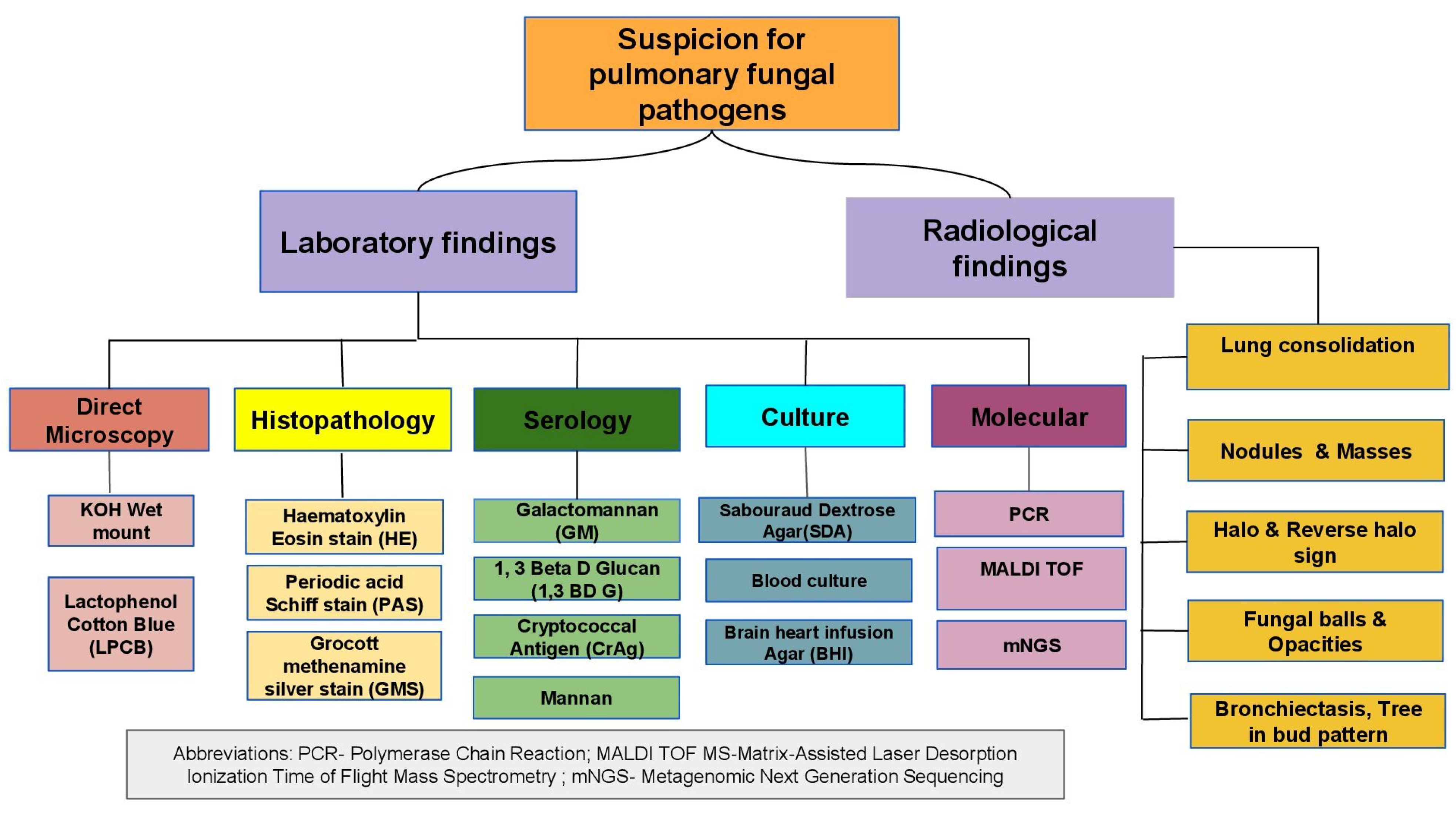
3. Diagnosis
3.1. Direct Microscopy and Histopathological Examination
3.2. Radiology
3.3. Culture
3.4. Serology
3.5. Molecular Diagnosis
3.5.1. Metagenomic Next-Generation Sequencing (mNGS)
3.5.2. Matrix-Assisted Laser Desorption–Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS)
3.6. Limitations of Fungal Diagnosis
4. Key Points
- Clinical suspicion of fungal pathogens in ATT non-responders should be considered before treating them further.
- Early differentiation of active PTB, PTLD, and PTB from fungal co-infection in settings with a high TB burden is the need of the hour.
- A broader screening strategy at the investigation stage itself should be proposed.
- Microbiological confirmation with an inclusion of simultaneous fungal and TB diagnostic methods for presumptive PTB patients with abnormal chest X-ray will facilitate appropriate diagnosis and treatment.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2023.
- Singh, J.; Dinkar, A.; Gupta, P. Uncommon manifestations in tuberculosis: An expanding clinical spectrum from North India. Int. J. Mycobacteriol. 2022, 11, 30–37. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Bitew, A.; Bati, S. Profiling of potential pulmonary fungal pathogens and the prevalence of the association between pulmonary tuberculosis and potential fungal pathogens in presumptive tuberculosis patients referred to Saint Peter’s Specialized Tuberculosis Referral Hospital, Addis Ababa, Ethiopia. SAGE Open Med. 2021, 9, 20503121211056164. [Google Scholar]
- Muni, S.; Rajpal, K.; Kumar, R.; Kumari, R.; Sinha, R.; Kumar, S.; Kumari, N. Identification of fungal isolates in patients with pulmonary tuberculosis treated at a tertiary care hospital. Cureus 2023, 15, e37664. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Meng, G. Pathogenic fungal infection in the lung. Front Immunol. 2019, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.T.; Pennington, K.M.; Limper, A.H. Advances in the diagnosis of fungal pneumonias. Expert Rev. Respir. Med. 2020, 14, 703–714. [Google Scholar] [CrossRef]
- Yan, X.; Zong, F.; Kong, H.; Wang, Y.; Zhao, X.; Liu, W.; Wang, Z.; Xie, W. Pulmonary Fungal Diseases in Immunocompetent Hosts: A Single-Center Retrospective Analysis of 35 Subjects. Mycopathologia 2016, 181, 513–521. [Google Scholar] [CrossRef]
- Campbell, A.P.; Qiu, L.; Dillman, J.R.; Trout, A.T.; Szabo, S.; Lopez-Nunez, O.F.; Pugmire, B.S.; Schapiro, A.H. Endemic mycoses in children in North America: A review of radiologic findings. Pediatr Radiol. 2023, 53, 984–1004. [Google Scholar] [CrossRef]
- Seo, W.; Kim, H.W.; Kim, J.S.; Min, J. Long term management of people with post-tuberculosis lung disease. Korean J. Intern. Med. 2024, 39, 7–24. [Google Scholar] [CrossRef]
- Ekeng, B.E.; Davies, A.A.; Osaigbovo, I.I.; Warris, A.; Oladele, R.O.; Denning, D.W. Pulmonary and extrapulmonary manifestations of fungal infections misdiagnosed as tuberculosis: The need for prompt diagnosis and management. J. Fungi 2022, 8, 460. [Google Scholar] [CrossRef]
- Lewis, R.E.; Stanzani, M.; Morana, G.; Sassi, C. Radiology-based diagnosis of fungal pulmonary infections in high-risk hematology patients: Are we making progress? Curr. Opin. Infect. Dis. 2023, 36, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Wang, B.; Petrik, M.; Beziere, N.; Hammoud, D.A. Radiotracer Development for Fungal-Specific Imaging: Past, Present, and Future. J. Infect. Dis. 2023, 228 (Suppl. 4), S259–S269. [Google Scholar] [CrossRef]
- Kanj, A.; Abdallah, N.; Soubani, A.O. The spectrum of pulmonary aspergillosis. Respir. Med. 2018, 141, 121–131. [Google Scholar] [CrossRef]
- Agarwal, R.; Sehgal, I.S.; Dhooria, S.; Muthu, V.; Prasad, K.T.; Bal, A.; Aggarwal, A.N.; Chakrabarti, A. Allergic bronchopulmonary aspergillosis. Indian J. Med. Res. 2020, 151, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Hebisawa, A.; Kitani, M.; Asano, K.; Neves, J.S. Allergic Bronchopulmonary Aspergillosis-A Luminal Hypereosinophilic Disease With Extracellular Trap Cell Death. Front. Immunol. 2018, 9, 2346. [Google Scholar] [CrossRef] [PubMed]
- Grenier, P.A.; Brun, A.L.; Longchampt, E.; Lipski, M.; Mellot, F.; Catherinot, E. Primary immunodeficiency diseases of adults: A review of pulmonary complication imaging findings. Eur. Radiol. 2023, 34, 4142–4154. [Google Scholar] [CrossRef]
- Khan, S.; Bilal, H.; Shafiq, M.; Zhang, D.; Awais, M.; Chen, C.; Khan, M.N.; Wang, Q.; Cai, L.; Islam, R.; et al. Distribution of Aspergillus species and risk factors for aspergillosis in mainland China: A systematic review. Ther. Adv. Infect. Dis. 2024, 11, 20499361241252536. [Google Scholar] [CrossRef]
- Heylen, J.; Vanbiervliet, Y.; Maertens, J.; Rijnders, B.; Wauters, J. Acute invasive pulmonary aspergillosis: Clinical presentation and treatment. Semin. Respir. Crit. Care Med. 2024, 45, 69–87. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, M.Y.; Hong, S.I.; Jung, J.; Lee, H.J.; Yun, S.-C.; Lee, S.-O.; Choi, S.-H.; Kim, Y.S.; Woo, J.H. Invasive Pulmonary Aspergillosis-mimicking Tuberculosis. Clin. Infect. Dis. 2015, 61, 9–17. [Google Scholar] [CrossRef]
- van der Torre, M.H.; Shen, H.; Rautemaa-Richardson, R.; Richardson, M.D.; Novak-Frazer, L. Molecular Epidemiology of Aspergillus fumigatus in Chronic Pulmonary Aspergillosis Patients. J. Fungi 2021, 7, 152. [Google Scholar] [CrossRef]
- Kosmidis, C. Special issue: Chronic pulmonary aspergillosis. J. Fungi 2022, 8, 714. [Google Scholar] [CrossRef]
- Denning, D.W.; Cole, D.C.; Ray, A. New estimation of the prevalence of chronic pulmonary aspergillosis (CPA) related to pulmonary TB—A revised burden for India. IJID Reg. 2022, 6, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zarif, A.; Thomas, A.; Vayro, A. Chronic pulmonary aspergillosis: A brief review. Yale J. Biol. Med. 2021, 94, 673–679. [Google Scholar]
- Jha, D.; Kumar, U.; Meena, V.P.; Sethi, P.; Singh, A.; Nischal, N.; Jorwal, P.; Vyas, S.; Singh, G.; Xess, I.; et al. Chronic pulmonary aspergillosis incidence in newly detected pulmonary tuberculosis cases during follow-up. Mycoses 2024, 67, e13747. [Google Scholar] [CrossRef] [PubMed]
- Rajpurohit, R.; Wagh, P.; Heda, M.; Dubey, G.; Gujar, P.S. Prevalence of chronic pulmonary aspergillosis in fibrocavitary pulmonary tuberculosis patients. J. Fam. Med. Prim. Care 2023, 12, 106–110. [Google Scholar] [CrossRef]
- Bharath, B.; Ray, A.; Jorwal, P.; Vyas, S.; Soneja, M.; Biswas, A.; Sinha, S.; Khan, M.A. Diagnostic utility of chest computerized tomography in the diagnosis of recurrence among sputum scarce and sputum negative previously treated pulmonary tuberculosis suspects. Lung India 2022, 39, 145–151. [Google Scholar] [CrossRef]
- Sehgal, I.S.; Dhooria, S.; Rudramurthy, S.M.; Prasad, K.T.; Muthu, V.; Aggarwal, A.N.; Garg, M.; Rastogi, P.; Agarwal, R. Role of C-Reactive Protein and Erythrocyte Sedimentation Rate in the Diagnosis and Monitoring of Treatment Response in Treatment Naïve Subjects with Chronic Pulmonary Aspergillosis. Mycopathologia 2023, 188, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, R.; Jhalani, I.; Kumar, A.; Goswami, A.G. Successful management of delayed postoperative lung collapse secondary to spillage of aspergilloma. BMJ Case Rep. 2023, 16, e254621. [Google Scholar] [CrossRef] [PubMed]
- Gandotra, A.; Mehtani, R.; Premkumar, M.; Duseja, A.; De, A.; Mallik, N.; Durgadevi, S.; Das, A.; Kalra, N. Invasive Pulmonary Aspergillosis and Tuberculosis Complicated by Hemophagocytic Lymphohistiocytosis—Sequelae of COVID-19 in a Liver Transplant Recipient. J. Clin. Exp. Hepatol. 2022, 12, 1007–1011. [Google Scholar] [CrossRef]
- Chaurasia, S.; Thimmappa, M.; Chowdhury, S. Case Report: Chronic Cavitatory Pulmonary Aspergillosis after COVID-19. Am. J. Trop. Med. Hyg. 2021, 106, 105–107. [Google Scholar] [CrossRef]
- Dong, S.; Wang, F.; Jin, H.; Dai, X. Five cases of pulmonary Aspergillus nodules diagnosed at surgery and by pathology in immunocompetent patients, with a literature review. Ther. Adv. Rare Dis. 2024, 5, 26330040241252450. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, Y.; Gu, Y.; Ni, Y.; Wang, Y.; Shen, K.; Shi, Y.; Su, X. Clinical features, diagnostic test performance, and prognosis in different subtypes of chronic pulmonary aspergillosis. Front. Med. 2022, 9, 811807. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.K.; Gilotra, T.S.; Tobin, E.H.; Baradhi, K.M. Aspergilloma; StatPearls Pulishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lamoth, F.; Calandra, T. Pulmonary aspergillosis: Diagnosis and treatment. Eur. Respir. Rev. 2022, 31, 220114. [Google Scholar] [CrossRef]
- Pekçolaklar, A.; Çıtak, N.; Aksoy, Y.; Erdoğu, V.; Metin, M. Is there any change in disease presentation and surgical outcomes in patients with pulmonary aspergilloma? An evaluation of the time trend. Turk. J. Thorac. Cardiovasc. Surg. 2022, 30, 241–249. [Google Scholar] [CrossRef]
- Uzair, A.; Waseem, M.; Bhatti, N.I.; Toor, Z.; Ishaq, A.; Ahmad, O. Chronic cavitary pulmonary aspergillosis as a sequela of pulmonary tuberculosis: A case report from Pakistan. SAGE Open Med Case Rep. 2024, 12, 2050313X241251777. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, K.; Tashiro, T.; Tashiro, M.; Takazono, T.; Kosai, K.; Morinaga, Y.; Kurihara, S.; Nakamura, S.; Imamura, Y.; Miyazaki, T.; et al. Pathogenesis and clinical features of chronic pulmonary aspergillosis—Is it possible to distinguish CNPA and CCPA clinically? J. Infect. Chemother. 2014, 20, 208–212. [Google Scholar] [CrossRef]
- Garg, M.; Bhatia, H.; Chandra, T.; Debi, U.; Sehgal, I.S.; Prabhakar, N.; Sandhu, M.S.; Agarwal, R. Imaging spectrum in chronic pulmonary aspergillosis. Am. J. Trop. Med. Hyg. 2023, 108, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-F.; Huang, C.-C.; Chou, K.-T.; Chan, Y.-J.; Yang, Y.-Y.; Wang, F.-D. Chronic Pulmonary Aspergillosis: Disease Severity Using Image Analysis and Correlation with Systemic Proinflammation and Predictors of Clinical Outcome. J. Fungi 2021, 7, 842. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.; Zhang, F. Clinical diagnosis, treatment, and laboratory detection of 50 cases of pulmonary cryptococcosis. Comput. Math. Methods Med. 2022, 2022, 7981472. [Google Scholar] [CrossRef]
- Kidd, S.E.; Abdolrasouli, A.; Hagen, F. Fungal nomenclature: Managing change is the name of the game. Open Forum Infect Dis. 2023, 10, ofac559. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.; Janbon, G.; Idnurm, A.; Bahn, Y.-S. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb. Perspect. Med. 2014, 4, a019760. [Google Scholar] [CrossRef]
- Hu, Y.; Ren, S.-Y.; Xiao, P.; Yu, F.-L.; Liu, W.-L. The clinical and radiological characteristics of pulmonary cryptococcosis in immunocompetent and immunocompromised patients. BMC Pulm. Med. 2021, 21, 262. [Google Scholar] [CrossRef]
- Xiong, C.; Lu, J.; Chen, T.; Xu, R. Comparison of the clinical manifestations and chest CT findings of pulmonary cryptococcosis in immunocompetent and immunocompromised patients: A systematic review and meta-analysis. BMC Pulm. Med. 2022, 22, 415. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, X.; Lu, Y.; Liu, X.; Lv, X. Clinical analysis in immunocompetent and immunocompromised patients with pulmonary cryptococcosis in western China. Sci. Rep. 2020, 10, 9387. [Google Scholar] [CrossRef] [PubMed]
- Meena, P.; Gupta, A.; Gaur, L.; Shingada, A.; Gupta, P.; Bhargava, V.; Rana, D.S. Cryptococcosis masquerading as disseminated tuberculosis in a patient on chronic hemodialysis. Saudi J. Kidney Dis. Transplant. 2019, 30, 1179–1183. [Google Scholar] [CrossRef]
- Ismail, J.; Chidambaram, M.; Sankar, J.; Agarwal, S.; Lodha, R. Disseminated cryptococcosis presenting as miliary lung shadows in an immunocompetent child. J. Trop. Pediatr. 2018, 64, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mahajan, V.; Kumar, A. Unusual case of coexistent pulmonary cryptococcosis and tuberculosis in an immuno-competent host. Indian J. Tuberc. 2017, 64, 228–231. [Google Scholar] [CrossRef]
- Pawar, S.; Ganakumar, V.; Jha, S.; Ragesh, R.; Ray, A.; Kakkar, A.; Sharma, M.C.; Sharma, S.K. Pulmonary cryptococcoma masquerading as lung cancer. J. Assoc. Physicians India 2016, 64, 66–68. [Google Scholar]
- Ranjan, P.; Jana, M.; Krishnan, S.; Nath, D.; Sood, R. Disseminated cryptococcosis with adrenal and lung involvement in an immunocompetent patient. J. Clin. Diagn. Res. 2015, 9, OD04-5. [Google Scholar] [CrossRef]
- Rafat, Z.; Ashrafi, K.; Hashemi, S.J.; Sasani, E.; Naserani, A.; Sarvestani, H.K.; Hashemi, F. The mycological and molecular study of Pneumocystis jiroveci pneumonia among HIV and non-HIV immunocompromised patients hospitalized in pulmonary units in Guilan, Northern Iran. Iran. J. Microbiol. 2021, 13, 518–524. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lu, C.-Y.; Lee, P.-I.; Chen, J.-M.; Huang, L.-M.; Chang, L.-Y. Pneumocystis jiroveci pneumonia in Taiwan from 2014 to 2017: Clinical manifestations and outcomes between pediatric and adult patients. J. Microbiol. Immunol. Infect. 2019, 52, 983–990. [Google Scholar] [CrossRef]
- Apostolopoulou, A.; Fishman, J.A. The Pathogenesis and Diagnosis of Pneumocystis jiroveci Pneumonia. J. Fungi 2022, 8, 1167. [Google Scholar] [CrossRef]
- Bateman, M.; Oladele, R.; Kolls, J.K. Diagnosing Pneumocystis jirovecii pneumonia: A review of current methods and novel approaches. Med. Mycol. 2020, 58, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Gandhi, S.; Rao, V. Clinical profile of human immunodeficiency virus patients with opportunistic infections: A descriptive case series study. Int. J. Appl. Basic Med. Res. 2015, 5, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.C.; Patil, H.V. Clinical manifestations and outcome of patients with human immunodeficiency virus infection at tertiary care teaching hospital. Indian J. Sex. Transm. Dis. AIDS 2016, 37, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Ghosh, S.; Halder, P.; Pal, D.; Modak, D.C.; Guha, S.K. Pulmonary tuberculosis and pneumocystis jirovecii concurrent pneumonia in HIV infected patients at a resource limited setting in Eastern India: A case series. Indian J. Tuberc. 2020, 67, 378–382. [Google Scholar] [CrossRef]
- Bongomin, F.; Ekeng, B.E.; Kibone, W.; Nsenga, L.; Olum, R.; Itam-Eyo, A.; Kuate, M.P.N.; Pebolo, F.P.; Davies, A.A.; Manga, M.; et al. Invasive fungal diseases in africa: A critical literature review. J. Fungi 2022, 8, 1236. [Google Scholar] [CrossRef]
- Goico, A.; Henao, J.; Tejada, K. Disseminated blastomycosis in a 36-year-old immunocompetent male from Chicago, IL. Oxf. Med. Case Rep. 2018, 2018, omy071. [Google Scholar] [CrossRef]
- Maphanga, T.G.; Birkhead, M.; Muñoz, J.F.; Allam, M.; Zulu, T.G.; Cuomo, C.A.; Schwartz, I.S.; Ismail, A.; Naicker, S.D.; Mpembe, R.S.; et al. Human Blastomycosis in South Africa Caused by Blastomyces percursus and Blastomyces emzantsi sp. nov., 1967 to 2014. J. Clin. Microbiol 2020, 58, e01661-19. [Google Scholar] [CrossRef]
- Bongomin, F.; Adetona Fayemiwo, S. Epidemiology of fungal diseases in Africa: A review of diagnostic drivers. Curr. Med. Mycol. 2021, 7, 63–70. [Google Scholar] [CrossRef]
- Abdallah, F.C.B.; Bachouch, I.; Belloumi, N.; Kacem, M.; Mlika, M.; Mezni, F.E.; Fenniche, S. Pulmonary blastomycosis. Pan Afr. Med. J. 2020, 36, 220. [Google Scholar] [CrossRef]
- Wang, N.; Luo, Z.; Deng, S.; Li, Q. A young male with chronic nonproductive cough diagnosed with blastomycosis in China: A case report. BMC Pulm. Med. 2020, 20, 189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kunoor, A.; Eapen, M.; Singh, P.K.; Chowdhary, A. Blastomycosis misdiagnosed as tuberculosis, india. Emerg. Infect. Dis. 2019, 25, 1776–1777. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.; Moura, S.; Castelo-Branco, D.; Rocha, M.F.; Lima-Neto, R.; Sidrim, J.J. Coccidioidomycosis in brazil: Historical challenges of a neglected disease. J. Fungi 2021, 7, 85. [Google Scholar] [CrossRef]
- Crum, N.F. Coccidioidomycosis: A contemporary review. Infect. Dis. Ther. 2022, 11, 713–742. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57 (Suppl. 1), S16–S20. [Google Scholar] [CrossRef]
- Johnson, R.H.; Sharma, R.; Kuran, R.; Fong, I.; Heidari, A. Coccidioidomycosis: A review. J. Investig. Med. 2021, 69, 316–323. [Google Scholar] [CrossRef]
- Scott, A.M.; Lim, J.R.; Randhawa, R.; Lee, J.; Yaddanapudi, K.; Rabe, B.; Malo, J. Examining Miliary Disease Etiology in a Coccidioides-Endemic Center: A Retrospective Cohort Study. J. Fungi 2023, 10, 29. [Google Scholar] [CrossRef]
- Sri, K.; Vaithy, A.; Kathirvelu, S.; Srinivasan, S. Coccidioidomycosis lymphadenopathy: An unusual presentation. BMJ Case Rep. 2023, 16, e253740. [Google Scholar] [CrossRef]
- Caceres, D.H.; Valdes, A. Histoplasmosis and Tuberculosis Co-Occurrence in People with Advanced HIV. J. Fungi 2019, 5, 73. [Google Scholar] [CrossRef]
- Kuate, M.P.N.; Ekeng, B.E.; Kwizera, R.; Mandengue, C.; Bongomin, F. Histoplasmosis overlapping with HIV and tuberculosis in sub-Saharan Africa: Challenges and research priorities. Ther. Adv. Infect. Dis. 2021, 8, 20499361211008676. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Kosmidis, C.; Rozaliyani, A.; Wahyuningsih, R.; Denning, D.W. Chronic Pulmonary Histoplasmosis-A Scoping Literature Review. Open Forum Infect. Dis. 2020, 7, ofaa119. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M.; Alsibai, K.D.; Epelboin, L.; Abboud, P.; About, F.; Demar, M.; Djossou, F.; Blaizot, R.; Douine, M.; Sabbah, N.; et al. A Simple Predictive Score to Distinguish between Disseminated Histoplasmosis and Tuberculosis in Patients with HIV. J. Fungi 2021, 8, 16. [Google Scholar] [CrossRef]
- Ekeng, B.E.; Oladele, R.O.; Emanghe, U.E.; Ochang, E.A.; Mirabeau, T.Y. Prevalence of Histoplasmosis and Molecular Characterization of Histoplasma species in Patients with Presumptive Pulmonary Tuberculosis in Calabar, Nigeria. Open Forum Infect. Dis. 2022, 9, ofac368. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Shadrach, B.J. Acute pulmonary histoplasmosis masquerading as miliary tuberculosis in a non-endemic region. Adv. Respir. Med. 2021, 89, 464–465. [Google Scholar] [CrossRef]
- Anot, K.; Sharma, S.; Gupta, M.; Kaur, D. Disseminated histoplasmosis and tuberculosis: Dual infection in a non-endemic region. BMJ Case Rep. 2020, 13, e235531. [Google Scholar] [CrossRef]
- Dutta, V.; Chopra, M.; Kovilapu, U.B.; Gahlot, G. Solitary pulmonary nodule: An interesting clinical mimicry of pulmonary tuberculosis. Med. J. Armed Forces India 2018, 75, 115–118. [Google Scholar] [CrossRef]
- Ramesh, V.; Narreddy, S.; Gowrishankar, S.; Barigala, R.; Nanda, S. A challenging case of pyrexia of unknown origin: Adrenal histoplasmosis mimicking tuberculosis in a patient with chronic hepatitis C. Trop. Dr. 2018, 51, 621–623. [Google Scholar] [CrossRef]
- Astekar, M.; Bhatiya, P.S.; Sowmya, G. Prevalence and characterization of opportunistic candidal infections among patients with pulmonary tuberculosis. J. Oral Maxillofac. Pathol. 2016, 20, 183–189. [Google Scholar] [CrossRef]
- Azim, A.; Ahmed, A. Diagnosis and management of invasive fungal diseases in non-neutropenic ICU patients, with focus on candidiasis and aspergillosis: A comprehensive review. Front. Cell. Infect. Microbiol. 2024, 14, 1256158. [Google Scholar] [CrossRef]
- Shweihat, Y.; Perry, J.; Shah, D. Isolated Candida infection of the lung. Respir. Med. Case Rep. 2015, 16, 18–19. [Google Scholar] [CrossRef][Green Version]
- Hadadi-Fishani, M.; Shakerimoghaddam, A.; Khaledi, A. Candida coinfection among patients with pulmonary tuberculosis in Asia and Africa; A systematic review and meta-analysis of cross-sectional studies. Microb. Pathog. 2020, 139, 103898. [Google Scholar] [CrossRef]
- Khanduri, R.; Khanduri, S.; Kumar, S.; Saini, A.; Kotwal, A. Drug-resistant tuberculosis coexisting with invasive candidiasis in an immunocompetent 30-year-old woman: A case report. Indian J. Tuberc. 2017, 64, 232–234. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwal, C.; Hooda, A.K.; Ojha, A.; Dhillon, M.; Kumar, K.V.S. Profile of infections in renal transplant recipients from India. J. Fam. Med. Prim. Care 2016, 5, 611–614. [Google Scholar] [CrossRef]
- Bao, J.; Liu, C.; Dong, Y.; Xu, Y.; Wang, Z.; Sun, K.; Xi, W.; Wang, K.; Gong, P.; Gao, Z. Clinical Manifestations of Pulmonary Mucormycosis in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation: A 21-Case Series Report and Literature Review. Can. Respir. J. 2022, 2022, 1237125. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Chakrabarti, A. Global epidemiology of mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef]
- Agrawal, R.; Yeldandi, A.; Savas, H.; Parekh, N.D.; Lombardi, P.J.; Hart, E.M. Pulmonary mucormycosis: Risk factors, radiologic findings, and pathologic correlation. Radiographics 2020, 40, 656–666. [Google Scholar] [CrossRef]
- Aggarwal, D.; Chander, J.; Janmeja, A.K.; Katyal, R. Pulmonary tuberculosis and mucormycosis co-infection in a diabetic patient. Lung India 2015, 32, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Dube, P.; Saroa, R.; Palta, S. Coinfections in Intensive Care Unit with pulmonary tuberculosis and mucormycosis: A clinical dilemma. Indian J. Crit. Care Med. 2016, 20, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Kaur, G.; Deepak, D.; Kumar, P. Disseminated pulmonary mucormycosis with concomitant tuberculosis infection in a diabetic patient. Int. J. Mycobacteriol. 2020, 9, 95–97. [Google Scholar] [CrossRef]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 5 October 2024).
- Mendonça, A.; Santos, H.; Franco-Duarte, R.; Sampaio, P. Fungal infections diagnosis—Past, present and future. Res. Microbiol. 2022, 173, 103915. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.N.; Gohil, M.R.; Navadiya, A.J.; Bapodra, M.K.; Patel, H.R. Comparative evaluation of histopathological analysis, KOH wet mount and fungal culture to diagnose fungal infections in post-COVID patients. Indian J. Pathol. Microbiol. 2023, 66, 540–544. [Google Scholar] [PubMed]
- Jandial, R.; Choudhary, M.; Singh, K. Histomorphological spectrum of fungal infections. Int. J. Res. Med. Sci. 2019, 7, 4166–4170. [Google Scholar] [CrossRef]
- Seth, R.; Xess, I.; Jana, M. Diagnosis of invasive fungal infections in children. Indian Pediatr. 2019, 56, 229–236. [Google Scholar] [CrossRef]
- Vaishnav, A.; Gurukiran, G.; Ighodaro, O.; Kandi, V. Radiological and imaging evidence in the diagnosis and management of microbial infections: An update. Cureus 2023, 15, e48756. [Google Scholar] [CrossRef]
- Ahmad, Z.; Bagchi, S.; Naranje, P.; Agarwal, S.K.; Das, C.J. Imaging spectrum of pulmonary infections in renal transplant patients. Indian J. Radiol. Imaging 2020, 30, 273–279. [Google Scholar] [CrossRef]
- Grover, S.B.; Grover, H.; Antil, N.; Patra, S.; Sen, M.K.; Nair, D. Imaging approach to pulmonary infections in the immunocompromised patient. Indian J. Radiol. Imaging 2022, 32, 081–112. [Google Scholar] [CrossRef]
- Ramanan, P.; Wengenack, N.L.; Theel, E.S. Laboratory diagnostics for fungal infections: A review of current and future diagnostic assays. Clin. Chest Med. 2017, 38, 535–554. [Google Scholar] [CrossRef]
- Standard Operating Procedures for Fungal Identification and Detection of Antifungal Resistance Antimicrobial Resistance Surveillance and Research Network; Indian Council of Medical Research: New Delhi, India, 2019.
- Hage, C.A.; Carmona, E.M.; Epelbaum, O.; Evans, S.E.; Gabe, L.M.; Haydour, Q.; Knox, K.S.; Kolls, J.K.; Murad, M.H.; Wengenack, N.L.; et al. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An official american thoracic society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2019, 200, 535–550. [Google Scholar] [CrossRef]
- Sandhar, T.K.; Chhina, D.K.; Gupta, V.; Chaudhary, J. Role of (1-3)-Β-D-Glucan Test in the Diagnosis of Invasive Fungal Infections among High-Risk Patients in a Tertiary Care Hospital. J. Lab. Physicians 2022, 14, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Chen, W.; Wei, Y.; Liang, J.; Liao, S.; Chen, Y.; Li, Y.; Wang, X.; Chen, W.; Qiu, Y.; et al. Comparison of diagnostic efficiency of detecting IgG and IgE with immunoassay method in diagnosing ABPA: A meta-analysis. BMC Pulm. Med. 2023, 23, 374. [Google Scholar] [CrossRef]
- Takazono, T.; Izumikawa, K. Recent advances in diagnosing chronic pulmonary aspergillosis. Front. Microbiol. 2018, 9, 1810. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.H.; Chiller, T.; Lindsley, M.D. Immunodiagnostic assays for the investigation of fungal outbreaks. Mycopathologia 2020, 185, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Gudisa, R.; Harchand, R.; Rudramurthy, S.M. Nucleic-Acid-Based Molecular Fungal Diagnostics: A Way to a Better Future. Diagnostics 2024, 14, 520. [Google Scholar] [CrossRef]
- Srinivas, S.; Kumari, P.; Gupta, D.K. Utility of Panfungal PCR in the diagnosis of invasive fungal infections in febrile neutropenia. J. Fam. Med. Prim. Care 2021, 10, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Poplin, V.; Smith, C.; Milsap, D.; Zabel, L.; Bahr, N.C. Diagnosis of pulmonary infections due to endemic fungi. Diagnostics 2021, 11, 856. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Xiao, Y.; Zou, H.; Hao, S.; Jiang, Y. Application of metagenomic next-generation sequencing and targeted metagenomic next-generation sequencing in diagnosing pulmonary infections in immunocompetent and immunocompromised patients. Front. Cell. Infect. Microbiol. 2024, 14, 1439472. [Google Scholar] [CrossRef]
- Naik, S.; Kashyap, D.; Deep, J.; Darwish, S.; Cross, J.; Mansoor, E.; Garg, V.K.; Honnavar, P. Utilizing Next-Generation Sequencing: Advancements in the Diagnosis of Fungal Infections. Diagnostics 2024, 14, 1664. [Google Scholar] [CrossRef]
- Ghosh, A.; Paul, S.; Sood, P.; Rudramurthy, S.; Rajbanshi, A.; Jillwin, T.; Chakrabarti, A. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for the rapid identification of yeasts causing bloodstream infections. Clin. Microbiol. Infect. 2015, 21, 372–378. [Google Scholar] [CrossRef]
- Paul, S.; Singh, P.; Rudramurthy, S.M.; Chakrabarti, A.; Ghosh, A.K. Matrix-assisted laser desorption/ionization-time of flight mass spectrometry: Protocol standardization and database expansion for rapid identification of clinically important molds. Future Microbiol. 2017, 12, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
| Type of Aspergillosis | Reported State and Year | Type of Study and Objectives | Study Population | Number of Isolates | Salient Findings | Reference |
|---|---|---|---|---|---|---|
| Chronic Pulmonary Aspergillosis (CPA) | New Delhi 2024 | Prospective Objective: To confirm the presence of CPA in newly diagnosed PTB at baseline and the end of TB therapy | 255—Recruited 158—Completed follow-up | 11.1% were positive at baseline, and 27.5% were positive at the end of anti-tubercular therapy | CPA can arise as Post-TB Lung Disease (PTLD) or exist as a co-infection with TB in new patients. | Jha et al. [25] |
| Chronic Pulmonary Aspergillosis | Maharashtra 2023 | Cross-sectional/Observational Objective: To determine the prevalence of CPA in patients with treated fibrocavitary PTB | 42 | 9.5% | Serological diagnosis is necessary for detecting CPA in patients with or without TB due to similar clinical features. | Rajpurohit et al. [26] |
| Chronic Pulmonary Aspergillosis | New Delhi 2022 | Prospective-Observational Objective: To investigate the diagnostic accuracy, sensitivity, and specificity of different computed tomography (CT) results in identifying recurrence in suspects of PTB. | 130 | 24.2% | The mediastinal necrotic lymph node is the appropriate CT finding for differentiating between recurrent TB and post-TB sequelae in CPA complications. | Bharath et al. [27] |
| Chronic Pulmonary Aspergillosis | India 2022 | Estimation Analysis Objective: To estimate the prevalence of CPA related to PTB. | - | - | Comprehensive estimation of total CPA burden in pulmonary TB patients | Denning et al. [23] |
| Chronic Pulmonary Aspergillosis | Chandigarh 2023 | Retrospective Objective: To determine the role of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) for diagnosing chronic pulmonary aspergillosis (CPA) | 434 subjects and 20 disease controls | - | Erythrocyte Sedimentation rate (ESR) and C-reactive protein (CRP) play a significant role as biomarkers in CPA diagnosis among Post-TB Lung Disease (PTLD) cases. | Sehgal et al. [28] |
| Aspergilloma | Uttarakhand 2023 | Case Report | - | 1 | Aspergilloma was found as a mass in the intrabronchial region instead of a lung cavity in a patient with TB a decade ago. | Lahiri et al. [29] |
| Invasive Pulmonary Aspergillosis | Chandigarh 2022 | Case Report | - | 1 | A 47-year-old male had a sequelae of Coronavirus Disease-2019 (COVID-19) complicated by TB and IPA | Gandotra et al. [30] |
| Chronic Cavitary Pulmonary Aspergillosis | Karnataka 2022 | Case Report | - | 1 | In a 57-year-old adult male, COVID-19 had reactivated latent aspergilloma, and the condition developed into CCPA, a more severe form of aspergillosis. This patient reportedly had a TB and aspergilloma co-infection 20 years ago. | Chaurasia et al. [31] |
| Reported State/Year | Site of Infection | Salient Findings | Reference |
|---|---|---|---|
| New Delhi 2019 | Lung | A 26-year-old female was initially diagnosed with TB, and treatment was initiated, but she was later diagnosed with cryptococcosis. | Meena et al. [47] |
| New Delhi 2018 | Lung and CNS | An immunocompetent child suspected of having TB was diagnosed with disseminated cryptococcosis at a later stage | Ismail et al. [48] |
| New Delhi 2016 | Lung | In a 36-year-old male, co-infection of pulmonary cryptococcosis and TB was reported. | Jain et al. [49] |
| New Delhi 2016 | Lung | A 45-year-old male with a history of TB was presented with a mass in the right lower lobe; similar to a lung tumor, but was eventually diagnosed as cryptococcoma through CT and histopathological examination. | Pawar et al. [50] |
| New Delhi 2015 | Lung/Adrenal gland | A 45-year-old male, with no TB history but initially diagnosed as disseminated TB, was later diagnosed with cryptococcosis by histopathological findings. | Ranjan et al. [51] |
| Reported State/Year | Site of Infection | Salient Findings | Reference |
|---|---|---|---|
| Rajasthan 2021 | Lung | A 28-year-old with pulmonary histoplasmosis was misdiagnosed as miliary TB | Agarwal et al. [77] |
| Chandigarh 2020 | Skin, Lung | Disseminated TB and histoplasmosis co-infection were reported in a 50-year-old male. | Anot et al. [78] |
| New Delhi 2019 | Lung | Multi-Drug Resistant-TB was suspected in a 59-year-old female with a history of cutaneous TB who presented with manifestations such as fever and dry cough. Eventually, a PET scan revealed a soft tissue nodule, and histopathological examination confirmed the presence of H. capsulatum. | Dutta et al. [79] |
| Telangana 2018 | Adrenal gland, Lung | Hepatitis C was confirmed; the case study presented with fever and was treated for four months with ATT for suspected TB, but was finally diagnosed with Histoplasmosis, which mimicked TB in clinical manifestations. | Ramesh et al. [80] |
| Reported State/Year | Type of Study | Study Population | Number of Isolates | Site of Infection | Salient Findings | Reference |
|---|---|---|---|---|---|---|
| Uttarakhand 2016 | Case Report | - | 1 | Lung | In a 30-year-old female, the coexistence of drug-resistant TB with invasive candidiasis was reported. | Khanduri et al. [85] |
| Rajasthan 2016 | Prevalence Study Objective: To determine the prevalence of opportunistic candidal infection in TB patients | 60 confirmed Pulmonary TB patients | 33 | Lung | The presence of Candida in sputum samples among PTB patients was reported using SDA and ChromAgar cultures. | Astekar et al. [81] |
| Maharashtra 2016 | Prospective Observational Study Objective: To study the clinical profile of renal transplant recipients. | 45 renal transplant recipients (RTRs) | 7 | - | Among renal transplant recipients, TB with candidiasis and CMV with TB were found in 7 patients. | Kumar et al. [86] |
| State | Year | Site of Infection | Salient Findings | Reference |
|---|---|---|---|---|
| Chandigarh 2015 | 2015 | Lung | Co-infection of TB and mucormycosis in a 30-year-old diabetic patient was reported. | Aggarwal et al. [90] |
| Puducherry 2016 | 2016 | Lung | Pulmonary TB with mucormycosis co-infection was demonstrated in a 72-year-old diabetic patient admitted to the intensive care Unit. | Dube et al. [91] |
| New Delhi 2020 | 2020 | Lung | Disseminated pulmonary mucormycosis and TB co-infection in a diabetic patient was reported. | Ramesh et al. [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regupathy, J.; Rajendran, P.; Kumar, V.; Shanmugam, S. Is Pulmonary Mycoses Shadowed by Tuberculosis? Mandate to Hit the Bull’s Eye—An Indian Perspective. Pathogens 2025, 14, 435. https://doi.org/10.3390/pathogens14050435
Regupathy J, Rajendran P, Kumar V, Shanmugam S. Is Pulmonary Mycoses Shadowed by Tuberculosis? Mandate to Hit the Bull’s Eye—An Indian Perspective. Pathogens. 2025; 14(5):435. https://doi.org/10.3390/pathogens14050435
Chicago/Turabian StyleRegupathy, Jeevarahini, Priya Rajendran, Vinod Kumar, and Sivakumar Shanmugam. 2025. "Is Pulmonary Mycoses Shadowed by Tuberculosis? Mandate to Hit the Bull’s Eye—An Indian Perspective" Pathogens 14, no. 5: 435. https://doi.org/10.3390/pathogens14050435
APA StyleRegupathy, J., Rajendran, P., Kumar, V., & Shanmugam, S. (2025). Is Pulmonary Mycoses Shadowed by Tuberculosis? Mandate to Hit the Bull’s Eye—An Indian Perspective. Pathogens, 14(5), 435. https://doi.org/10.3390/pathogens14050435






