Invasive Candidiasis Coinfection in Patients with Severe COVID-19 Disease: Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Literature Selection Criteria
2.3. Data Collection Process
2.4. Quality Evaluation
2.5. Data Analysis
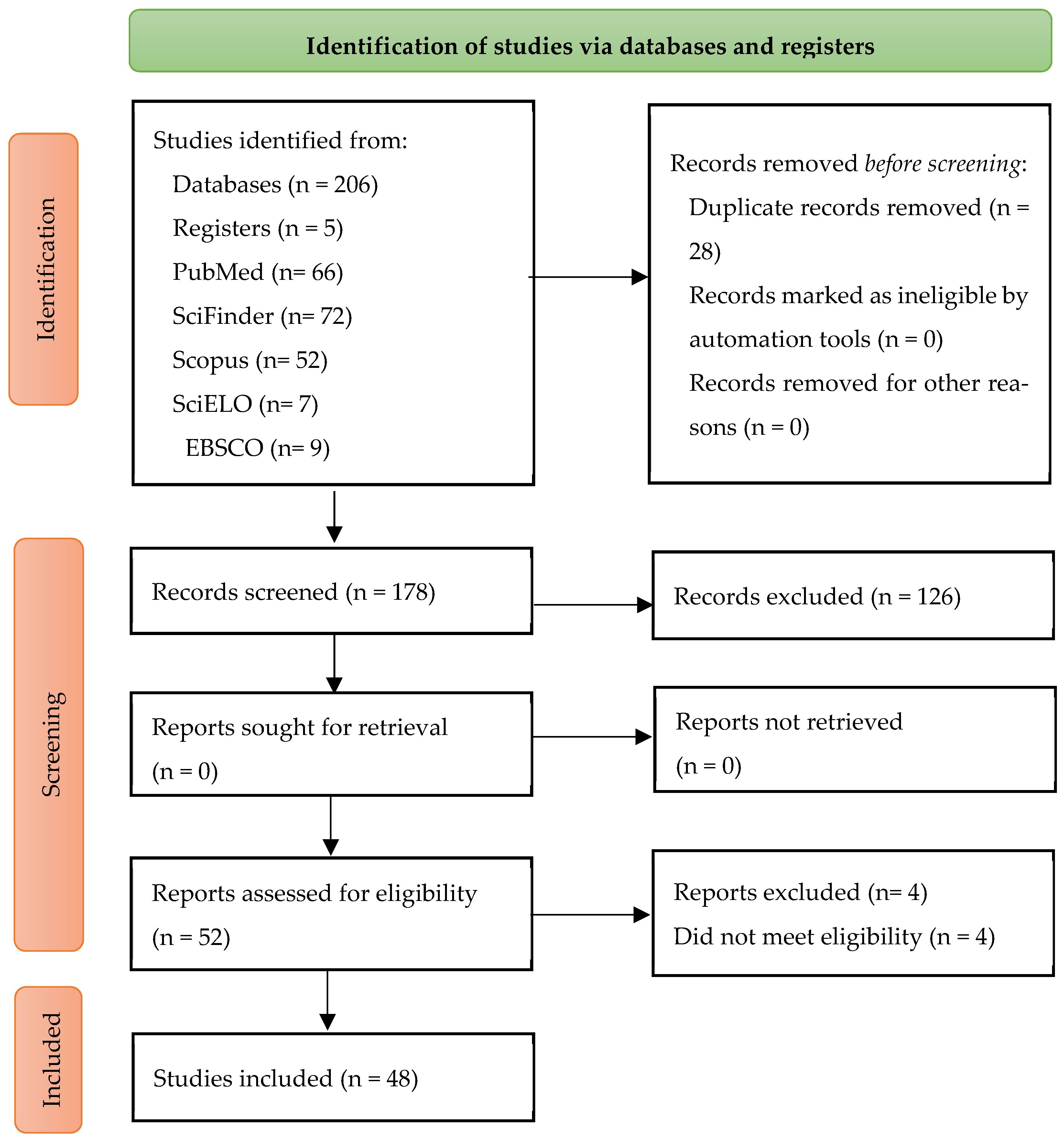
3. Results
Selection of Sources of Evidence
4. Discussion
4.1. Coinfection Incidence
4.2. Candidiasis Diagnosis
4.3. Candidiasis’ Causal Agent
4.4. Develop and Treatment of Candidiasis
4.5. Treatment Duration and Its Effects
4.6. Relation Between COVID-19 and Invasive Candidiasis
4.7. Study Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morey-Olivé, M.; Espiau, M.; Mercadal-Hally, M.; Lera-Carbasllo, E.; García-Patos, V. Manifestaciones cutáneas en contexto del brote actual de enfermedad por coronavirus 2019. An. Pediatr. (Engl. Ed.) 2020, 92, 374–375. [Google Scholar] [CrossRef] [PubMed]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; Czaja, C.A.; Kayalioglu, H.; et al. The Landscape of Candidemia During the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin. Infect. Dis. 2022, 74, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Estévez, A.; Sánchez-Carrillo, C.; Guinea, J.; Escribano, P.; Alonso, R.; Valerio, M.; Padilla, B.; Bouza, E.; Muñoz, P. Incidence of Candidemia Is Higher in COVID-19 versus Non-COVID-19 Patients, but Not Driven by Intrahospital Transmission. J. Fungi 2022, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Shishido, A.A.; Mathew, M.; Baddley, J.W. Overview of COVID-19-Associated Invasive Fungal Infection. Curr. Fungal Infect. Rep. 2022, 16, 87–97. [Google Scholar] [CrossRef]
- Szabo, B.G.; Lakatos, B.; Bobek, I.; Szabo, E.; Szlavik, J.; Vályi-Nagy, I. Invasive fungal infections among critically ill adult COVID-19 patients: First experiences from the national centre in Hungary. J. Mycol. Med. 2021, 31, 101198. [Google Scholar] [CrossRef]
- Koukaki, E.; Rovina, N.; Tzannis, K.; Sotiropoulou, Z.; Loverdos, K.; Koutsoukou, A.; Dimopoulos, G. Fungal Infections in the ICU during the COVID-19 Era: Descriptive and Comparative Analysis of 178 Patients. J. Fungi 2022, 8, 881. [Google Scholar] [CrossRef]
- Kayaaslan, B.; Eser, F.; Kalem, A.K.; Kaya, G.; Kaplan, B.; Kacar, D.; Hasanoglu, I.; Coskun, B.; Guner, R. Post-COVID syndrome: A single-center questionnaire study on 1007 participants recovered from COVID-19. J. Med. Virol. 2021, 93, 6566–6574. [Google Scholar] [CrossRef]
- Moser, D.; Biere, K.; Han, B.; Hoerl, M.; Schelling, G.; Choukér, A.; Woehrle, T. COVID-19 Impairs Immune Response to Candida albicans. Front. Immunol. 2021, 12, 640644. [Google Scholar] [CrossRef]
- Ahmed, N.; Mahmood, M.S.; Ullah, M.d.A.; Araf, Y.; Rahaman, T.I.; Moin, A.T.; Hosen, M.J. COVID-19-Associated Candidiasis: Possible Patho-Mechanism, Predisposing Factors, and Prevention Strategies. Curr. Microbiol. 2022, 79, 127. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-associated fungal infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The Levels of Evidence and Their Role in Evidence-Based Medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Segrelles-Calvo, G.; de S. Araújo, G.R.; Llopis-Pastor, E.; Carrillo, J.; Hernández-Hernández, M.; Rey, L.; Melean, N.; Escribano, I.; Antón, E.; Zamarro, C.; et al. Candida spp. co-infection in COVID-19 patients with severe pneumonia: Prevalence study and associated risk factors. Respir. Med. 2021, 188, 106619. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, N.; Park, Y.; Kim, J.; Jeon, K.; Park, M.-J.; Song, W. Prevalence and Clinical Impact of Coinfection in Patients with Coronavirus Disease 2019 in Korea. Viruses 2022, 14, 446. [Google Scholar] [CrossRef]
- Porto, A.P.M.; Borges, I.C.; Buss, L.; Machado, A.; Bassetti, B.R.; Cocentino, B.; Bicalho, C.S.; Carrilho, C.; Rodrigues, C.; Neto, E.; et al. Healthcare-associated infections on the intensive care unit in 21 Brazilian hospitals during the early months of the coronavirus disease 2019 (COVID-19) pandemic: An ecological study. Infect. Control Hosp. Epidemiol. 2023, 44, 284–290. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef]
- Pemán, J.; Ruiz-Gaitán, A.; García-Vidal, C.; Salavert, M.; Ramírez, P.; Puchades, F.; García-Hita, M.; Alastruey-Izquierdo, A.; Quindós, G. Fungal co-infection in COVID-19 patients: Should we be concerned? Rev. Iberoam. Micol. 2020, 37, 41–46. [Google Scholar] [CrossRef]
- Abdoli, A.; Falahi, S.; Kenarkoohi, A. COVID-19-associated opportunistic infections: A snapshot on the current reports. Clin. Exp. Med. 2021, 22, 327–346. [Google Scholar] [CrossRef]
- Chiurlo, M.; Mastrangelo, A.; Ripa, M.; Scarpellini, P. Invasive fungal infections in patients with COVID-19: A review on pathogenesis, epidemiology, clinical features, treatment, and outcomes. New Microbiol. 2021, 44, 71–83. [Google Scholar]
- Rajendra Santosh, A.B.; Muddana, K.; Bakki, S.R. Fungal Infections of Oral Cavity: Diagnosis, Management, and Association with COVID-19. SN Compr. Clin. Med. 2021, 3, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Hilmioğlu-Polat, S.; Daneshnia, F.; Pan, W.; Hafez, A.; Fang, W.; Liao, W.; Şahbudak-Bal, Z.; Metin, D.; Junior, J.M.; et al. Clonal Candidemia Outbreak by Candida parapsilosis Carrying Y132F in Turkey: Evolution of a Persisting Challenge. Front. Cell Infect. Microbiol. 2021, 11, 676177. [Google Scholar] [CrossRef] [PubMed]
- Frías-De-León, M.G.; Pinto-Almazán, R.; Hernández-Castro, R.; García-Salazar, E.; Meza-Meneses, P.; Rodríguez-Cerdeira, C.; Arenas, R.; Conde-Cuevas, E.; Acosta-Altamirano, G.; Martínez-Herrera, E. Epidemiology of Systemic Mycoses in the COVID-19 Pandemic. J. Fungi 2021, 7, 556. [Google Scholar] [CrossRef]
- Katz, J. Prevalence of candidiasis and oral candidiasis in COVID-19 patients: A cross-sectional pilot study from the patients’ registry in a large health center. Quintessence Int. 2021, 52, 714–718. [Google Scholar] [CrossRef]
- Coskun, A.S.; Durmaz, Ş.Ö. Fungal Infections in COVID-19 Intensive Care Patients. Pol. J. Microbiol. 2021, 70, 395–400. [Google Scholar] [CrossRef]
- Erami, M.; Mirhendi, H.; Momen-Heravi, M.; Hezaveh, S.J.H.; Ahsaniarani, A.H.; Sabet, S.S.; Aboutalebian, S. A case of COVID-19-associated rhino-orbito-cerebral mucormycosis caused by Apophysomyces variabilis with a review of the literature. Front. Cell Infect. Microbiol. 2022, 12, 898477. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Badali, H.; Khodavaisy, S. Opportunistic Fungal Infections in the Epidemic Area of COVID-19: A Clinical and Diagnostic Perspective from Iran. Mycopathologia 2020, 185, 607–611. [Google Scholar] [CrossRef]
- Vitale, R.G.; Afeltra, J.; Seyedmousavi, S.; Giudicessi, S.L.; Romero, S.M. An overview of COVID-19 related to fungal infections: What do we know after the first year of pandemic? Braz. J. Microbiol. 2022, 53, 759–775. [Google Scholar] [CrossRef]
- White, P.L.; Price, J.S.; Cordey, A.; Backx, M. Molecular Diagnosis of Yeast Infections. Curr. Fungal Infect. Rep. 2021, 15, 67–80. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Manshadi, S.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef]
- Senok, A.; Alfaresi, M.; Khansaheb, H.; Nassar, R.; Hachim, M.; Al Suwaidi, H.; Almansoori, M.; Alqaydi, F.; Afaneh, Z.; Mohamed, A.; et al. Coinfections in Patients Hospitalized with COVID-19: A Descriptive Study from the United Arab Emirates. Infect. Drug Resist. 2021, 14, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Xi, Y.; Lin, Z.; Pan, Y.; Song, B.; Li, C.; Zheng, X.; Zhong, M.; Jiang, L.; Pan, C.; et al. Secondary infection in severe and critical COVID-19 patients in China: A multicenter retrospective study. Ann. Palliat. Med. 2021, 10, 8557–8570. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, A.; Germinario, B.N.; Ferrante, M.; Frangi, C.; Li Voti, R.; Muccini, C.; Ripa, M.; COVID-BioB Study Group. Candidemia in Coronavirus Disease 2019 (COVID-19) Patients: Incidence and Characteristics in a Prospective Cohort Compared with Historical Non–COVID-19 Controls. Clin. Infect. Dis. 2021, 73, e2838–e2839. [Google Scholar] [CrossRef] [PubMed]
- Amorim dos Santos, J.; Normando, A.G.C.; Carvalho da Silva, R.L.; Acevedo, A.C.; De Luca Canto, G.; Sugaya, N.; Santos-Silva, A.R.; Guerra, E.N.S. Oral Manifestations in Patients with COVID-19: A 6-Month Update. J. Dent. Res. 2021, 100, 1321–1329. [Google Scholar] [CrossRef]
- Roudbary, M.; Kumar, S.; Kumar, A.; Černáková, L.; Nikoomanesh, F.; Rodrigues, C.F. Overview on the Prevalence of Fungal Infections, Immune Response, and Microbiome Role in COVID-19 Patients. J. Fungi 2021, 7, 720. [Google Scholar] [CrossRef]
- Denny, S.; Abdolrasouli, A.; Elamin, T.; Gonzalo, X.; Charani, E.; Patel, A.; Donaldson, H.; Hughes, S.; Armstrong-James, D.; More, L.; et al. A retrospective multicenter analysis of candidaemia among COVID-19 patients during the first UK pandemic wave. J. Infect. 2021, 82, 276–316. [Google Scholar] [CrossRef]
- Norberg, C.M.; Norberg, P.R.; Norberg, A.; Lopes de Matos, A.A.; Sanches, F.; Manhães, F.; Mangiavacchi, B.; Faial, L. Candida infections associated with COVID-19: An underestimated risk. WJPPS 2021, 10, 48–64. [Google Scholar]
- Samaranayake, L.P.; Seneviratne, C.J.; Fakhruddin, K.S. Coronavirus disease 2019 (COVID-19) vaccines: A concise review. Oral. Dis. 2022, 28, 2326–2336. [Google Scholar] [CrossRef]
- Brandi, N.; Ciccarese, F.; Balacchi, C.; Rimondi, M.R.; Modolon, C.; Sportoletti, C.; Capozzi, C.; Renzulli, M.; Paccapelo, A.; Castelli, A.; et al. Co-Infections and Superinfections in COVID-19 Critically Ill Patients Are Associated with CT Imaging Abnormalities and the Worst Outcomes. Diagnostics 2022, 12, 1617. [Google Scholar] [CrossRef]
- Rafat, Z.; Ramandi, A.; Khaki, P.A.; Ansari, S.; Ghaderkhani, S.; Haidar, H.; Tajari, F.; Roostaei, D.; Ghazvini, R.; Hashemi, S.; et al. Fungal and bacterial co-infections of the respiratory tract among patients with COVID-19 hospitalized in intensive care units. Gene Rep. 2022, 27, 101588. [Google Scholar] [CrossRef]
- Ayalon, O.; Cohen, M.J.; Orenbuch-Harroch, E.; Sviri, S.; van Heerden, P.V.; Korem, M. Invasive fungal infections in critically ill COVID-19 patients in a large tertiary university hospital in Israel. J. Crit. Care 2022, 69, 154004. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Zakeri, A.; Zandi, M.; Kesheh, M.M.; Tabibzadeh, A.; Dastranj, M.; Faramarzi, S.; Didehdar, M.; Hafezi, H.; Hosseini, P.; et al. The Role of Bacterial and Fungal Human Respiratory Microbiota in COVID-19 Patients. Biomed. Res. Int. 2021, 2021, 6670798. [Google Scholar] [CrossRef] [PubMed]
- Kamali Sarvestani, H.; Mahmoudi, S.; Afarinesh Khaki, P.; Ansari, S.; Ghaderkhani, S.; Roostaei, D.; Ghazvini, R.; Hashemi, S.; Rafat, Z.; Abollahi, A. Epidemiology, risk factors, species distribution, and antifungal susceptibility of candidemia among hospitalized patients with COVID-19. Curr. Med. Mycol. 2022, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Kubin, C.J.; McConville, T.H.; Dietz, D.; Zucker, J.; May, M.; Nelson, B.; Istorico, E.; Bartram, L.; Small-Saunders, J.; Sobieszczyk, M.; et al. Characterization of Bacterial and Fungal Infections in Hospitalized Patients with Coronavirus Disease 2019 and Factors Associated with Health Care-Associated Infections. Open Forum Infect. Dis. 2021, 8, ofab201. [Google Scholar] [CrossRef]
- Cataldo, M.A.; Tetaj, N.; Selleri, M.; Marchioni, L.; Capone, A.; Caraffa, E.; Di Caro, A.; Petrosillo, N.; INMICOVID-19 Co-infection Group. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: An alarming “collateral effect”. J. Glob. Antimicrob. Resist. 2020, 23, 290–291. [Google Scholar] [CrossRef]
- Agrifoglio, A.; Cachafeiro, L.; Figueira, J.C.; Añón, J.M.; García de Lorenzo, A. Critically ill patients with COVID-19 and candidaemia: We must keep this in mind. J. Mycol. Med. 2020, 30, 101012. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Antinori, S.; Bonazzetti, C.; Gubertini, G.; Capetti, A.; Pagani, C.; Morena, V.; Rimoldi, S.; Galimberti, L.; Sarzi-Puttini, P.; Ridolfo, A. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: An increased risk for candidemia? Autoimmun. Rev. 2020, 19, 102564. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Kolonitsiou, F.; Kefala, S.; Spiliopoulou, A.; Aretha, D.; Bartzavali, C.; Siapika, A.; Marangos, M.; Fligou, F. Increased incidence of candidemia in critically ill patients during the Coronavirus Disease 2019 (COVID-19) pandemic. Braz. J. Infect. Dis. 2022, 26, 102353. [Google Scholar] [CrossRef]
- Baddley, J.W.; Thompson, G.R.; Chen, S.C.-A.; White, P.L.; Johnson, M.D.; Nguyen, M.H.; Schwartz, I.; Spec, A.; Ostrosky-Zeichner, L.; Jackson, B.; et al. Coronavirus Disease 2019–Associated Invasive Fungal Infection. Open Forum Infect. Dis. 2021, 8, ofab510. [Google Scholar] [CrossRef]
- Macauley, P.; Martin, A.; Epelbaum, O. Corticosteroids in the Treatment of Severe COVID-19 Lung Disease: The Pulmonology Perspective from the First United States Epicenter. Int. J. Infect. Dis. 2020, 100, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Basile, K.; Halliday, C.; Kok, J.; Chen, S.C.-A. Fungal Infections Other Than Invasive Aspergillosis in COVID-19 Patients. J. Fungi 2022, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, M.; Korem, M.; Ayalon, O.; Wiener-Well, Y.; Shachor-Meyouhas, Y.; Cohen, R.; Bishara, J.; Atamna, A.; Brosh-Nissimov, T.; Maaravi, N.; et al. Invasive Fungal Diseases in Hospitalized Patients with COVID-19 in Israel: A Multicenter Cohort Study. J. Fungi 2022, 8, 721. [Google Scholar] [CrossRef]
- Santosh, A.B.R.; Muddana, K.; Bakki, S.R. Response to Commentary: Fungal Infections of Oral Cavity: Diagnosis, Management, and Association with COVID-19. SN Compr. Clin. Med. 2021, 3, 2205–2206. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef]
- Lamers, M.M.; Beumer, J.; Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.; Schayck, J.P.; Mykytyn, A.; Duimel, H.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef]
- Torres-Estrella, C.U.; Reyes-Montes Mdel, R.; Duarte-Escalante, E.; Sierra Martínez, M.; Frías-De-León, M.G.; Acosta-Altamirano, G. Vaccines Against COVID-19: A Review. Vaccines 2022, 10, 414. [Google Scholar] [CrossRef]
- Acosta-Altamirano, G. Nasal Mask: An Alternative to Prevent Contagion during Essential Activities. Biomed. J. Sci. Tech. Res. 2021, 34, 27018–27022. [Google Scholar] [CrossRef]
- Feng, Y.; Ling, Y.; Bai, T.; Xie, Y.; Huang, J.; Li, J.; Xiong, W.; Yang, D.; Chen, R.; Lu, F.; et al. COVID-19 with Different Severities: A Multicenter Study of Clinical Features. Am. J. Respir. Crit. Care Med. 2020, 201, 1380–1388. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934. [Google Scholar] [CrossRef]
- Amorim dos Santos, J.; Normando, A.G.C.; Carvalho da Silva, R.L.; Acevedo, A.C.; De Luca Canto, G.; Sugaya, N.; Santos-Silva, A.R.; Guerra, E.N.S. Oral Manifestations in Patients with COVID-19: A Living Systematic Review. J. Dent. Res. 2021, 100, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Denny, S.; Rawson, T.M.; Hart, P.; Satta, G.; Abdulaal, A.; Hughes, S.; Gilchrist, M.; Mughal, N.; Moore, L. Bacteraemia variation during the COVID-19 pandemic; A multi-centre UK secondary care ecological analysis. BMC Infect. Dis. 2021, 21, 556. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Daneshnia, F.; Najafzadeh, M.J.; Hagen, F.; Mahmoudi, S.; Salehi, M.; Zarrinfar, H.; Namvar, Z.; Zareshahrabadi, Z.; Khodavaisy, S.; et al. Evaluation of Molecular Epidemiology, Clinical Characteristics, Antifungal Susceptibility Profiles, and Molecular Mechanisms of Antifungal Resistance of Iranian Candida parapsilosis Species Complex Blood Isolates. Front. Cell Infect. Microbiol. 2020, 10, 206. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Farahyar, S.; Fang, W.; Salimi, M.; Salehi, M.; Hagen, F.; Weihua, P.; Roudbary, M.; Boekhout, T. Incidence and spectrum of yeast species isolated from the oral cavity of Iranian patients suffering from hematological malignancies. J. Oral. Microbiol. 2019, 11, 1601061. [Google Scholar] [CrossRef]
- Omrani, A.S.; Koleri, J.; Ben Abid, F.; Daghfel, J.; Odaippurath, T.; Peediyakkal, M.Z.; Baiou, A.; Sarsak, E.; Elayana, M.; Kaleeckal, A.; et al. Clinical characteristics and risk factors for COVID-19-associated Candidemia. Med. Mycol. 2021, 59, 1262–1266. [Google Scholar] [CrossRef]
- Erami, M.; Raiesi, O.; Momen-Heravi, M.; Getso, M.I.; Fakhrehi, M.; Mehri, N.; Yarahmadi, M.; Amiri, S.; Raissi, V.; Hashemi, S. Clinical impact of Candida respiratory tract colonization and acute lung infections in critically ill patients with COVID-19 pneumonia. Microb. Pathog. 2022, 166, 105520. [Google Scholar] [CrossRef]
- Bishburg, E.; Okoh, A.; Nagarakanti, S.R.; Lindner, M.; Migliore, C.; Patel, P. Fungemia in COVID-19 ICU patients, a single medical center experience. J. Med. Virol. 2021, 93, 2810–2814. [Google Scholar] [CrossRef]
- Cannon, R.D. Oral Fungal Infections: Past, Present, and Future. Front. Oral Health. 2022, 3, 838639. [Google Scholar] [CrossRef]
- Suleyman, G.; Alangaden, G.J. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect. Dis. Clin. N. Am. 2021, 35, 1027–1053. [Google Scholar] [CrossRef]
- Adzic-Vukicevic, T.; Velickovic, J.; Radovanovic-Spurnic, A.; Velickovic, D.; Milenkovic, S.; Petrovic, F.; Micic, J.; Dragutinovic, N. Fatal invasive candidiasis in COVID-19 patient with severe bleeding and extensively drug-resistant Klebsiella enterobacter. J. Infect. Dev. Ctries. 2022, 16, 1025–1029. [Google Scholar] [CrossRef]
- Ezeokoli, O.T.; Pohl, C.H. Opportunistic pathogenic fungal co-infections are prevalent in critically ill COVID-19 patients: Are they risk factors for disease severity? S. Afr. Med. J. 2020, 110, 1081. [Google Scholar] [CrossRef]
| First Author (Year) | Admission | N Patients/Age | Study Design and Settings | Observation Period | Prevalence | Candida Species Found | Risk Factors in COVID-19/Candida coinfection |
|---|---|---|---|---|---|---|---|
| Segrelles-Calvo G. (2021) [14] | ICU | 215/>18 | Systematic review Observational and prospective study | February–April 2022 | Invasive candidiasis 14.4% | C. albicans M. parapsilosis | All patients were positive to Candida spp. Remaining for longer periods in the ICU in comparison to those who tested negative |
| Jeong S (2022) [15] | ICU | 57 admitted 379 outpatient/advanced age | More recent prevalence in coinfection by virus, bacteria and fungi. Observational and prospective study | August 2020– October 2021 | Fungal rate 10.5% 6/57 | C. albicans M. parapsilosis C. tropicalis | Advanced age Coinfection involving more than one virus, bacteria or fungi Neutrophil and lymphocyte count, as well as lactate dehydrogenase, were associated with higher mortality rate. |
| Porto Ana P.M (2022) [16] | ITU, ICU | 4563/adults | Ecological observational and prospective study | April–June 2020 | CLABSI’s incidence: 1.60 (IQR, 0.44–4.20) | Candida spp. | Higher incidence of Central Line-Associated Bloodstream Infection (CLABSI) due to Candida spp. |
| Nucci M (2021) [17] | ICU, | 41/mean age 62 years | Review study Retrospective | January 2019–February 2020 / March–September 2020 | Ranged from 0.7% to 23.5% | C. albicans | All patients with candidemia associated with COVID-19 were on mechanical ventilation with a central venous catheter, broad-spectrum antibiotics, steroids, and parental nutrition. |
| Peman J (2020) [18] | ICU | 1095/NR | Review study | 2019–2020 | NR | C. albicans M. parapsilosis C. tropicalis N. glabratus C. auris M. guilliermondii | Higher levels of pro-inflammatory (IL-1, IL-2, IL-6, TNF-α) and anti-inflammatory (IL-4, IL-10) cytokines. Less IFN-γ, CD4, and CD8 cell expression, thus raising the risk for severe fungi infections. |
| Abdoli A (2022) [19] | ICU | NR | Review study | NR | NR | C. albicans C. tropicalis M. parapsilosis, N. glabratus M. orthopsilosis | Prolonged ICU stay, central venous catheters, and steroid use as main risk factors in fungal infection. |
| Chiurlo M (2021) [20] | ICU | NR | Review study Systematic review | 2020–2021 | 10% of admitted patients due to COVID-19 infection High mortality rate >50% | C. albicans Candida spp. | Physical barriers’ alterations, vascular catheters, mucositis, GI surgery, immunosuppression, microbiota alterations, severe lung disease, diabetes or advanced age |
| Rajendra Santosh A.B. (2021) [21] | ICU | NR | Review study Systematic review | 2019–2020 | Frequency in infections due to fungi is rising due to the human immunodeficiency virus and immunosuppressive drugs | Candida spp. | Organ-transplanted patients, steroid use, azole drug use, control of systemic underlying pathologies, and prophylactic antibiotic regimen. Diabetes mellitus, broad-spectrum antibiotics, neutropenia, steroids, and voriconazole. |
| Arastehfar A. (2021) [22] | ICU | 1988/NR | Ecological observational and retrospective study | November 2020–January 2021 | C. albicans (57%) N. glabratus (28%) | C. albicans N. glabratus M. parapsilosis | Candidemia as a worsening factor in COVID-19 severity, broad-spectrum antibiotics, central venous catheter, mechanical ventilation, IL-6 inhibitor, and tocilizumab use. |
| Frías-De-León M. G. (2021) [23] | ICU | NR/>40 | Bibliographic research | January 2020–February 2021 | NR | C. albicans M. parapsilosis N. glabratus C. tropicalis C. auris P. kudriavzevii C. lusitaniae C. inconspicua C. dubliniensis M. orthopsilosis | COVID-19 pathophysiology characteristics (high levels of inflammatory cytokines and reduced T-Cells) favor fungal colonization and infection along with mechanical ventilation, central venous catheters, and prolonged hospitalization stay. |
| Katz J. (2021) [24] | Several hospitals and clinics | 889/NR | Using i2b2 database | Year 2019–2021 | Disproportionate compromised in Afro-American population (40% of COVID-19 cases and/invasive candidiasis) | C. albicans | Invasive candidiasis was associated with a higher risk in COVID-19 |
| Coskun A (2021) [25] | ICU | 627/mean of 73.5 | Review study Electronic clinical archives | March 2020–February 2021 | Ranging from 5% to 70% in mortality due to fungal infection in the ICU | C. albicans M. parapsilosis C. tropicalis | High scores in APACHE II, diabetes mellitus, neutropenia, kidney disease, abdominal surgery, broad-spectrum antibiotics, parenteral nutrition, hemodialysis, mechanical ventilation, central venous catheter, and immunosuppression treatments. |
| Machado M (2022) [3] | ICU | 47,048 on 2019 | Retrospective study | January 2019– December 2020 | Candidemia’s incidence: 4.73 patients with COVID in 1000 admissions, 0.85 patients without COVID in 1000 admissions | C. albicans M. parapsilosis C. tropicalis N. glabratus P. kudriavzevii K. marxianus | Central venous catheter-related candidemia was the most common entry way for patients with COVID-19 |
| Shishido AA (2022) [4] | ICU | 65 /NR | Review study | NR | High mortality rates | C. albicans | High incidence and mortality in patients with COVID-19, longer stays in the ICU and CVC longer stay in place, steroids use, sepsis, age higher than 65 years |
| Szabo BG (2021) [5] | ICU | 90 /advanced age (mean of 75.0 ± 13.0 years old) | Case series Retrospective observational study | March–July 2020 | C. albicans 50%, N. glabratus 37.5%, M. parapsilosis 12.5% and M. metapsilosis 12.5% | C. albicans N. glabratus M. parapsilosis M. metapsilosis | Candidemia increases morbidity and mortality in adult patients with severe COVID-19 disease. |
| Seagle EE (2022) [2] | NR | 251/NR | Case analysis | April–August 2020 | Up to 25.5% of all patients had a coinfection of Candida and SARS-CoV-2 | C. albicans, N. glabratus M. parapsilosis C. tropicalis C. dubliniensis C. lusitaniae P. kudriavzevii M. guilliermondii | Patients with COVID-19 had a higher risk of coinfection due to candidemia even when they did not have a commonly associated risk factor for candidemia |
| Koukaki E (2022) [6] | ICU | 178/66 | Retrospective observational study | August 2020– November 2021 | 5 out of 178 patients developed candidemia associated with COVID-19 but only one more patient was affected by candidemia and aspergillosis | M. parapsilosis (one patient) C. auris (one patient) N. glabratus(one patient) Candida spp. (three patients) | Higher incidence rate of fungal infections in patients admitted in the ICU due to COVID-19 disease |
| Erami M (2022) [26] | ICU | 69 a 100/61.1 (range = 21–88) | Descriptive study | NR | C. albicans (55; 79.7%) N. glabratus (12; 17.4%) and two more patients due to (2.9%) C. africana | C. albicans N. glabratus C. africana | Infection due to Candida spp. did not influence the variables of infection and death due to COVID-19. Airway colonization by C. albicans was commonly found, especially in patients with comorbidities such as diabetes, malignancy, and affected by renal alterations. |
| Kayaaslan B (2021) [7,27] | ICU | 2487/72 | Retrospective study | March 2020–March 2021 | Candidemia’s incidence was higher in the COVID-19 group (2.16, IC 95% 1.77–2.60) than those without COVID-19 (1.06, IC 95% 0.89–0.125) | C. albicans M. parapsilosis N. glabratus C. tropicalis and others | Higher incidence and early presentation with increased mortality rate due to candidemia in patients with COVID-19 |
| Salehi M (2020) [27] | ICU | NR | Review study | 2020 | Until May 25 2020, up to 133,521 confirmed cases of COVID-19 and 7359 deaths were reported in Iran * | C. albicans | Inadequate treatment increases the probability to develop a fungal infection, thus increasing the mortality rate. |
| Vitale RG (2022) [28] | ICU | 146/35–88 | Review study | 2021 | Estimated mortality due to invasive candidiasis ranged from 19% to 40% and up to 70% in the ICU | C. albicans C. auris N. glabratus C. tropicalis M. parapsilosis C. dubliniensis M. orthopsilosis P. kudriavzevii | In patients with COVID-19, fungal infections could worsen the prognosis and recovery |
| White, PL (2021) [29] | ICU | 51/mean age of: 57, M/F: 2.2/1 | Evaluation of a prospective cohort study | NR | Incidence of 26.7% (14.1% in aspergillosis and 12.6% in invasive candidiasis) | C. albicans | Invasive fungal disease associated with COVID-19 |
| Salehi M (2020) [30] | Several hospitals and clinics | 53/27 to 90 | Transversal study | March 2020– April 2020 | During the study, up to 53 (5%) out of 1059 iranian patients with COVID-19 confirmed infections had OPC* | C. albicans N. glabratus C. dubliniensis M. parapsilosis M. tropicalis P. kudriavzevii | * Invasive candidiasis (OPC) in patients with COVID-19 |
| Senok, A. (2021) [31] | Dubai’s hospital electronic system | 29.802/49.3 ± 12.5 | Retrospective review | February 31–July 2020 | 1.3% presented coinfection | C. auris M. parapsilosis | Coinfection in patients with COVID-19 |
| Sang, L. (2021) [32] | Several hospitals and clinics | 190/NR | Retrospective review of medical records of adult patients | January 2020 –April 2020 | C. albicans (6.8%) | C. albicans | Secondary infection in patients with COVID-19 |
| Jeong, S. (2022) [15] | Several hospitals and clinics | 436 samples of 57 admitted patients and 379 outpatients/65.7% were >60 years old | Prevalence evaluation in coinfection due to virus, bacteria and fungi in patients with COVID-19 | August 2020–October 2021 | Incidence rate in coinfections due to bacteria or fungi were 52.6% and 10.5%, respectively, in patients admitted due to COVID-19 | C. albicans | Higher coinfection rate in patients with COVID-19 disease |
| Mastrangelo A. (2021) [33] | ICU | 72/NR | Prospective cohort study comparing historical control patients without COVID-19 | February 2020–June 2020 | 35 (48.6%) | C. albicans | A characteristics description of candidemia in patients affected by SARS-CoV-2 |
| Amorim dos Santos J (2021) [34] | Worldwide study | 64,876/NR | Systematic review | January 2021, six months after the initial research (June 2020) | Eight studies reported invasive candidiasis | C. albicans | It reported oral signs and symptoms in patients with COVID-19 disease |
| Roudbary, M. (2021) [35] | Several hospitals and clinics | NR | Literature research | Between 2020 and 2021 | Common fungal infections were invasive candidiasis and aspergillosis | C. albicans | It reported opportunistic fungal diseases in patients with COVID-19 disease |
| Denny S. (2021) [36] | Several hospitals and clinics | 11/> 17 years old | Retrospective review in candidemia | March 2020–May 2020 | C. albicans in 63.6% | C. albicans M. parapsilosis N. glabratus C. dubliniensis | It describes the high incidence of candidemia in patients with COVID-19 disease |
| Norberg, C M. (2021) [37] | Scientific literature analysis, different regions of the world | NR | Bibliographic review | 2021 | 8 out of 9 patients had a coinfection due to Candida spp. (N. glabratus (4), C. auris (3) and C. albicans (1) | C. auris | Despite the high risk of developing fungal coinfection in patients infected by SARS-CoV-2, the data are scarce in relation to incidence and risks of secondary infections |
| Samaranayake, L. P. (2022) [38] | Database (Pubmed, OVID, SCOPUS and Web of Science) | 292/NR | Systematic review | March 2020– October 2021 | Candida infection was the most common coinfection, 64% (n = 96) | C. albicans | Orofacial mycoses in COVID-19 disease |
| Brandi, N. (2022) [39] | ICU | 95/NR | One center observational and retrospective study | October 2020–January 2021 | 27 (42.9%) patients tested positive for bacterial and fungal infections and 3 patients (4.8%) were affected exclusively by fungi | Candida spp. | Fungal coinfections are frequent in patients with COVID-19 admitted in the ICU and are associated with poor outcomes |
| Rafat, Z. (2022) [40] | ICU | 73/NR | Transversal study in which sputum samples and endotracheal aspirate of patients with COVID-19 in the ICU were collected | May to October 2020 | 15 cases (20.5%) confirmed with fungal coinfections | C. albicans | Patients with severe COVID-19 disease in the ICU were prone to develop fungal infections |
| Ayalon, O. (2022) [41] | ICU | 311/NR | Case–control study | 1 September 2020–31 March 2021 | Candidemia 3.5% | C. albicans | Incidence of invasive candidiasis in patients with COVID-19 disease |
| Soltani S. (2021) [42] | NR | 2246 patients | Systematic review and meta-analysis | 1 December 2019–30 December 2020 | Grouped prevalence of fungal coinfection 12.6% | Aspergillus 2.39% Candida 0.39% | NR |
| Kamali Sarvestani (2021) [43] | ICU | 153 patients | Transversal review | March 2020–March 2021 | NR | C. albicans (7/12, 58.3%), C. dubliniensis (2/12, 16.6%), C. tropicalis (1/12, 8.3%), N. glabratus(1/12, 8.3%), P. kudriavzevii (1 /12, 8.3%) | Presence and treatment of candidemia due to C. albicans and related species (C. dubliniensis) in Iranian patients with COVID-19 |
| Kubin CJ. (2021) [44] | Manhattan, New York, EEUU | 516/3028 patients | Retrospective cohort study | 2 March and 31 May 2020 | NR | NR | Fungal infections manly due to healthcare-related Candida spp. |
| Cataldo MA (2020) [45] | ICU | 2 | Retrospective cohort study | March–April 2020 | The incidence of invasive candidiasis in patients admitted in the ICU was higher in those affected by COVID-19 than prior the pandemic | C. albicans M. parapsilosis N. glabratus | Patients with COVID-19 had a higher risk to develop candidemia during stay in the ICU |
| Agrifoglio A (2020) [46] | ICU | 139 | Retrospective analysis | February to June 2020 | The four months candidemia incidence was 10.8%, much higher in comparison to the seven years prior data | C. albicans M. parapsilosis N. glabratus | It was identified an exponential raise in invasive candidiasis cases |
| Hughes S (2020) [47] | Several hospitals | 836 | Observational study | February 20– April 20 2020 | The incidence of bacterial and fungal coinfection was observed in patients admitted with severe acute respiratory distress syndrome | C. albicans | The main pathogen involved in fungal coinfection was C. albicans |
| Antinori S. (2020) [48] | ICU | 99 | Review article | 2020 and January 2022 | NR | N. glabratus C. albicans | Evidence reveals bacterial and fungal coinfection in COVID-19 patients |
| Papadimitriou-Olivgeris M. (2022) [49] | ICU | 3572 | Retrospective study | 2010–August 2021 | Steroid therapy was evaluated in relation to develop candidemia during the COVID-19 pandemic | M. parapsilosis C. auris | A significant increase in candidemia incidence was evaluated during the COVID-19 pandemic in patients with and without COVID-19 |
| Baddley JW. (2021) [50] | ICU | 37 studies | Retrospective study | June 2021 and November 2021 | Fungal coinfection’s incidence varies and it is related to the population heterogeneity, surveillance protocols, and fungal infection definition | Invasive candidiasis and endemic mycoses | Invasive fungal infections are associated with severe lung injury and immunological deficits such as HIV or immunomodulatory drugs |
| Macauley P. (2022) [51] | ICU | 3568 | Overall analysis and comparison | May 2021 and October 2021 | 12 cases in COVID-19 group (5.1% incidence) 51/1.000 admissions | C. albicans accounted for a minority of isolates | Increase in cases in the SARS-CoV-2 pandemic. |
| Basile K. (2022) [52] | ICU | Not specified | Review article | 6 December 2021 and 6 January 2022 | Not specified | Aspergillus fungal infections including invasive candidiasis, cryptococcosis, pneumocystosis, mucormycosis, and endemic mycoses | Increase in fungal infection associated with COVID-19 disease |
| Kayaaslan B. (2021) [7] | ICU | 1229 | Retrospective study | August 2020 to August 2021 | The candidemia incidence was evaluated in critical patients affected by COVID-19 with risk factors | C. albicans | Patients with severe COVID-19 disease had a higher risk of developing candidemia due to exposure to classical risk factors and specific risks in COVID-19 in the ICU |
| Elbaz M- (2022) [53] | ICU | 1000 | Multicenter Cohort Study | February 2020 and May 2021. | Variation in incidence of lung disease due to mold, ranging between 0 and 51.2 per 1000 critical hospitalizations. | Lung disease due to mold associated with COVID-19 and invasive candidiasis | Very variable data on mold conditions have been reported. |
| First Author (Year) | Country | Diagnosis Type COVID-19 | Diagnosis Type Invasive Candidiasis | Treatment |
|---|---|---|---|---|
| Segrelles-Calvo G. (2021) [14] | Spain | PCR and IgG | Blood culture | Immunosuppressant/anti-inflammatory (tocilizumab (TCZ)) immunosuppressants/systemic corticosteroids (tocilizumab and systemic steroids (SS)) immunomodulator/antiviral (interferon 1β (IFN-1 β)) antiviral (lopinavir–ritonavir) |
| Jeon S (2022) [15] | United Kingdom | NR | RT-PCR multiplex Matrix-assisted laser desorption ionization mass spectrometry (Vitek-MS) (MALDI) | Immunosuppressors including steroids and TCZ |
| Porto Ana PM (2022) [16] | Brazil | NR | NR | Antibacterial (piperacillin–tazobactam (PIP-TZ) meropenem and vancomycin) |
| Nucci M (2021) [17] | Brazil | NR | NR | Antifungal (anidulafungin and fluconazole) |
| Peman J (2020) [18] | USA Brazil, India, Russia, Peru, Chile, Mexico y South Africa | NR | NR | Antifungal (anidulafungin and isavuconazole) |
| Abdoli A (2022) [19] | NR | NR | Serological test with β-D-glucan (BDG) and mannan antigen | Echinocandins Azoles (voriconazole/fluconazole/ posaconazole/isavuconazole) Polyenes (liposomal amphotericin b) |
| Chiurlo M (2021) [20] | NR | RT-PCR-antigen test | Pathogen isolation serological test with β-D-glucan (BDG) and mannan antigen | Immunosuppressor drugs, TCZ use, steroids, and anti-IL-6 receptor agents |
| Rajendra Santosh AB. (2021) [21] | India | NR | Exfoliative cytology Pathogen culture Saliva test and oral mucosa biopsy | Polyenes (nystatin and b-amphotericin) Azoles (fluconazole, itraconazole and pozaconazole) Antimetabolites (flucytosine) |
| Rajendra Santosh AB. (2021) [54] | India | NR | Special care must be given to patients with a recent diagnosis of COVID-19 to detect and prevent mucormycosis | NR |
| Arastehfar A. (2021) [55] | Iran | RT-PCR | Positive blood culture, 21-plex PCR and sequencing | Antifungals (fluconazole or caspofungin) |
| Frías-De-León M. G. (2021) [23] | Several areas | RT-Q PCR | Molecular and microbiological | Azole antifungal drugs (fluconazole, voriconazole, isavuconazole) echinocandins (caspofungin, anidulafungin, micafungin) Polyenes (b-amphotericin, nystatin) |
| Katz J. (2021) [24] | Africa | NR | NR | NR |
| Coskun A (2021) [25] | Turkey | COVID-19 through electronic medical records and blood cultures | Blood culture | Carbapenem and glycopeptides 27 remaining patients with combination of carbapenem and oxazolidinone or glycopeptide family drug |
| Machado M (2022) [3] | Spain | PCR | Blood culture | Antifungal (echinocandins and fluconazole) |
| Shishido AA (2022) [4] | NR | NR | NR | Steroids and immunosuppressor therapy |
| Szabo BG (2021) [5] | Hungary | PCR | Blood culture | Antifungals (caspofungin, fluconazole, voriconazole, itraconazole, isavuconazole, B- amphotericin) |
| Seagle EE (2022) [6] | USA | PCR | Blood culture | NR |
| Koukaki E (2022) [6] | Greek | PCR | Blood culture | Half of all patients were treated with TCZ and a high dose of dexamethasone, two more received additional monoclonal antibody therapy |
| Erami M (2022) [26] | Iran | Diagnosed based on symptoms, radiological signs, PCR | Microbiological tests | Steroid dosage > 2 mg/kg dexamethasone Antifungals (b-amphotericin, voriconazole, itraconazole, fluconazole, caspofungin) |
| Kayaaslan B (2021) [7] | Turkey | PCR or common finding of COVID-19 in CT-SCAN with a positive antigen test | Blood culture | Antifungals (fluconazole, voriconazole, caspofungin and micafungin) |
| Salehi M (2020) [27] | Iran | Physical examination and PCR | Blood culture, MALDI-TOF (blood culture) and RT-PCR | Broad-spectrum antibiotics, immunosuppressors or steroids, invasive or non-invasive mechanical support Antifungals (fluconazole and nystatin) |
| Vitale RG (2022) [28] | India, Brazil, China, Italy, Iran, UK, USA, Mexico, Colombia | PCR | Blood culture | Antifungal and antibiotic treatment Steroids Antifungals (B-amphotericin, anidulafungin, liposomal, isavuconazole, micafungin, voriconazole) |
| White, P. L, 2021 [29] | NR | PCR | PCR for Pneumocystis NBL-BAL. Serological BDG proposed if positive more test should be run for fungi (PCR- GM-EIA) | Antifungal therapy (AFT) in this cohort study could be beneficial for survival if started early, but it needs prospective validation. Prophylactic AFT could be beneficial in this group |
| Salehi M. (2020) [27] | Iran | PCR and sequencing technique of internal transcribed spacing region (ITS1–5.8S-ITS2) | Presence of gemmating yeast and pseudo-hyphae in a 10% KOH preparation and culture | Most of the isolated Candida species were sensible for three antifungal drug families: azole (fluconazole, voriconazole and itraconazole), polyenes (B-amphotericin), and echinocandins (caspofungin, anidulafungin and micafungin). |
| Senok, A. (2021) [31] | Arab Emirates United | RT-PCR test for SARS-CoV-2 | Coinfection confirmed by laboratorial test and culture | Mean lapse to empiric antibiotic star was 1.2 ± 3.6 days after admission, with ceftriaxone, azithromycin, and piperacillin–tazobactam being the most common used drugs |
| Sang, L., (2021) [32] | China | NR | Bacterial and fungal frequency was measured in cultures of airway and blood samples | Antifungal and antibiotic treatment was administrated in 71 (43.8%) patients |
| Jeong, S., (2022) [15] | South Korea | PCR-RT | Culture with antibiogram to detect pathogens were carried out and underwent a Multiplex test | Poor immune response due to SARS-CoV-2 infection and immunosuppressor treatment (steroids and tocilizumab) and COVID-19 therapeutics may boost fungal infection |
| Mastrangelo, A. (2021) [33] | Italy | NR | NR | Antifungal |
| Amorim dos Santos J, (2021) [34] | Worldwide | PCR | Invasive candidiasis infection was confirmed in presence of germ tube; positive presence of pseudo-hyphae in a 10% KOH preparation and culture | NR |
| Roudbary, M., (2021) [35] | Several regions worldwide | PCR | MALDI-TOF (blood culture) Molecular sequencing technique | Antifungals (intravenous fluconazole, caspofungin, micafungin, anidulafungin, and b-amphotericin) |
| Denny, S. (2021) [36] | United Kingdom | PCR | Blood culture identified throughout spectroscopy | All isolated pathogens were sensitive to fluconazole, with the exception of one case, N. glabratus, that showed moderate sensibilization |
| Norberg, C. M. B. M. (2021) [37] | Brazil | PCR | Blood culture IgG test and germ tube test | An association exists between tocilizumab treatment and the development of candidemia in patients with COVID-19 |
| Vitale, R. G. (2022) [28] | Brazil | PCR | Culture | In Brazil, all species of C. auris were reported as sensible to azole, amphotericin, and echinocandins |
| Samaranayake, L. P. (2022) [38] | 14 countries | PCR | Clinical observation of sites with systemic candidiasis manifestation | Infections due to Candida spp. were treated with antifungals (oral nystatin, miconazole, or systemic fluconazole) |
| Brandi, N. (2022) [39] | Italy | PCR | Radiological images are a key component to detect coinfections | Non-specific therapy recorded |
| Rafat, Z. (2022) [40] | Iran | PCR | Direct microscopic observation 10% KOH preparation and culture | NR |
| Ayalon, O. (2022) [41] | Israel | PCR | Spectometry MALDI-TOF (blood culture) | NR |
| Salehi M (2020) [27] | Iran | PCR | Sequencing technique of internal transcribed spacing region (ITS1-5.8S-ITS2) and microbiological methods | Antifungals (fluconazole, itraconazole, voriconazole, B-amphotericin, caspofungin, micafungin, and anidulafungin) |
| Soltani S. (2021) [42] | Iran | NR | NR | Antifungals (amphotericin B, micafungin, and fluconazole) |
| Kamali sarvestani h. (2021) [43] | Iran | PCR | Blood cultures, mycological test and sequencing technique of internal transcribed spacing region | Antifungals (caspofungin alone, B-amphotericin, voriconazole, fluconazole, itraconazole) |
| Kubin CJ. (2021) [44] | USA | PCR | Blood cultures | Hydroxychloroquine, azithromycin, low-dosage methyl-prednisone, and fluconazole. Remdesivir (antiviral), vancomycin, and carbapenem (antibacterials). |
| Antinori S. (2020) [48] | Italy | NR | NR | Antifungals (voriconazole, voriconazole switched to isavuconazole, isavuconazole, caspofungin followed by voriconazole, liposomal amphotericin B) |
| Papadimitriou-olivgeris M. (2022) [49] | NR | NR | NR | Antifungals (fluconazole, voriconazole, echinocandins, anidulafungin, caspofungin, micafungin, liposomal-amphotericin b) |
| Basile K. (2022) [52] | Australia | PCR | Blood cultures | Antiviral Treatment |
| Kayaaslan B. (2022) [7] | Turkey | NR | NR | NR |
| Elbaz M. (2022) [53] | Israel | PCR | Blood cultures | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Ledezma, O.E.; Reyes-Montes, M.d.R.; Acosta-Altamirano, G.; Frías-De-León, M.G.; García-Salazar, E.; Duarte-Escalante, E.; Santiago-Abundio, J.; González-Miguel, Z.; García-Hernández, M.d.L.; Martínez-Quezada, R.; et al. Invasive Candidiasis Coinfection in Patients with Severe COVID-19 Disease: Scoping Review. Pathogens 2025, 14, 466. https://doi.org/10.3390/pathogens14050466
Valencia-Ledezma OE, Reyes-Montes MdR, Acosta-Altamirano G, Frías-De-León MG, García-Salazar E, Duarte-Escalante E, Santiago-Abundio J, González-Miguel Z, García-Hernández MdL, Martínez-Quezada R, et al. Invasive Candidiasis Coinfection in Patients with Severe COVID-19 Disease: Scoping Review. Pathogens. 2025; 14(5):466. https://doi.org/10.3390/pathogens14050466
Chicago/Turabian StyleValencia-Ledezma, Omar Esteban, María del Rocío Reyes-Montes, Gustavo Acosta-Altamirano, María Guadalupe Frías-De-León, Eduardo García-Salazar, Esperanza Duarte-Escalante, Jesús Santiago-Abundio, Zuleyma González-Miguel, María de Lourdes García-Hernández, Rebeca Martínez-Quezada, and et al. 2025. "Invasive Candidiasis Coinfection in Patients with Severe COVID-19 Disease: Scoping Review" Pathogens 14, no. 5: 466. https://doi.org/10.3390/pathogens14050466
APA StyleValencia-Ledezma, O. E., Reyes-Montes, M. d. R., Acosta-Altamirano, G., Frías-De-León, M. G., García-Salazar, E., Duarte-Escalante, E., Santiago-Abundio, J., González-Miguel, Z., García-Hernández, M. d. L., Martínez-Quezada, R., Torres-Páez, O. U., Galindo-Oseguera, E., Meza-Meneses, P., & Santiago-González, N. (2025). Invasive Candidiasis Coinfection in Patients with Severe COVID-19 Disease: Scoping Review. Pathogens, 14(5), 466. https://doi.org/10.3390/pathogens14050466








