Abstract
Toxoplasmosis, caused by Toxoplasma gondii, is a widespread parasitic infection affecting humans and animals. The genetic diversity of T. gondii varies across regions, with type I, II, and III strains predominantly circulating in Europe and North America. This study genotyped 67 (78.8%) T. gondii DNA isolates from cats using nested and multilocus PCR-RFLP, identifying type I, genotype #10 (ToxoDB#10), for the first time in Poland. The other 18 (21.2%) stool samples containing T. gondii-like oocysts were confirmed as Hammondia hammondi. Comparative analysis with data from other countries highlights notable regional differences in genotype prevalence. The high occurrence of genotype 3 (ToxoDB#3) in central Europe may be linked to its presence in wild rodents and insectivores, key reservoirs in the parasite’s life cycle. Additionally, genetic analysis of meat products and livestock indicates a potential transmission pathway to felines through raw or undercooked meat consumption. These findings contribute to a better understanding of T. gondii epidemiology and its implications for public health and veterinary medicine.
1. Introduction
Toxoplasma gondii is an intracellular protozoan parasite capable of infecting all warm-blooded animals, in contrast to Hammondia hammondi, which can naturally infect only a limited range of animals like mice, rats, and felids [1,2,3]. House cats and other felids are the only animals that can excrete T. gondii and H. hammondi oocysts into the environment through their feces. T. gondii oocysts become infective after sporulating for 3 to 5 days and can remain viable in water and soil for many months [4,5,6]. However, the sporulation period can be as short as one day under optimal environmental conditions, such as those found in cat litter [7]. Approximately one-third of the global human population is infected with T. gondii; however, clinical symptoms, such as fever, ocular inflammation, and lymphadenopathy, occur in fewer than 20% of infected individuals [8,9]. Toxoplasmosis poses a significant challenge in immunocompromised patients, where generalized infection may develop, leading to neurological, lymphatic, or ocular complications [10]. The primary sources of T. gondii infection in humans include raw meat, raw milk, or mature cheese containing viable tachyzoites or bradyzoites of the parasite [11,12]. Cats and other felids become infected after consuming raw meat or tissues from any warm-blooded animal that can act as an intermediate host [13]. In young cats, during their first infection, clinical signs such as diarrhea, lethargy, polydipsia, jaundice, dyspnea, and fever may be observed [14]. Infected cats can shed between 3 and 8 million oocysts during the shedding period, although in some cases, no oocysts are excreted at all [15]. Zulpo et al. confirmed that the shedding of T. gondii oocysts may remain elevated in experimentally re-infected cats even years after the primary infection. Approximately 10% and 71% of cats re-infected with different strains were found to excrete oocysts following secondary and tertiary infections, respectively [16].
Dubey demonstrated a relationship between T. gondii infection and Cystoisospora felis. In cats initially infected with T. gondii and subsequently with C. felis, reactivation of toxoplasmosis and oocyst shedding could occur. However, when the order of infection was reversed, the local intestinal immunity induced by C. felis was sufficiently strong to prevent the shedding of T. gondii oocysts. Moreover, in adult cats, a reshedding may be induced by co-infection with other infectious diseases or long-term treatment with immunosuppressive drugs [17].
The oocysts of T. gondii and Hammondia hammondi are very similar in morphology, and the parasites themselves are closely related as tissue cyst-forming coccidians [18]. However, the bioassay procedure can differentiate them. T. gondii tissue cysts are more commonly found in neural tissue, whereas H. hammondi cysts predominantly localize in skeletal muscle. Additionally, tachyzoites and bradyzoites of H. hammondii are not infective after oral administration. Moreover, H. hammondi appears to be non-pathogenic and does not cause clinical disease in cats or any naturally infected hosts, including humans [6]. Molecular techniques based on the polymerase chain reaction (PCR) are the methods for differentiating Hammondia-like organisms from T. gondii oocysts [19,20]. Due to variations in virulence among T. gondii strains, multilocus PCR-RFLP (restriction fragment length polymorphism) genotyping plays a crucial role in determining the geographic distribution and genetic diversity of clonal lineages [21,22,23,24,25]. In this study, we present the results of T. gondii and H. hammondi oocyst differentiation using PCR methods and a molecular analysis of T. gondii strains isolated from feline feces collected in Poland between 2020 and 2024.
2. Materials and Methods
2.1. Sample Collection
Between 2020 and 2024, a total of 61,648 feline fecal samples were examined using the zinc flotation method (ZnSO4, Specific Gravity = 1.31) and direct smear microscopy in a 0.9% saline solution. All stool samples were examined in a commercial veterinary laboratory as part of the diagnosis and prevention of gastrointestinal parasitic infestation. Stool consistency was evaluated in the laboratory prior to microscopic examination. Samples containing oocysts smaller than 15 μm in diameter were frozen for further molecular analysis (Figure 1).
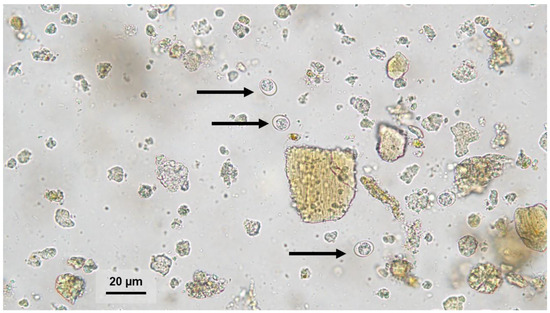
Figure 1.
Oocyst (black arrows) morphologically resembling Toxoplasma gondii or Hammondia hammondi in a feline fecal sample detected by zinc sulfate flotation. Magnification 400×.
2.2. DNA Isolation and Molecular Analysis
DNA extraction was performed using the commercial Genomic Mini AX Stool kit (A&A Biotechnology, Gdańsk, Poland). The extracted DNA was suspended in 200 μL of elution buffer and stored at −20 °C for further analysis.
To differentiate T. gondii and H. hammondii, two different PCR tests were used [19,26]. Samples positive for T. gondii were subsequently analyzed by the nested and multiplex multilocus PCR-RFLP (Mn-PCR-RFLP) using various genetic markers: the B1 gene [21,27] and SAG1, 5′SAG2, 3′SAG2, alt.SAG2, SAG3, BTUB, GRA6, Apico, C22-8, C29-2, L358, and PK1 [8,24,28,29,30,31]. The primers and restriction enzymes applied in this study are presented in Table 1 and Table 2. Both PCR and nested PCR were carried out via a MultiGene optiMAX thermal cycler (Labnet International, Inc., Taoyuan, Taiwan) using a commercial StartWarm HS-PCR Mix kit (A&A Biotechnology, Gdańsk, Poland). The 530 bp products of B1 gene amplification were sequenced and analyzed using Chromas 2.6.6 (Technelysium Pty Ltd., South Brisbane, Australia) and MEGA version 7 software. To compare the obtained nucleotide sequences with the NCBI GenBank database, the Basic Local Alignment Search Tool (BLAST®, https://blast.ncbi.nlm.nih.gov/Blast.cgi; accessed on 3 December 2024) was employed. Amplification products from nested and Mn-PCR-RFLP reactions were visualized on a 1.2% agarose gel under ultraviolet light. Subsequently, Mn-PCR-RFLP products were digested with restriction enzymes in a 20 μL volume and separated on a 3.5% agarose gel. The obtained band patterns were compared with data from ToxoDB (https://toxodb.org/toxo/; accessed on 16 December 2024) and two research papers [32,33] to determine the T. gondii genotype (Table 3).

Table 1.
List of primers used in the screening test to differentiate Hammondia hammondi and Toxoplasma gondii oocysts from feline stool samples.

Table 2.
List of primers and corresponding restriction enzymes used in molecular characterization of Toxoplasma gondii strains obtained from feline stool samples.

Table 3.
Toxoplasma gondii genotype number based on the RFLP patterns presented in ToxoDB and referenced from Brennan et al., 2016 [32] and Chen et al., 2025 [33].
2.3. Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics version 29.0 (Armonk, NY, USA) for the following variables: sex (female, male), age (in years), co-infection (e.g., presence of other intestinal parasites such as Giardia duodenalis or Cystoisospora spp.), stool consistency (formed, diarrhetic), and parasite genotype (T. gondii type I or II, H. hammondi). Only statistically significant results (p ≤ 0.05) are reported. Given that most variables were measured on a nominal scale and the age variable did not meet the assumption of normality, non-parametric tests were applied.
3. Results
Between 2020 and 2024, oocysts smaller than 15 μm in diameter were found in 0.14% (85/61,648) of feline stool samples. The 67 (0.11%) samples were tested positive for T. gondii and 18 (0.03%) for H. hammondi (Figure 2 and Figure 3). No co-infection of T. gondii and H. hammondi was observed. The annual detection rates of T. gondii and H. hammondi are presented in Table 4. Among 67 T. gondii DNA isolates analyzed, 13 belong to clonal lineage I and 54 to lineage II (Figure 4, Figure 5 and Figure 6). Additional information on co-infections, stool consistency, sex, age of cats, and T. gondii strain types is provided in Table 5. Nested and Mn-PCR-RFLP enabled the identification of three T. gondii genotypes: ToxoDB#1 genotype (11.9%; 8/67), ToxoDB#3 (68.7%; 46/67), and ToxoDB#10 (19.4%; 13/67). All 13 genetic markers were successfully amplified, which can be explained by the large number of oocysts excreted in the cat feces (Table 3).
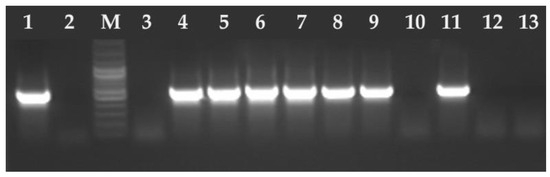
Figure 2.
Electrophoresis results of the PCR products on a 2% agarose gel. The amplified PCR product 530 bp T. gondii B1 gene fragment. Lane 1—positive control RH strain of T. gondii, lane 2—negative control (ddH2O), M—marker (Marker 3, A&A Biotechnology, Gdańsk, Poland), lanes 3, 10, 12–13 negative samples, lanes 4–9 and 11 positive samples.
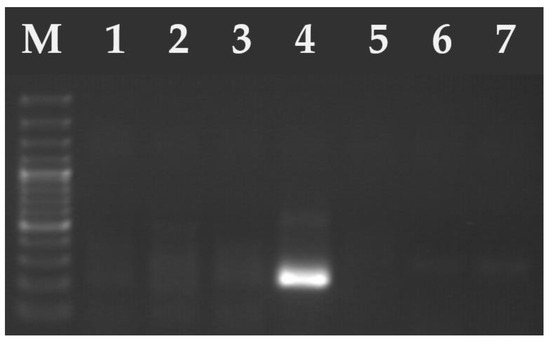
Figure 3.
Electrophoresis results of the PCR products on a 2% agarose gel. The amplified PCR product ~240 bp of H. hammondi (lane 4—positive sample). Lanes 1–3 and 5–6 samples were negative for H. hammondi. M—marker (Marker 3, A&A Biotechnology, Gdańsk, Poland).

Table 4.
Annual distribution of feline stool samples testing positive for T. gondii and H. hammondi in Poland.
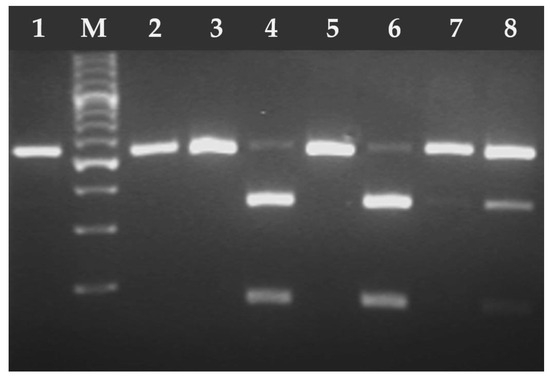
Figure 4.
Electrophoresis results of the RFLP-PCR products of T. gondii B1 gene fragment digested with PmlI (Eco72) endonuclease. Lane 1 T. gondii RH strain (type I), lanes 2–8 T. gondii DNA isolates from feline feces; lanes 2–3, 5, 7 (type I), lanes 4, 6, and 8 (type II/III). M—marker (Marker 3, A&A Biotechnology, Gdańsk, Poland).
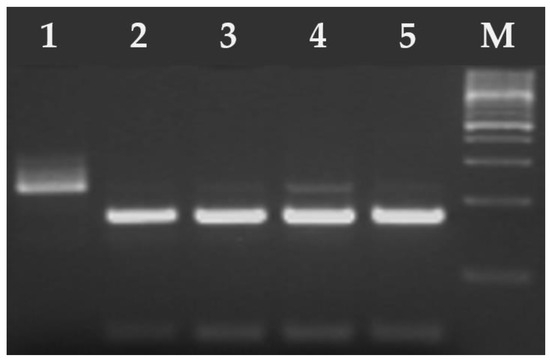
Figure 5.
Electrophoresis results of the RFLP-PCR products of T. gondii SAG2-3′ digested with HhaI endonuclease. Lane 1 T. gondii RH strain (type I), lanes 2–5 T. gondii DNA isolates from feline feces (type II/III). M—marker (Marker 3, A&A Biotechnology, Gdańsk, Poland).
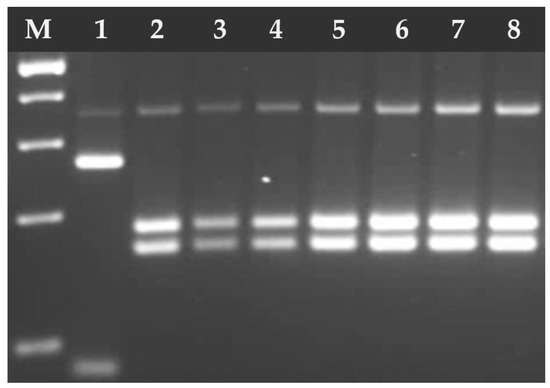
Figure 6.
Electrophoresis results of the RFLP-PCR products of T. gondii GRA6 digested with MseI endonuclease. Lane 1 T. gondii RH strain (type I), lanes 2–8 T. gondii DNA isolates from feline feces (type II). M—marker (Marker 3, A&A Biotechnology, Gdańsk, Poland).

Table 5.
Comparison of T. gondii and H. hammondi positive samples according to host age, sex, stool consistency, co-infections, and identified clonal lineage.
The statistical analyses were based on data obtained from 85 domestic cats (45 females and 40 males), aged approximately 2 months to 18 years. The overall mean age was 2.71 years (SD = 4.09); female cats had a mean age of 1.59 years (SD = 2.43), while males averaged 3.97 years (SD = 5.12).
A chi-square goodness-of-fit test revealed no significant difference in T. gondii prevalence between females (n = 36) and males (n = 31), χ2(1) = 0.37, p = 0.541. Among cats under 1 year of age, T. gondii (n = 45) was significantly more prevalent than H. hammondi (n = 12), χ2(1) = 19.11, p < 0.001. This trend was consistent across the full sample: T. gondii (n = 67) occurred significantly more frequently than H. hammondi (n = 18), χ2(1) = 28.25, p < 0.001. A chi-square test for independence indicated a significant association between age group and the occurrence of diarrhea, with younger cats (<1 year) exhibiting diarrhea more frequently than older individuals, χ2(1) = 7.11, p = 0.008, Cramer’s V = 0.317. Cats infected with T. gondii type I (ToxoDB#10) were significantly younger (mean rank = 20.54, n = 13) compared to those infected with type II (ToxoDB#1 and 3), (mean rank = 37.24, n = 54), as revealed by a Mann–Whitney U test, U = 176.00, Z = –2.78, p = 0.005, r = 0.34. The type of parasite was also significantly associated with the presence of diarrhea, which occurred more frequently in cats shedding T. gondii than in those shedding H. hammondi, χ2(1) = 13.08, p < 0.001, Cramer’s V = 0.424. However, there was no significant association between parasite type and the presence of co-infections, χ2(1) = 0.041, p = 0.839 (Table 4). The lack of detailed information regarding the cats’ origin, lifestyle, and diet limits the possibility of providing a more comprehensive context for the findings.
4. Discussion
Cats and other felids are the only mammals in which the sexual phase of the life cycle of T. gondii or H. hammondi occurs [2,34]. Domestic cats living in close proximity to humans are susceptible to T. gondii infection regardless of age, sex, or breed [35]. During the shedding period, cats can excrete over three million oocysts into the environment via feces [15].
However, the shedding of T. gondii oocysts typically precedes the development of specific antibodies, rendering serological tests ineffective for detecting active oocyst excretion [36]. Lappin et al. emphasize that diagnosing toxoplasmosis in cats through coproscopic examination is unreliable, due to both the brief duration of oocyst shedding and the low sensitivity of this diagnostic method [36]. The oocysts of T. gondii and H. hammondi are morphologically very similar, making them difficult to distinguish through routine microscopic examination [37]. However, certain features observable in histopathological analysis and pathophysiological differences following bioassays in mice, such as the infectivity of tachyzoites and bradyzoites or the ability for congenital transmission, are present in T. gondii but absent during H. hammondi infections [3]. Consequently, correct differentiation of T. gondii oocysts from H. hammondi is essential for epidemiological studies and ensuring public health safety. Therefore, molecular biology techniques are employed as they allow for the rapid identification of the protozoan without performing a bioassay [19].
Berger-Schoch et al. detected small oocysts in 2 out of 252 feline fecal samples. Molecular analysis confirmed T. gondii in one sample and H. hammondi in the other. The T. gondii-positive case was an 11-year-old indoor individual with chronic pneumonia [38]. In our study, T. gondii shedding was also detected in an 18-year-old cat, which had been treated with prednisolone 1 mg/kg, twice daily (Prednicortone 20 mg tablets for dogs and cats, Dechra Regulatory B.V., Best, The Netherlands) for intestinal lymphoma. Researchers suggest that oocyst shedding and acute systemic toxoplasmosis in older cats may be associated with compromised immunity resulting from comorbidities, including infectious diseases such as Feline Immunodeficiency Virus (FIV), Feline Leukemia Virus (FeLV), Feline Infectious Peritonitis (FIP), or immunosuppressive therapy [39,40,41,42,43,44].
Diarrhea is a frequently reported symptom of T. gondii and other coccidian intestinal parasitosis [45,46]. Diarrhea was also reported in 2 out of 60 cats in Latvia and 6 out of 903 cats in Germany, shedding T. gondii oocyst [47,48]. In our research, diarrhea was observed in 80.6% (54/67) of cats shedding T. gondii and 33.3% (6/18) of those shedding H. hammondi.
T. gondii oocysts are rarely diagnosed via coproscopy due to the short duration of shedding. In Poland, Wąsiatycz identified T. gondii oocysts in 0.67% (1/149) of cat stool samples in Poznań [49]. Similarly, in Germany, coproscopic examination conducted between 2004 and 2006 in a private laboratory revealed a comparably low shedding rate (0.1%; 22/20,317). Additionally, only a few individual cats shedding oocysts were identified in studies from France, Austria, and Switzerland: 2/858, 1/994, and 1/5, respectively. In contrast, no oocyst-positive fecal samples were found in the feline population from the Netherlands (n = 966), Denmark (n = 437), or Italy (n = 257) [50]. In Brazil, T. gondii oocyst shedding was observed in 4 out of 237 stray animals (two kittens and two adult cats). Notably, these animals did not have detectable specific IgG antibodies, which suggests a primary infection [51].
The genetic diversity of T. gondii circulating in Europe and North America includes three major clonal lineages: type I, II, and III, which are capable of infecting humans and animals and are also commonly found in the environment (soil and water) [52,53,54,55,56,57]. Although these strains do not differ morphologically, their virulence varies significantly in experimental murine models [55]. Genotyping conducted by Lehmann et al. [58] identified more virulent atypical strains and hybrid isolates of T. gondii, which are significantly more prevalent in South America than in Europe or North America.
In the current study, 67 T. gondii DNA isolates from feline feces were genotyped using nested and multilocus PCR-RFLP. Thirteen of these isolates were identified as type I (ToxoDB#10). The genetic structure of T. gondii strains in cats has been analyzed across various regions globally. In Southern Thailand, among eight T. gondii isolates from cat feces, two were atypical, two were recombinant, one belonged to type I, two to type III, and one to either type II or III [59]. In Iran (Mashhad region), T. gondii type II genotype was identified in the feces of 8 out of 175 stray cats. Additionally, T. gondii DNA was detected in brain tissue and in the feces of 2 out of 31 deceased stray cats [60].
Numerous European studies utilizing multilocus PCR-RFLP have shown that type II is the predominant clonal lineage identified in domestic cats [38,61,62]. However, type I isolates have also been particularly reported in Spain and Italy [63,64]. In our study, type I (T. gondii clonal lineage I) was detected in 13 out of 67 feline fecal samples. For the first time, atypical T. gondii genotypes, distinct from clonal lineages I, II, and III, were identified in Germany in feces from naturally infected cats [65]. Molecular analysis of T. gondii strains responsible for systemic toxoplasmosis in cats also suggests genotype 3 (type II) [66]. The same genotype has also been documented in Germany [65]. In the present study, ToxoDB#3 was detected in 46 out of 67, indicating it as the dominant genotype infecting cats in Central Europe [62].
The high prevalence of this genotype may be related to its widespread occurrence in wild rodents and insectivores, which act as intermediate or paratenic hosts in the life cycle of T. gondii [67]. In Poland, T. gondii DNA, primarily genotype II and III, was detected in tissue samples from 10 wild rodents and insectivores captured in the Lublin Province [68]. In the Mazury Lake District (northeastern Poland), Grzybek et al. reported a T. gondii seroprevalence of 5.5% (32/577) among four rodent species [69]. However, a separate study failed to detect T. gondii DNA in the tissues of seropositive animals [70].
Consumption of raw or undercooked meat is considered one of the primary risk factors for T. gondii infection in domestic cats [71,72,73]. Genetic analysis of T. gondii strains isolated from sheep in Italy revealed infection with the type II clonal lineage [74]. In 19 out of 57 pork meat samples from grocery stores in the United Kingdom, T. gondii DNA of types II and I was detected [75]. In studies conducted in Poland by Sroka et al., T. gondii DNA was detected in 5.4% (175/3223) of meat product samples retailed in Poland. Genetic multilocus analysis was possible for only 61 PCR-positive samples amplified for the B1 gene. Finally, type I, type II, and type III T. gondii lineages were identified in 10 (10.2%), 17 (17.3%), and 48 (49.0%) samples, respectively [76]. These findings suggest that the same genotypes circulating among livestock are likely responsible for infections in domestic cats, particularly those with access to raw or undercooked meat.
5. Conclusions
Based on our study, the detection rate of T. gondii oocysts in feline feces with coproscopy methods was below 1%, and distinguishing them with H. hammondi oocyst without the use of molecular techniques is not feasible. The results also demonstrated considerable genetic diversity of T. gondii among cats across Poland. To the best of our knowledge, this is the first study confirming the presence of type I clonal lineage (ToxoDB#10) in naturally infected cats in Poland. This finding underscores the importance of implementing advanced diagnostics tools for toxoplasmosis, not only in definitive hosts, but also in susceptible species, including humans. The observed prevalence of T. gondii type I raises concerns regarding its ecological impact and potential risks to both human and animal health, highlighting the urgent need for further research into environmental reservoirs, transmission pathways, and the epidemiological significance of different genotypes.
Author Contributions
Conceptualization, D.J.; methodology, D.J.; formal analysis, R.S.; resources, D.J., A.K.M. and J.O.; data curation, D.J., A.K.M. and J.O.; writing original draft preparation, D.J.; writing review and editing, D.J., O.S.-J. and R.S.; visualization, D.J. and R.S.; supervision, O.S.-J., R.S. and D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was carried out in accordance with the standards recommended by the EU Directive 2010/63/EU for animal experiments and Good Laboratory Practice and the Act of Polish Parliament of 15 January 2015 on protection of animals used for scientific purposes (Journal of Laws 2015, item 266). All procedures were in line with Polish law regulations. Samples were submitted by veterinarians as a part of the laboratory service tests; therefore, the Ethic Commission Agreement was not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dubey, J.P. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 1998, 28, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 2009, 39, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Sreekumar, C. Redescription of Hammondia hammondi and its differentiation from Toxoplasma gondii. Int. J. Parasitol. 2003, 33, 1437–1453. [Google Scholar] [CrossRef]
- Dubey, J.P.; Miller, N.L.; Frenkel, J.K. The Toxoplasma gondii oocyst from cat feces. J. Exp. Med. 1970, 132, 636–662. [Google Scholar] [CrossRef]
- Dubey, J.P.; Frenkel, J.K. Cyst-Induced Toxoplasmosis in Cats. J. Protozool. 1972, 19, 155–177. [Google Scholar] [CrossRef]
- Dubey, J.P.; Steitel, R.H. Further studies on the transmission of Hammondia hammondi in cats. J. Parasitol. 1976, 62, 548–551. [Google Scholar] [CrossRef]
- Dubey, J.P.; Ferreira, L.R.; Martins, J.; Jones, J.L. Sporulation and survival of Toxoplasma gondii oocysts in different types of commercial cat litter. J. Parasitol. 2011, 97, 751–754. [Google Scholar] [CrossRef]
- Holland, G.N. Ocular Toxoplasmosis: A Global Reassessment. Part I: Epidemiology and Course of Disease. Am. J. Ophthalmol. 2003, 136, 973–988. [Google Scholar] [CrossRef]
- Kijlstra, A.; Jongert, E. Control of the Risk of Human Toxoplasmosis Transmitted by Meat. Int. J. Parasitol. 2008, 38, 1359–1370. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Darde, M.L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Cook, A.J.C.; Gilbert, R.E.; Buffolano, W.; Petersen, E.; Jenum, P.A.; Foulon, W.; Semprini, A.E.; Dunn, D.T. Sources of Toxoplasma Infection in Pregnant Women: European Multicentre Case-Control Study. BMJ 2000, 321, 142–147. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, H.K.; Salah-Eldin, H.; Khodeer, S.; Allah, A.A. Prevalence of Toxoplasma gondii Infection in Antenatal Population in Menoufia Governorate. Egypt. Acta Trop. 2012, 124, 185–191. [Google Scholar] [CrossRef]
- Tong, W.H.; Pavey, C.; O’Handley, R.; Vyas, A. Behavioral Biology of Toxoplasma gondii Infection. Parasit. Vectors 2021, 14, 77. [Google Scholar] [CrossRef]
- Dubey, J.P.; Carpenter, J.L. Histologically Confirmed Clinical Toxoplasmosis in Cats: 100 Cases (1952–1990). J. Am. Vet. Med. Assoc. 1993, 203, 1556–1566. [Google Scholar] [CrossRef]
- Dabritz, H.A.; Conrad, P.A. Cats and Toxoplasma: Implications for Public Health. Zoonoses Public Health 2010, 57, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Zulpo, D.L.; Sammi, A.S.; Dos Santos, J.R.; Sasse, J.P.; Martins, T.A.; Minutti, A.F.; Cardim, S.T.; de Barros, L.D.; Navarro, I.T.; Garcia, J.L. Toxoplasma gondii: A study of oocyst re-shedding in domestic cats. Vet. Parasitol. 2018, 249, 17–20. [Google Scholar] [CrossRef]
- Dubey, J.P. Effect of immunization of cats with Isospora felis and BCG on immunity to reexcretion of Toxoplasma gondii oocysts. J. Protozool. 1978, 25, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Toxoplasma, Hammondia, Sarcocystis, and Other Tissue Cyst-Forming Coccidia of Humans and Animals. In Parasitic Protozoa; Kreier, J.P., Ed.; Academic Press: New York, NY, USA, 1993; Volume 6, pp. 1–158. [Google Scholar]
- Schares, G.; Herrmann, D.C.; Beckert, A.; Schares, S.; Hosseininejad, M.; Pantchev, N.; Globokar Vrhovec, M.; Conraths, F.J. Characterization of a repetitive DNA fragment in Hammondia hammondi and its utility for the specific differentiation of H. hammondi from Toxoplasma gondii by PCR. Mol. Cell Probes 2008, 22, 244–251. [Google Scholar] [CrossRef]
- Monteiro, R.M.; Pena, H.F.; Gennari, S.M.; de Souza, S.O.; Richtzenhain, L.J.; Soares, R.M. Differential Diagnosis of Oocysts of Hammondia-like Organisms of Dogs and Cats by PCR-RFLP Analysis of 70-Kilodalton Heat Shock Protein (HSP70). Gene. Parasitol. Res. 2008, 103, 235–238. [Google Scholar] [CrossRef]
- Grigg, M.E.; Boothroyd, J.C. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-Restriction Fragment Length Polymorphism analysis at the B1 gene. J. Clin. Microbiol. 2001, 39, 398–400. [Google Scholar] [CrossRef]
- Ajzenberg, D.; Banuls, A.L.; Su, C.; Dumetre, A.; Demar, M.; Carme, B.; Darde, M.L. Genetic Diversity, Clonality and Sexuality in Toxoplasma gondii. Int. J. Parasitol. 2004, 34, 1185–1196. [Google Scholar] [CrossRef]
- Howe, D.K.; Sibley, L.D. Toxoplasma gondii Comprises Three Clonal Lineages: Correlation of Parasite Genotype with Human Disease. J. Infect. Dis. 1995, 172, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, X.; Dubey, J.P. Genotyping of Toxoplasma gondii by Multilocus PCR-RFLP Markers: A High-Resolution and Simple Method for Identification of Parasites. Int. J. Parasitol. 2006, 36, 841–848. [Google Scholar] [CrossRef]
- Sroka, J.; Kusyk, P.; Bilska-Zając, E.; Karamon, J.; Dutkiewicz, J.; Wójcik-Fatla, A.; Zajac, V.; Stojecki, K.; Rozycki, M.; Cencek, T. Seroprevalence of Toxoplasma gondii Infection in Goats from the South-West Region of Poland and the Detection of T. gondii DNA in Goat Milk. Folia Parasitol. 2017, 64, 23. [Google Scholar] [CrossRef] [PubMed]
- Homan, W.L.; Vercammen, M.; Debraekeleer, J.; Verschueren, H. Identification of a 200-to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 2000, 30, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Grigg, M.E.; Ganatra, J.; Boothroyd, J.C.; Margolis, T.P. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J. Infect. Dis. 2001, 184, 633–639. [Google Scholar] [CrossRef]
- Howe, D.K.; Honore, S.; Derouin, F.; Sibley, L.D. Determination of genotypes of Toxoplasma gondii strains isolated from patient with toxoplasmosis. J. Clin. Microbiol. 1997, 35, 1411–1414. [Google Scholar] [CrossRef]
- Khan, A.; Su, C.; German, M.; Storch, G.A.; Clifford, D.B.; Sibley, L.D. Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J. Clin. Microbiol. 2005, 43, 5881–5887. [Google Scholar] [CrossRef]
- Fazaeli, A.; Carter, P.E.; Dardé, M.L.; Pennington, T.H. Molecular typing of Toxoplasma gondii strains by GRA6 gene sequence analysis. Int. J. Parasitol. 2000, 30, 637–642. [Google Scholar] [CrossRef]
- Su, C.; Shwab, E.K.; Zhou, P.; Zhu, X.Q.; Dubey, J.P. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology 2010, 137, 1–11. [Google Scholar] [CrossRef]
- Brennan, A.; Donahoe, S.L.; Beatty, J.A.; Belov, K.; Lindsay, S.; Briscoe, K.A.; Šlapeta, J.; Barrs, V.R. Comparison of genotypes of Toxoplasma gondii in domestic cats from Australia with latent infection or clinical toxoplasmosis. Vet. Parasitol. 2016, 228, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Fenton, H.; Hall, J.; Rose, K.; Peddemors, V.M.; Šlapeta, J. Dolphins share Toxoplasma gondii Type II genotypes with terrestrial animals: Evidence of terrestrial T. gondii contamination in marine environments. Vet. Parasitol. 2025, 335, 110439. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Ferguson, D.J. Life Cycle of Hammondia hammondi (Apicomplexa: Sarcocystidae) in Cats. J. Eukaryot. Microbiol. 2015, 62, 346–352. [Google Scholar] [CrossRef]
- Dubey, J.P.; Hoover, E.A.; Walls, K.W. Effect of age and sex on the acquisition of immunity to toxoplasmosis in cats. J. Protozool. 1977, 24, 184–186. [Google Scholar] [CrossRef]
- Lappin, M.R.; Greene, C.E.; Winston, S.; Toll, S.L.; Epstein, M.E. Clinical feline toxoplasmosis: Serological diagnosis and therapeutic management of 15 cases. J. Vet. Intern. Med. 1989, 3, 139–143. [Google Scholar] [CrossRef]
- Frenkel, J.K.; Dubey, J.P. Hammondia hammondi gen. nov., sp.nov., from domestic cats, a new coccidian related to Toxoplasma and Sarcocystis. Z. Parasitenkd. 1975, 46, 3–12. [Google Scholar] [CrossRef]
- Berger-Schoch, A.E.; Herrmann, D.C.; Schares, G.; Müller, N.; Bernet, D.; Gottstein, B.; Frey, C.F. Prevalence and genotypes of Toxoplasma gondii in feline faeces (oocysts) and meat from sheep, cattle and pigs in Switzerland. Vet. Parasitol. 2011, 177, 290–297. [Google Scholar] [CrossRef]
- Dubey, J.P. Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. J. Parasitol. 1995, 81, 410–415. [Google Scholar] [CrossRef]
- Elmore, S.A.; Jones, J.L.; Conrad, P.A.; Patton, S.; Lindsay, D.S.; Dubey, J.P. Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010, 26, 190–196. [Google Scholar] [CrossRef]
- Lappin, M.R.; VanLare, K.A.; Seewald, W.; Roycroft, L.M.; Scorza, A.V.; King, S.; Roberts, E.S. Effect of oral administration of cyclosporine on Toxoplasma gondii infection status of cats. Am. J. Vet. Res. 2015, 76, 351–357. [Google Scholar] [CrossRef]
- Last, R.D.; Suzuki, Y.; Manning, T.; Lindsay, D.; Galipeau, L.; Whitbread, T.J. A case of fatal systemic toxoplasmosis in a cat being treated with cyclosporin A for feline atopy. Vet. Dermatol. 2004, 15, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.M.; Blois, S.; Vince, A.R. Fatal extraintestinal toxoplasmosis in a young male cat with enlarged mesenteric lymph nodes. Can. Vet. J. 2016, 57, 483–486. [Google Scholar]
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 7–56. [Google Scholar]
- Scorza, A.V.; Tyrrell, P.; Wennogle, S.; Chandrashekar, R.; Lappin, M.R. Experimental infection of cats with Cystoisospora felis. J. Vet. Intern. Med. 2021, 35, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Prowell, M. Ante-mortem diagnosis, diarrhea, oocyst shedding, treatment, isolation, and genetic typing of Toxoplasma gondii associated with clinical toxoplasmosis in a naturally infected cat. J. Parasitol. 2013, 99, 158–160. [Google Scholar] [CrossRef]
- Deksne, G.; Petrusēviča, A.; Kirjušina, M. Seroprevalence and factors associated with Toxoplasma gondii infection in domestic cats from urban areas in Latvia. J. Parasitol. 2013, 99, 48–50. [Google Scholar] [CrossRef]
- Raue, K.; Heuer, L.; Böhm, C.; Wolken, S.; Epe, C.; Strube, C. 10-year parasitological examination results (2003 to 2012) of faecal samples from horses, ruminants, pigs, dogs, cats, rabbits and hedgehogs. Parasitol. Res. 2017, 116, 3315–3330. [Google Scholar] [CrossRef]
- Wąsiatycz, G. Prevalence of Toxoplasma gondii infection of cats in Poznań and its district in the aspect of the danger of parasite invasion of people. Wiad. Parazytol. 1998, 44, 693–704. [Google Scholar]
- Schares, G.; Vrhovec, M.G.; Pantchev, N.; Herrmann, D.C.; Conraths, F.J. Occurrence of Toxoplasma gondii and Hammondia hammondi oocysts in the faeces of cats from Germany and other European countries. Vet. Parasitol. 2008, 152, 34–45. [Google Scholar] [CrossRef]
- Pena, H.F.; Soares, R.M.; Amaku, M.; Dubey, J.P.; Gennari, S.M. Toxoplasma gondii infection in cats from São Paulo state, Brazil: Seroprevalence, oocyst shedding, isolation in mice, and biologic and molecular characterization. Res. Vet. Sci. 2006, 81, 58–67. [Google Scholar] [CrossRef]
- Frenkel, J.; Ruiz, A.; Chinchilla, M. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. Am. J. Trop. Med. Hyg. 1975, 24, 439–443. [Google Scholar] [CrossRef]
- Grigg, M.E.; Bonnefoy, S.; Hehl, A.B.; Suzuki, Y.; Boothroyd, J.C. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science 2001, 294, 161–165. [Google Scholar] [CrossRef]
- Długońska, H. Toxoplasma gondii—known and unknown parasite. Ann. Parasitol. 2008, 54, 199–204. [Google Scholar]
- Sibley, L.D.; Boothroyd, J.C. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 1992, 359, 82–85. [Google Scholar] [CrossRef]
- Khan, A.; Fux, B.; Su, C.; Dubey, J.P.; Dardé, M.L.; Ajioka, J.W.; Rosenthal, B.M.; Sibley, L.D. Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc. Natl. Acad. Sci. USA 2007, 104, 14872–14877. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, H.; Wang, H.; Qin, H.; Xiao, J. Land use and soil contamination with Toxoplasma gondii oocysts in urban areas. Sci. Total Environ. 2016, 568, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, T.; Marcet, P.L.; Graham, D.H.; Dahl, E.R.; Dubey, J.P. Globalization and the population structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 2006, 103, 11423–11428. [Google Scholar] [CrossRef] [PubMed]
- Chemoh, W.; Sawangjaroen, N.; Nissapatorn, V.; Sermwittayawong, N. Genotyping of Toxoplasma gondii isolated from cat feces in Songkhla, Southern Thailand. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 105–109. [Google Scholar] [CrossRef]
- Khodaverdi, M.; Razmi, G. Prevalence and genotyping of Toxoplasma gondii in stray cats in Mashhad area, Iran. BMC Vet. Res. 2019, 15, 463. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Schares, G.; Maksimov, P.; Joeres, M.; Ortega-Mora, L.M.; Calero-Bernal, R. Toxoplasma gondii genotyping: A closer look into Europe. Front. Cell. Infect. Microbiol. 2022, 12, 842595. [Google Scholar] [CrossRef]
- Spycher, A.; Geigy, C.; Howard, J.; Posthaus, H.; Gendron, K.; Gottstein, B.; Debache, K.; Herrmann, D.C.; Schares, G.; Frey, C.F. Isolation and genotyping of Toxoplasma gondii causing fatal systemic toxoplasmosis in an immunocompetent 10-year-old cat. J. Vet. Diagn. Invest. 2011, 23, 104–108. [Google Scholar] [CrossRef]
- Mancianti, F.; Nardoni, S.; Mugnaini, L.; Zambernardi, L.; Guerrini, A.; Gazzola, V.; Papini, R.A. A retrospective molecular study of select intestinal protozoa in healthy pet cats from Italy. J. Feline Med. Surg. 2015, 17, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Miró, G.; Mateo, M.; Ramírez, C.; Fuentes, I. Molecular characterization of Toxoplasma gondii isolates from cats in Spain. J. Parasitol. 2008, 94, 1044–1046. [Google Scholar] [CrossRef]
- Herrmann, D.C.; Pantchev, N.; Vrhovec, M.G.; Barutzki, D.; Wilking, H.; Fröhlich, A.; Lüder, C.G.; Conraths, F.J.; Schares, G. Atypical Toxoplasma gondii genotypes identified in oocysts shed by cats in Germany. Int. J. Parasitol. 2010, 40, 285–292. [Google Scholar] [CrossRef]
- Sroka, J.; Wójcik-Fatla, A.; Bilska-Zając, E.; Karamon, J.; Zdybel, J.M.; Piotrowska, W.; Dąbrowska, J.; Samorek-Pieróg, M.; Korpysa-Dzirba, W.; Cencek, T. Molecular confirmation of systemic toxoplasmosis in a cat. Ann. Agric. Environ. Med. 2023, 30, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Pardo Gil, M.; Hegglin, D.; Briner, T.; Ruetten, M.; Müller, N.; Moré, G.; Frey, C.F.; Deplazes, P.; Basso, W. High prevalence rates of Toxoplasma gondii in cat-hunted small mammals—Evidence for parasite-induced behavioural manipulation in the natural environment? Int. J. Parasitol. Parasites Wildl. 2023, 20, 108–116. [Google Scholar] [CrossRef]
- Sroka, J. Occurrence of Toxoplasma gondii infection among free-living small rodents and insectivores in the Lublin Province—the role of these animals in the epidemiology of toxoplasmosis. Med. Og. Nauk. Zdr. 2021, 27, 448–452. [Google Scholar] [CrossRef]
- Grzybek, M.; Antolová, D.; Tołkacz, K.; Alsarraf, M.; Behnke-Borowczyk, J.; Nowicka, J.; Paleolog, J.; Biernat, B.; Behnke, J.M.; Bajer, A. Seroprevalence of Toxoplasma gondii among sylvatic rodents in Poland. Animals 2021, 11, 1048. [Google Scholar] [CrossRef]
- Nowicka, J.; Antolova, D.; Lass, A.; Biernat, B.; Baranowicz, K.; Goll, A.; Krupinska, M.; Ferra, B.; Strachecka, A.; Behnke, J.M.; et al. Identification of Toxoplasma gondii in wild rodents in Poland by molecular and serological techniques. Ann. Agric. Environ. Med. 2024, 31, 626–630. [Google Scholar] [CrossRef]
- Must, K.; Hytonen, M.K.; Orro, T.; Lohi, H.; Jokelainen, P. Toxoplasma gondii seroprevalence varies by cat breed. PLoS ONE 2017, 12, e0184659. [Google Scholar] [CrossRef]
- Gyorke, A.; Opsteegh, M.; Mircean, V.; Iovu, A.; Cozma, V. Toxoplasma gondii in Romanian household cats: Evaluation of serological test, epidemiology and risk factors. Prev. Vet. Med. 2011, 102, 321–328. [Google Scholar] [CrossRef]
- Brennan, A.; Hawley, J.; Dhand, N.; Boland, L.; Beatty, J.A.; Lappin, M.R.; Barrs, V.R. Seroprevalence and risk factors for Toxoplasma gondii infection in owned domestic cats in Australia. Vector Borne Zoonotic Dis. 2020, 20, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Chessa, G.; Chisu, V.; Porcu, R.; Masala, G. Molecular characterization of Toxoplasma gondii Type II in sheep abortion in Sardinia, Italy. Parasite 2014, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, T.V.; Marlee, D.; Hyde, J.E.; Sims, P.F. Prevalence of Toxoplasma gondii in commercial meat products as monitored by polymerase chain reaction—Food for thought? Int. J. Parasitol. 2002, 32, 1193–1199. [Google Scholar] [CrossRef]
- Sroka, J.; Bilska-Zając, E.; Wójcik-Fatla, A.; Zając, V.; Dutkiewicz, J.; Karamon, J.; Piotrowska, W.; Cencek, T. Detection and Molecular Characteristics of Toxoplasma gondii DNA in Retail Raw Meat Products in Poland. Foodborne Pathog. Dis. 2019, 16, 195–204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).