Detectability and Persistence of Cyclospora cayetanensis Oocysts in Artificially Contaminated Soil and Fresh Herbs Grown Under Controlled Climatic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants Growth and Oocysts Inoculation in Leaves and Soil
2.2. Sample Collection and Processing in Soil and Leaves from Herbs
3. Results
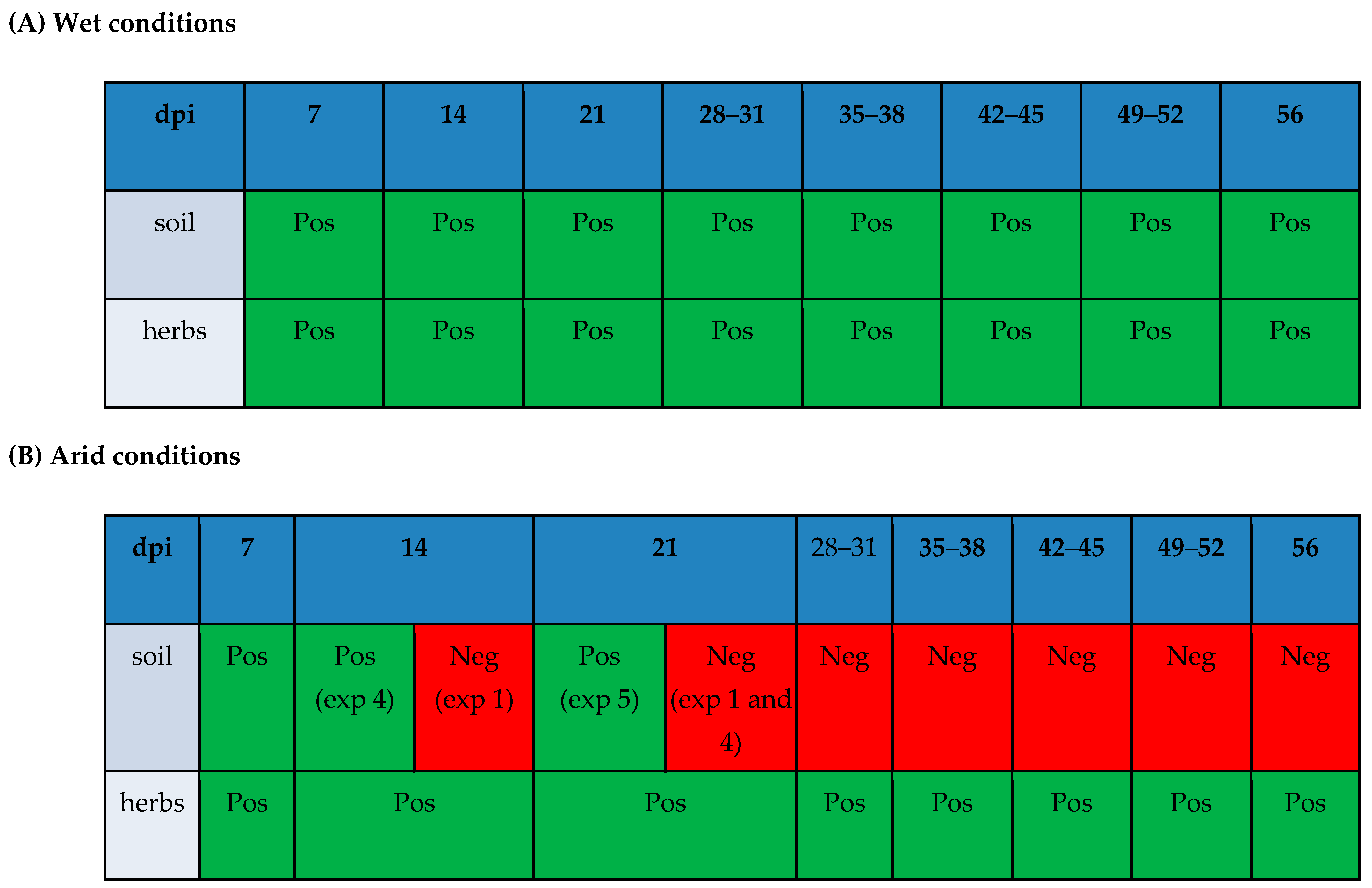
3.1. Experiments in Sandy Clay Loam (SCL)
Analysis of Presence of Oocysts in Leachate Water in Experiment 2 in Sandy Clay Loam
3.2. Experiment on Silt Loam Soil (Experiment 5)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badri, M.; Olfatifar, M.; Karim, R.; Modirian, E.; Houshmand, E.; Abdoli, A.; Nikoonejad, A.; Sotoodeh, S.; Zargar, A.; Samimi, R.; et al. Global prevalence of intestinal protozoan contamination in vegetables and fruits: A systematic review and meta-analysis. Food Control 2022, 133, 108656. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Z.; Li, J.; Xiao, L.; Zhang, L. The global prevalence of Cyclospora cayetanensis infection: A systematic review, meta-analysis, and meta-regression. Acta Trop. 2024, 253, 107175. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.; Cinar, H.N.; Dubey, J.P. Cyclospora cayetanensis and cyclosporiasis: An update. Microorganisms 2019, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.; Chacin-Bonilla, L.; Maloney, J.G.; Santin, M. Cyclospora cayetanensis: A perspective (2020–2023) with emphasis on epidemiology and detection methods. Microorganisms 2023, 11, 2171. [Google Scholar] [CrossRef]
- Marques, D.F.P.; Alexander, C.L.; Chalmers, R.M.; Elson, R.; Freedman, J.; Hawkins, G.; Lo, J.; Robinson, G.; Russell, K.; Smith-Palmer, A.; et al. Cyclosporiasis in returning to the United Kingdom from Mexico in summer 2017: Lessons from the recent past to inform the future. Euro Surveill. 2017, 22, 30592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortiz-Pineda, C.; Temesgen, T.T.; Robertson, L.J. Multiplex quantitative PCR analysis of strawberries from Bogota, Colombia, for contamination with three parasites. J. Food Protect. 2020, 83, 1679–1684. [Google Scholar] [CrossRef]
- Resendiz-Nava, C.N.; Orozco-Mosqueda, G.E.; Mercado-Silva, E.M.; Flores-Robles, S.; Silva-Rojas, H.V.; Nava, G.M. A molecular tool for rapid detection and traceability of Cyclospora cayetanensis in fresh berries and berry farm soils. Foods 2020, 9, 261. [Google Scholar] [CrossRef]
- Temesgen, T.T.; Stigum, V.M.; Robertson, L.J. Surveillance of berries sold on the Norwegian market for parasite contamination using molecular methods. Food Microbiol. 2022, 104, 103980. [Google Scholar] [CrossRef]
- Lalonde, L.; Oakley, J.; Fries, P. Verification and Use of the US-FDA BAM 19b method for detection of Cyclospora cayetanensis in a survey of fresh produce by CFIA laboratory. Microorganisms 2022, 10, 559. [Google Scholar] [CrossRef]
- Herwaldt, B.L. Cyclospora cayetanensis: A review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin. Infect. Dis. 2000, 31, 1040–1057. [Google Scholar] [CrossRef]
- Ortega, Y.R.; Sanchez, R. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 2010, 23, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Morton, V.; Meghnath, K.; Gheorghe, M.; Fitzgerald-Husek, A.; Hobbs, J.; Honish, L.; David, S. Use of a case-control study and control bank to investigate an outbreak of locally acquired cyclosporiasis in Canada, 2016. Can. Comm. Dis. Rep. 2019, 45, 225–229. [Google Scholar] [CrossRef]
- Kozak, G.K.; MacDonald, D.; Landry, L.; Farber, J.M. Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J. Food Prot. 2013, 76, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.; Grocholl, J.; Mullins, J.; Durigan, M.; Ewing-Peeples, L.; Rogers, E.L.; Hirneisen, K.; Madson, S.; Wang, S.S. Multi-laboratory validation of a modified real-time PCR assay (Mit1C) for the detection of Cyclospora cayetanensis in fresh produce. Food Microbiol. 2025, 128, 104727. [Google Scholar] [CrossRef]
- FDA. FDA Releases Cyclospora Prevention, Response, and Research Action Plan. FDA Food and Drug Administration. 1 July 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-releases-cyclospora-prevention-response-and-research-action-plan (accessed on 20 September 2024).
- Institute of Medicine (US) Forum on Microbial Threats. Addressing Foodborne Threats to Health: Policies, Practices, and Global Coordination: Workshop Summary; 3, Investigating Foodborne Threats; National Academies Press (US): Washington, DC, USA, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK57087/ (accessed on 6 March 2025).
- Ortega, Y.R.; Sterling, C.R.; Gilman, R.H.; Cama, V.A.; Díaz, F. Cyclospora species: A new protozoan pathogen of humans. N. Engl. J. Med. 1993, 328, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.V.; Paton, C.A.; Mtambo, M.M.; Girdwood, R.W.A. Sporulation of Cyclospora sp. oocysts. Appl. Environ. Microbiol. 1997, 63, 1631–1632. [Google Scholar] [CrossRef]
- Dawson, D. Foodborne protozoan parasites. Int. J. Food Microbiol. 2005, 103, 207–227. [Google Scholar] [CrossRef]
- Wang, K.X.; Li, C.P.; Wang, J.; Tian, Y. Cyclospora cayetanensis in Anhui, China. World J. Gastroenterol. 2002, 8, 1144–1148. [Google Scholar] [CrossRef]
- Lopez, A.S.; Bendik, J.M.; Alliance, J.Y.; Roberts, J.M.; da Silva, A.J.; Moura, I.N.S.; Arrowood, M.J.; Eberhard, M.L.; Herwaldt, B.L. Epidemiology of Cyclospora cayetanensis and other intestinal parasites in a community in Haiti. J. Clin. Microbiol. 2003, 41, 2047–2054. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, B.; Wang, Q.; Wang, R.; Jian, F.; Zhang, L.; Ning, C.; Fu, K.; Wang, Y.; Qi, M.; et al. Prevalence and molecular characterization of Cyclospora cayetanensis, Henan, China. Emerg. Infect. Dis. 2011, 17, 1887–1890. [Google Scholar] [CrossRef]
- Dumetre, A.; Aubert, D.; Puech, P.-H.; Hohweyer, J.; Azas, N.; Villena, I. Interaction forces drive the environmental transmission of pathogenic protozoa. Appl. Environ. Microbiol. 2012, 78, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, L.S.; Gajadhar, A.A. Cyclospora cayetanensis, a food- and waterborne coccidian parasite. Vet. Parasitol. 2004, 126, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Alum, A.; Absar, I.M.; Asaad, H.; Rubino, J.R.; Ijaz, M.K. Impact of environmental conditions on the survival of Cryptosporidium and Giardia on environmental surfaces. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 210385. [Google Scholar] [CrossRef]
- Frenkel, J.K.; Ruiz, A.; Chinchilla, M. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. Am. J. Trop. Med. Hyg. 1975, 24, 439–443. [Google Scholar] [CrossRef]
- Dumetre, A.; Darde, M.L. How to detect Toxoplasma gondii oocysts in environmental samples? FEMS Microbiol. Rev. 2003, 27, 651–661. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Long-Term Survival of Toxoplasma gondii Sporulated Oocysts in Seawater. J. Parasitol. 2009, 95, 1019–1020. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis in Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–313. [Google Scholar]
- Petersen, H.H.; Enemark, H.L.; Olsen, A.; Amin, M.G.; Dalsgaard, A. Transport of Cryptosporidium parvum oocysts in soil columns following applications of raw and separated liquid slurries. Appl. Environ. Microbiol. 2012, 78, 5994–6000. [Google Scholar] [CrossRef]
- Tamburrini, A.; Pozio, E. Long-term survival of Cryptosporidium parvum oocysts in seawater and in experimentally infected mussels (Mytilus galloprovincialis). Int. J. Parasitol. 1999, 29, 711–715. [Google Scholar] [CrossRef]
- King, B.J.; Monis, P.T. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology 2007, 134, 309–323. [Google Scholar] [CrossRef]
- Sathyanarayanan, L.; Ortega, Y. Effects of temperature and different food matrices on Cyclospora cayetanensis oocyst sporulation. J. Parasitol. 2006, 92, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, L.; Ortega, Y. Effects of pesticides on sporulation of Cyclospora cayetanensis and viability of Cryptosporidium parvum. J. Food Prot. 2004, 67, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Escotte-Binet, S.; Da Silva, A.M.; Cancès, B.; Aubert, D.; Dubey, J.; La Carbona, S.; Villena, I.; Poulle, M.L. A rapid and sensitive method to detect Toxoplasma gondii oocysts in soil samples. Vet. Parasitol. 2019, 274, 108904. [Google Scholar] [CrossRef]
- Shipley, A.; Arida, J.; Almeria, S. Comparative evaluation of an easy laboratory method for the concentration of oocysts and commercial DNA isolation kits for the molecular detection of Cyclospora cayetanensis in silt loam soil samples. Microorganisms 2022, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Durigan, M.; Ewing-Peeples, L.; Almeria, S.; Balan, K.V.; Grocholl, J.; Irizawa, S.; Mammel, M. Detection of Cyclospora cayetanensis in Food and Water Samples: Optimized Protocols for Specific and Sensitive Molecular Methods from a Regulatory Agency Perspective. J. Food Prot. 2024, 87, 100291. [Google Scholar] [CrossRef]
- Arida, J.; Shipley, A.; Almeria, S. Molecular detection of Cyclospora cayetanensis in two main types of farm soil using real-time PCR assays and method modification for commercial potting mix. Microorganisms 2023, 11, 1506. [Google Scholar] [CrossRef]
- Abanyie, F.; Harvey, R.R.; Harris, J.; Wiegand, R.; Gaul, L.; des Vignes-Kendrick, M.; Irvin, K.; Williams, I.; Hall, R.; Herwaldt, B.; et al. 2013 multistate outbreaks of Cyclospora cayetanensis infections associated with fresh produce: Focus on the Texas investigations. Epidemiol. Infect. 2015, 143, 3451–3458. [Google Scholar] [CrossRef]
- Lopez, A.S.; Dodson, D.R.; Arrowood, M.J.; Orlandi, P.A.; da Silva, A.J.; Bier, J.W.; Hanauer, S.D.; Kuster, R.L.; Oltman, S.; Baldwin, M.S.; et al. Outbreak of cyclosporiasis associated with basil in Missouri in 1999. Clin. Infect. Dis. 2001, 32, 1010–1071. [Google Scholar] [CrossRef]
- Barratt, J.; Houghton, K.; Richins, T.; Straily, A.; Threlkel, R.; Bera, B.; Kenneally, J.; Clemons, B.; Madison-Antenucci, S.; Cebelinski, E.; et al. Investigation of US Cyclospora cayetanensis outbreaks in 2019 and evaluation of an improved Cyclospora genotyping system against 2019 cyclosporiasis outbreak clusters. Epidemiol. Infect. 2021, 149, e214. [Google Scholar] [CrossRef]
- Dixon, B.R. Parasitic illnesses associated with the consumption of fresh produce—An emerging issue in developed countries. Curr. Opinion Food Sci. 2016, 8, 104–109. [Google Scholar] [CrossRef]
- Döller, P.C.; Dietrich, K.; Filipp, N.; Brockmann, S.; Dreweck, C.; Vonthein, R.; Wagner-Wiening, C.; Wiedenmann, A. Cyclosporiasis outbreak in Germany associated with the consumption of salad. Emerg. Infect. Dis. 2002, 8, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R.; Almeria, S.; da Silva, A.J. BAM Chapter 19b: Molecular Detection of Cyclospora Cayetanensis in Fresh Produce Using Real-Time PCR. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm553445.htm (accessed on 11 October 2024).
- Almeria, S.; da Silva, A.J.; Blessington, T.; Cloyd, T.C.; Cinar, H.N.; Durigan, M.; Murphy, H.R. Evaluation of the U.S. Food and Drug Administration validated method for detection of Cyclospora cayetanensis in high-risk fresh produce matrices and a method modification for a prepared dish. Food Microbiol. 2018, 76, 497–503. [Google Scholar] [CrossRef]
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef]
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States-unspecified agents. Emerg. Infect. Dis. 2011, 17, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.J.; Thomas, M.K.; Pintar, K.D.M. Expert elicitation as a means to attribute 28 enteric pathogens to foodborne, waterborne, animal contact, and person-to-person transmission routes in Canada. Foodborne Pathog. Dis. 2015, 12, 335–344. [Google Scholar] [CrossRef]
- Koumans, E.H.; Katz, D.J.; Malecki, J.M.; Kumar, S.; Wahlquist, S.P.; Arrowood, M.J.; Hightower, A.W.; Herwaldt, B.L. An outbreak of cyclosporiasis in Florida in 1995: A harbinger of multistate outbreaks in 1996 and 1997. Am. J. Trop. Med. Hyg. 1998, 59, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Arrowood, M.J.; Eberhard, M.; Maguire, J.H. Cyclospora in Guatemala: Further considerations. J. Clin. Microbiol. 2002, 40, 731–732. [Google Scholar] [CrossRef]
- Chacín-Bonilla, L. Transmission of Cyclospora cayetanensis infection: A review focusing on soil-borne cyclosporiasis. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 215–216. [Google Scholar] [CrossRef]
- Chacín-Bonilla, L.; Barrios, F.; Sanchez, Y. Epidemiology of Cyclospora cayetanensis infection in San Carlos Island, Venezuela: Strong association between socio-economic status and infection. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 1018–1024. [Google Scholar] [CrossRef]
- Assurian, A.; Murphy, H.; Ewing, L.; Cinar, H.N.; da Silva, A.; Almeria, S. Evaluation of the U.S. Food and Drug Administration validated molecular method for detection of Cyclospora cayetanensis oocysts on fresh and frozen berries. Food Microbiol. 2020, 87, 103397. [Google Scholar] [CrossRef]
- Chacin-Bonilla, L.; Sanchez, Y. Seasonal trends of Cyclospora cayetanensis infections in a community of falcon state, northwestern Venezuela. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 673–675. [Google Scholar] [CrossRef]
- Davies, C.M.; Altavilla, N.; Krogh, M.; Ferguson, C.M.; Deere, D.A.; Ashbolt, N.J. Environmental inactivation of Cryptosporidium oocysts in catchment soils. J. Appl. Microbiol. 2005, 98, 308–317. [Google Scholar] [CrossRef]
- Jenkins, M.B.; Bowman, D.D.; Fogarty, E.A.; Ghiorse, W.C. Cryptosporidium parvum oocyst inactivation in three soil types at various temperatures and water potentials. Soil Biol. Biochem. 2002, 34, 1101–1109. [Google Scholar] [CrossRef]
- Lélu, M.; Gilot-Fromont, E.; Aubert, D.; Richaume, A.; Afonso, E.; Dupuis, E.; Gotteland, C.; Marnef, F.; Poulle, M.L.; Dumètre, A.; et al. Development of a sensitive method for Toxoplasma gondii oocyst isolation in soil. Vet. Parasitol. 2011, 183, 59–67. [Google Scholar] [CrossRef]
- de Wit, L.A.; Kilpatrick, A.M.; VanWormer, E.; Croll, D.A.; Tershy, B.R.; Kim, M.; Shapiro, K. Seasonal and spatial variation in Toxoplasma gondii contamination in soil in urban public spaces in California, United States. Zoonoses Public Health 2020, 67, 70–78. [Google Scholar] [CrossRef]
- Deng, H.; Exel, K.E.; Swart, A.; Bonacic Marinovic, A.A.; Dam-Deisz, C.; van der Giessen, J.W.B.; Opsteegh, M. Digging into Toxoplasma gondii infections via soil: A quantitative microbial risk assessment approach. Sci. Total Environ. 2021, 755, 143232. [Google Scholar] [CrossRef] [PubMed]
- Krusor, C.; Smith, W.A.; Tinker, M.T.; Silver, M.; Conrad, P.A.; Shapiro, K. Concentration and retention of Toxoplasma gondii oocysts by marine snails demonstrate a novel mechanism for transmission of terrestrial zoonotic pathogens in coastal ecosystems. Environ. Microbiol. 2015, 17, 4527–4537. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.J.; Campbell, A.T.; Smith, H.V. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl. Envir. Microbiol. 1992, 58, 3494–3500. [Google Scholar] [CrossRef]
- Daugschies, A.; Najdrowski, M. Eimeriosis in cattle: Current understanding. J. Vet. Med. B Infect. Dis. Vet. Public. Health 2005, 52, 417–427. [Google Scholar] [CrossRef]
- Frenkel, J.K.; Dubey, J.P. Toxoplasmosis and its prevention in cats and man. J. Infect. Dis. 1972, 126, 664–673. [Google Scholar] [CrossRef]
- Kato, S.; Jenkins, M.; Fogarty, E.; Bowman, D. Cryptosporidium parvum oocyst inactivation in field soil and its relation to soil characteristics: Analyses using the geographic information systems. Sci. Total Environ. 2004, 321, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, A.; Zimmels, Y.; Starosvetsky, J.; Zuckerman, U.; Armon, R. A Two-phase separation method for recovery of Cryptosporidium oocysts from soil samples. Water Air Soil Pollut. 2009, 203, 325–334. [Google Scholar] [CrossRef]
- Ochsner, T. Open Book Initiative. 2019. Available online: https://open.library.okstate.edu/rainorshine/chapter/1-5-factors-affecting-saturated-hydraulic-conductivity/#:~:text=As%20you%20may%20have%20expected,values%20of%20saturated%20hydraulic%20conductivity (accessed on 13 March 2025).
- Jenkins, M.C.; Parker, C.; O’Brien, C.; Miska, K.; Fetterer, R. Differing susceptibilities of Eimeria acervulina, Eimeria maxima, and Eimeria tenella oocysts to desiccation. J. Parasitol. 2013, 99, 899–902. [Google Scholar] [CrossRef]
- Martinaud, G.; Billaudelle, M.; Moreau, J. Circadian variation in shedding of the oocysts of Isospora turdi (Apicomplexa)in blackbirds (Turdus merula): An adaptative trait against desiccation and ultraviolet radiation. Int. J. Parasit. 2009, 39, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Tucker, M.S.; Yeager, C.; Fournet, V.; Jenkins, M.C.; Dubey, J.P.; Kniel, K.E.; Rosenthal, B.M.; Sharma, M. Zero-Valent Iron and Sand Filtration Reduces Levels of Cyclospora cayetanensis Surrogates, Eimeria tenella and Eimeria acervulina, in Water. Microorganisms 2024, 12, 2344. [Google Scholar] [CrossRef]
- Yeager, C.; Tucker, M.; Gutierrez, A.; O’Brien, C.; Sharma, M.; Fournet, V.; Dubey, J.P.; Jenkins, M.; Kniel, K.; Rosenthal, B.M. Filters comprised of sand and Zero Valent Iron hold promise as tools to mitigate risk posed by Cyclospora cayetanensis oocysts. Food Waterborne Parasitol. 2024, 37, e00243. [Google Scholar] [CrossRef]




| Experiment No. | Water Conditions | Temperature Conditions | Type of Soil |
|---|---|---|---|
| Experiment 1 | Arid (watering once a week) | Moderate (21–25 °C) | Sandy Clay Loam |
| Experiment 2 | Wet (watering every 2–3 days) | Moderate (21–25 °C) | Sandy Clay Loam |
| Experiment 3 | Wet (watering every 2–3 days) | High (24–28 °C) | Sandy Clay Loam |
| Experiment 4 | Arid (watering once a week) | High (24–28° C) | Sandy Clay Loam |
| Experiment 5 | Arid (watering once a week) | High (24–28° C) | Silt Loam |
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | |
|---|---|---|---|---|
| Collection Day | Average CT ± Stdev | Average CT ± Stdev | Average CT ± Stdev | Average CT ± Stdev |
| 7 dpi | 33.24 ± 1.47 | 32.45 ± 0.76 | 33.73 ± 1.76 | 34.52 ± 1.71 |
| 14 dpi | 34.41 ± 0.97 | 37.07 ± 0.52 | 35.67 ± 1.63 | 30.49 ± 0.06 |
| 21 dpi | 35.06 ± 1.37 | 34.37 ± 1.38 | 33.25 ± 0.55 | 33.06 ± 0.46 |
| 28–31 dpi | 33.93 ± 1.22 | 32.86 ± 0.34 | 34.02 ± 2.03 | 34.65 ± 1.20 |
| 35–38 dpi | 33.70 ± 0.56 | 33.34 ± 0.21 | 32.88 ± 0.85 | 35.23 ± 2.57 |
| 42–45 dpi | 37.38 ± 3.57 | 35.39 ± 1.14 | 33.30 ± 1.85 | 35.73 ± 0.65 |
| 49–52 dpi | 33.58 ± 0.39 | 35.38 ± 1.40 | 33.60 ± N/A **# | 35.88 ± 0.50 |
| 56 dpi | N/A * | 35.62 ± 1.41 | 33.70 ± N/A **# | 37.62 ± N/A **& |
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | |
|---|---|---|---|---|
| Collection Day | Average CT ± Stdev | Average CT ± Stdev | Average CT ± Stdev | Average CT ± Stdev |
| 7 dpi | 34.47 ± 1.86 | 31.67 ± 1.02 | 38.0 ± N/A | 36.4 ± 0.09 |
| 14 dpi | und | 32.43 ± 0.37 | 36.6 ± N/A | 37.44 ± 0.76 |
| 21 dpi | und | 29.57 ± 0.56 | 37.5 ± N/A | und |
| 28–31 dpi | und | 33.74 ± N/A | 35.15 ± 0.73 | und |
| 35–38 dpi | und | 35.43 ± N/A | und ** | und |
| 42–45 dpi | und | 35.62 ± 0.93 | 35.33 ± 1.30 | und |
| 49–52 dpi | und | 35.99 ± 1.33 | 31.7 ± 0.50 | und |
| 56 dpi | und | und * | 30.4 ± 0.26 | und |
| Experiment | Condition of Leaf Collected | Average CT Values and Standard Deviation (3 Replicates) |
|---|---|---|
| Experiment 3 | Normal leaf 21 dpi | 32.7 ± 1.2 |
| Experiment 3 | Wilted leaf 21 dpi | 31.7 ± 0.13 |
| Experiment 4 | Normal leaf 31 dpi | 35.5 ± 1.5 |
| Experiment 4 | Mostly dried leaf 38 dpi | 32.3 ± 0.5 |
| Experiment 4 | Mostly dried leaf 45 dpi | 35.1 ± 1.1 |
| Experiment 4 | Mostly dried leaf 52 dpi | 35.5 ± 1.7 |
| Experiment 4 | Completely dried leaf 56 dpi | und |
| Leaves | Soil | |||
|---|---|---|---|---|
| Sandy Clay Loam Soil Experiment 4 | Silt Loam Soil Experiment 5 | Sandy Clay Loam Soil Experiment 4 | Silt Loam Soil Experiment 5 | |
| Collection Day | Average CT ± Stdev | Average CT ± Stdev | Average CT ± Stdev | Average CT ± Stdev |
| 7 dpi | 34.52 ± 1.71 | 35.98 ± 0.61 | 36.4 ± 0.09 | 35.90 ± 0.31 |
| 14 dpi | 30.49 ± 0.06 | 36.95 ± 0.12 | 37.44 ± 0.76 | 36.44 ± 0.45 |
| 21 dpi | 33.06 ± 0.46 | 36.76 ± 1.46 | und | 36.56 ± 1.17 |
| 28–31 dpi | 34.65 ± 1.20 | 34.88 ± 0.21 | und | und |
| 35–38 dpi | 35.23 ± 2.57 | 36.35 ± 1.28 | und | und |
| 42–45 dpi | 35.73 ± 0.65 | 37.24 ± 1.07 | und | und |
| 49–52 dpi | 35.88 ± 0.50 | 37.56 ± N/A | und | und |
| 56 dpi | 37.62 ± N/A | N/A * | und | und |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, E.L.; Arida, J.; Grocholl, J.; Njoroge, J.; Almeria, S. Detectability and Persistence of Cyclospora cayetanensis Oocysts in Artificially Contaminated Soil and Fresh Herbs Grown Under Controlled Climatic Conditions. Pathogens 2025, 14, 430. https://doi.org/10.3390/pathogens14050430
Rogers EL, Arida J, Grocholl J, Njoroge J, Almeria S. Detectability and Persistence of Cyclospora cayetanensis Oocysts in Artificially Contaminated Soil and Fresh Herbs Grown Under Controlled Climatic Conditions. Pathogens. 2025; 14(5):430. https://doi.org/10.3390/pathogens14050430
Chicago/Turabian StyleRogers, Ellie L., Joseph Arida, John Grocholl, Joyce Njoroge, and Sonia Almeria. 2025. "Detectability and Persistence of Cyclospora cayetanensis Oocysts in Artificially Contaminated Soil and Fresh Herbs Grown Under Controlled Climatic Conditions" Pathogens 14, no. 5: 430. https://doi.org/10.3390/pathogens14050430
APA StyleRogers, E. L., Arida, J., Grocholl, J., Njoroge, J., & Almeria, S. (2025). Detectability and Persistence of Cyclospora cayetanensis Oocysts in Artificially Contaminated Soil and Fresh Herbs Grown Under Controlled Climatic Conditions. Pathogens, 14(5), 430. https://doi.org/10.3390/pathogens14050430







