Programmed Cell Death-1 Expression in T-Cell Subsets in Chickens Infected with Marek’s Disease Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Viruses Preparation
2.3. Experimental Design
2.4. Flow Cytometric Analysis
2.4.1. Antibodies Against Meq and Chicken PD-1
2.4.2. Expression of Meq and PD-1 in DF-1 Cells
2.4.3. CD4+ T-Cell Staining
2.4.4. CD8+ T-Cell and γδ T-Cell Staining
2.5. Western Blotting
2.6. Statistical Analyses
3. Results
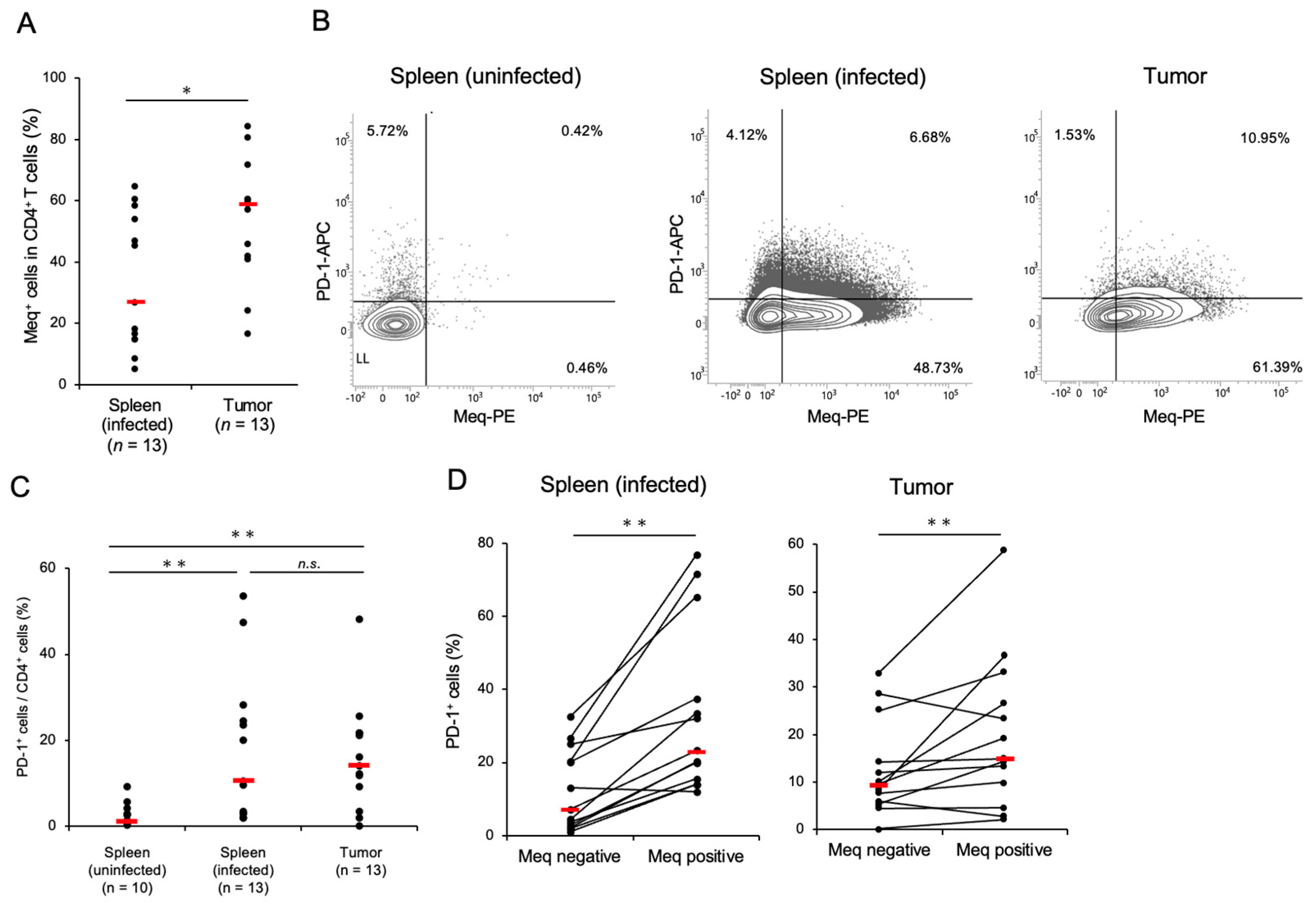
3.1. CD4+ T-Cells Transformed by MDV Express a High Proportion of PD-1
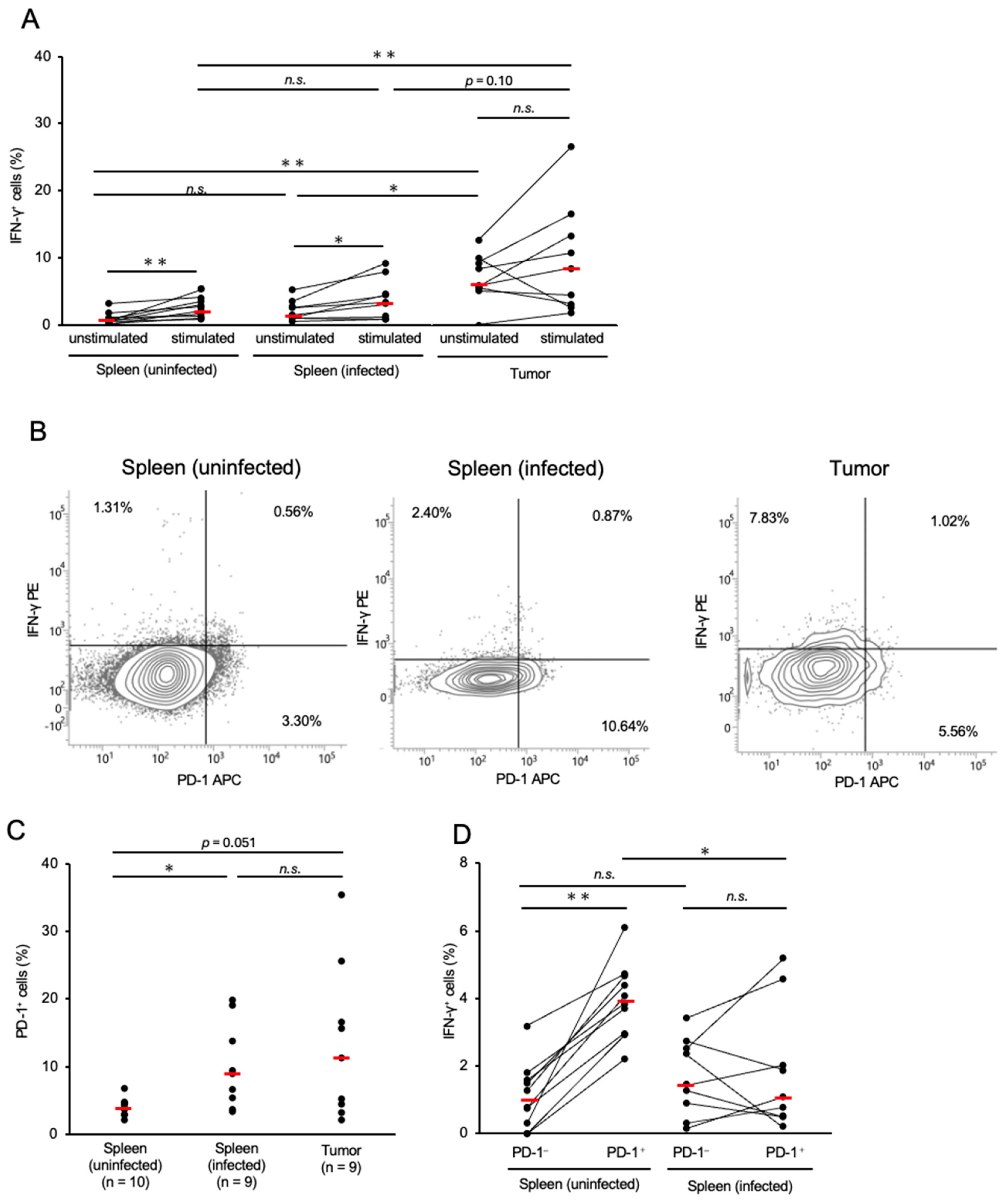
3.2. Expressions of IFN-γ and PD-1 in CD8+ T-Cells in Chickens with MD
3.3. Expressions of IFN-γ and PD-1 in γδ T-Cells in Chickens with MD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calnek, B.W. Pathogenesis of Marek’s Disease Virus Infection. In Marek’s Disease; Hirai, K., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2001; Volume 255, pp. 25–55. ISBN 978-3-540-67798-7. [Google Scholar]
- Abdul-Careem, M.F.; Javaheri-Vayeghan, A.; Shanmuganathan, S.; Haghighi, H.R.; Read, L.R.; Haq, K.; Hunter, D.B.; Schat, K.A.; Heidari, M.; Sharif, S. Establishment of an aerosol-based Marek’s disease virus infection model. Avian Dis. 2009, 53, 387–391. [Google Scholar] [CrossRef]
- Barrow, A.D.; Burgess, S.C.; Baigent, S.J.; Howes, K.; Nair, V.K. Infection of macrophages by a lymphotropic herpesvirus: A new tropism for Marek’s disease virus. J. Gen. Virol. 2003, 84 Pt 10, 2635–2645. [Google Scholar] [CrossRef]
- Davison, F.; Nair, V. Use of Marek’s disease vaccines: Could they be driving the virus to increasing virulence? Expert Rev. Vaccines 2005, 4, 77–88. [Google Scholar] [CrossRef]
- Baaten, B.J.; Staines, K.A.; Smith, L.P.; Skinner, H.; Davison, T.F.; Butter, C. Early replication in pulmonary B cells after infection with Marek’s disease herpesvirus by the respiratory route. Viral Immunol. 2009, 22, 431–444. [Google Scholar] [CrossRef]
- Morimura, T.; Ohashi, K.; Kon, Y.; Hattori, M.; Sugimoto, C.; Onuma, M. Apoptosis and CD8-down-regulation in the thymus of chickens infected with Marek’s disease virus. Arch. Virol. 1996, 141, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Berthault, C.; Larcher, T.; Härtle, S.; Vautherot, J.-F.; Trapp-Fragnet, L.; Denesvre, C. Atrophy of primary lymphoid organs induced by Marek’s disease virus during early infection is associated with increased apoptosis, inhibition of cell proliferation and a severe B-lymphopenia. Vet. Res. 2018, 49, 31. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; van Haarlem, D.A.; Härtle, S.; Kaufer, B.B.; Jansen, C.A. Marek’s Disease Virus Infection of Natural Killer Cells. Microorganisms 2019, 7, 588. [Google Scholar] [CrossRef]
- Mwangi, W.N.; Smith, L.P.; Baigent, S.J.; Beal, R.K.; Nair, V.; Smith, A.L. Clonal Structure of Rapid-Onset MDV-Driven CD4+ Lymphomas and Responding CD8+ T Cells. Osterrieder N, editor. PLoS Pathog. 2011, 7, e1001337. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Kung, H.J. Marek’s disease herpesvirus transforming protein MEQ: A c-Jun analogue with an alternative life style. Virus Genes 2000, 21, 51–64. [Google Scholar] [CrossRef]
- Qian, Z.; Brunovskis, P.; Lee, L.; Vogt, P.K.; Kung, H.J. Novel DNA binding specificities of a putative herpesvirus bZIP oncoprotein. J. Virol. 1996, 70, 7161–7170. [Google Scholar] [CrossRef]
- Levy, A.M.; Gilad, O.; Xia, L.; Izumiya, Y.; Choi, J.; Tsalenko, A.; Yakhini, Z.; Witter, R.; Lee, L.; Cardona, C.J.; et al. Marek’s disease virus Meq transforms chicken cells via the v-Jun transcriptional cascade: A converging transforming pathway for avian oncoviruses. Proc. Natl. Acad. Sci. USA 2005, 102, 14831–14836. [Google Scholar] [CrossRef] [PubMed]
- Umthong, S.; Dunn, J.R.; Cheng, H.H. Depletion of CD8αβ+ T Cells in Chickens Demonstrates Their Involvement in Protective Immunity towards Marek’s Disease with Respect to Tumor Incidence and Vaccinal Protection. Vaccines 2020, 8, 557. [Google Scholar] [CrossRef]
- Hao, X.; Li, S.; Li, J.; Yang, Y.; Qin, A.; Shang, S. An Anti-Tumor Vaccine Against Marek’s Disease Virus Induces Differential Activation and Memory Response of γδ T Cells and CD8 T Cells in Chickens. Front. Immunol. 2021, 12, 645426. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Sadler, A.J.; Williams, B.R. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Harlin, O.; Härtle, S.; Fehler, F.; Vychodil, T.; Kaufer, B.B.; Kaspers, B. IFNα and IFNγ Impede Marek’s Disease Progression. Viruses 2019, 11, 1103. [Google Scholar] [CrossRef]
- Haq, K.; Elawadli, I.; Parvizi, P.; Mallick, A.I.; Behboudi, S.; Sharif, S. Interferon-γ influences immunity elicited by vaccines against very virulent Marek’s disease virus. Antivir. Res. 2011, 90, 218–226. [Google Scholar] [CrossRef]
- Meijerink, N.; van Haarlem, D.A.; Velkers, F.C.; Stegeman, A.J.; Rutten, V.P.M.G.; Jansen, C.A. Analysis of chicken intestinal natural killer cells, a major IEL subset during embryonic and early life. Dev. Comp. Immunol. 2021, 114, 103857. [Google Scholar] [CrossRef]
- Sabsabi, M.A.; Kheimar, A.; You, Y.; von La Roche, D.; Härtle, S.; Göbel, T.W.; von Heyl, T.; Schusser, B.; Kaufer, B.B. Unraveling the role of γδ T cells in the pathogenesis of an oncogenic avian herpesvirus. mBio 2024, 15, e0031524. [Google Scholar] [CrossRef]
- Matsuyama-Kato, A.; Iseki, H.; Boodhoo, N.; Bavananthasivam, J.; Alqazlan, N.; Abdul-Careem, M.F.; Plattner, B.L.; Behboudi, S.; Sharif, S. Phenotypic characterization of gamma delta (γδ) T cells in chickens infected with or vaccinated against Marek’s disease virus. Virology 2022, 568, 115–125. [Google Scholar] [CrossRef]
- Matsuyama-Kato, A.; Shojadoost, B.; Boodhoo, N.; Raj, S.; Alizadeh, M.; Fazel, F.; Fletcher, C.; Zheng, J.; Gupta, B.; Abdul-Careem, M.F.; et al. Activated Chicken Gamma Delta T Cells Are Involved in Protective Immunity against Marek’s Disease. Viruses 2023, 15, 285. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama-Kato, A.; Boodhoo, N.; Raj, S.; Abdul-Careem, M.F.; Plattner, B.L.; Behboudi, S.; Sharif, S. The tumor microenvironment generated by Marek’s disease virus suppresses interferon-gamma-producing gamma delta T cells. Vet. Microbiol. 2023, 285, 109874. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Matsuyama-Kato, A.; Murata, S.; Isezaki, M.; Kano, R.; Takasaki, S.; Ichii, O.; Konnai, S.; Ohashi, K. Molecular characterization of immunoinhibitory factors PD-1/PD-L1 in chickens infected with Marek’s disease virus. Virol. J. 2012, 9, 94. [Google Scholar] [CrossRef]
- Kleffel, S.; Posch, C.; Barthel, S.R.; Mueller, H.; Schlapbach, C.; Guenova, E.; Elco, C.P.; Lee, N.; Juneja, V.R.; Zhan, Q.; et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 2015, 162, 1242–1256. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; et al. Immunohistochemical Analysis of PD-L1 Expression in Canine Malignant Cancers and PD-1 Expression on Lymphocytes in Canine Oral Melanoma. PLoS ONE 2016, 11, e0157176. [Google Scholar] [CrossRef] [PubMed]
- Ikebuchi, R.; Konnai, S.; Sunden, Y.; Onuma, M.; Ohashi, K. Molecular cloning and expression analysis of bovine programmed death-1. Microbiol. Immunol. 2010, 54, 291–298. [Google Scholar] [CrossRef]
- Kozako, T.; Yoshimitsu, M.; Fujiwara, H.; Masamoto, I.; Horai, S.; White, Y.; Akimoto, M.; Suzuki, S.; Matsushita, K.; Uozumi, K.; et al. PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia 2009, 23, 375–382. [Google Scholar] [CrossRef]
- Parvizi, P.; Andrzejewski, K.; Read, L.R.; Behboudi, S.; Sharif, S. Expression profiling of genes associated with regulatory functions of T-cell subsets in Marek’s disease virus-infected chickens. Avian Pathol. 2010, 39, 367–373. [Google Scholar] [CrossRef]
- Sato, J.; Murata, S.; Yang, Z.; Kaufer, B.B.; Fujisawa, S.; Seo, H.; Maekawa, N.; Okagawa, T.; Konnai, S.; Osterrieder, N.; et al. Effect of Insertion and Deletion in the Meq Protein Encoded by Highly Oncogenic Marek’s Disease Virus on Transactivation Activity and Virulence. Viruses 2022, 14, 382. [Google Scholar] [CrossRef] [PubMed]
- Conradie, A.M.; Bertzbach, L.D.; Bhandari, N.; Parcells, M.; Kaufer, B.B. A Common Live-Attenuated Avian Herpesvirus Vaccine Expresses a Very Potent Oncogene. Goodrum F, editor. mSphere 2019, 4, e00658-19. [Google Scholar] [CrossRef]
- Schumacher, D.; Tischer, B.K.; Fuchs, W.; Osterrieder, N. Reconstitution of Marek’s Disease Virus Serotype 1 (MDV-1) from DNA Cloned as a Bacterial Artificial Chromosome and Characterization of a Glycoprotein B-Negative MDV-1 Mutant. J. Virol. 2000, 74, 11088–11098. [Google Scholar] [CrossRef] [PubMed]
- Jarosinski, K.W.; Schat, K.A. Multiple alternative splicing to exons II and III of viral interleukin-8 (vIL-8) in the Marek’s disease virus genome: The importance of vIL-8 exon I. Virus Genes 2007, 34, 9–22. [Google Scholar] [CrossRef]
- Kurokawa, A.; Yamamoto, Y. Development of monoclonal antibodies specific to Marek disease virus-EcoRI-Q (Meq) for the immunohistochemical diagnosis of Marek disease using formalin-fixed, paraffin-embedded samples. J. Vet. Diagn. Investig. 2022, 34, 458–464. [Google Scholar] [CrossRef]
- Kurokawa, A.; Yamamoto, Y. Immunohistochemical Diagnosis of Marek’s Disease Based on Meq Detection in 104 Field Cases of Chicken Lymphoma. Avian Dis. 2024, 68, 293–298. [Google Scholar] [CrossRef]
- Chen, D.; Guo, Y.; Jiang, J.; Wu, P.; Zhang, T.; Wei, Q.; Huang, J.; Wu, D. γδ T cell exhaustion: Opportunities for intervention. J. Leukoc. Biol. 2022, 112, 1669–1676. [Google Scholar] [CrossRef]
- Raimondi, G.; Shufesky, W.J.; Tokita, D.; Morelli, A.E.; Thomson, A.W. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J. Immunol. 2006, 176, 2808–2816. [Google Scholar] [CrossRef]
- Burgess, S.C.; Davison, T.F. Identification of the Neoplastically Transformed Cells in Marek’s Disease Herpesvirus-Induced Lymphomas: Recognition by the Monoclonal Antibody AV37. J. Virol. 2002, 76, 7276–7292. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Sheng, M.K.; Vick, L.; Collins, C.; Yoon, D.J.; Murphy, W.J. Asymmetrical Expression of PD1 and CD25 in T-Cells Post-Initial Activation. J. Immunol. 2023, 210, 226.18. [Google Scholar] [CrossRef]
- Gurung, A.; Kamble, N.; Kaufer, B.B.; Pathan, A.; Behboudi, S. Association of Marek’s Disease induced immunosuppression with activation of a novel regulatory T cells in chickens. Cheng HH, editor. PLoS Pathog. 2017, 13, e1006745. [Google Scholar] [CrossRef] [PubMed]
- Buza, J.J.; Burgess, S.C. Modeling the proteome of a Marek’s disease transformed cell line: A natural animal model for CD30 overexpressing lymphomas. Proteomics 2007, 7, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, J.S.; Jeong, Y.H.; Son, J.; Ban, Y.H.; Lee, B.H.; Chen, L.; Chang, J.; Chung, D.H.; Choi, I.; et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J. Immunol. 2015, 194, 5801–5811. [Google Scholar] [CrossRef]
- Brauneck, F.; Weimer, P.; Schulze zur Wiesch, J.; Weisel, K.; Leypoldt, L.; Vohwinkel, G.; Fritzsche, B.; Bokemeyer, C.; Wellbrock, J.; Fiedler, W. Bone Marrow-Resident Vδ1 T Cells Co-express TIGIT with PD-1, TIM-3 or CD39 in AML and Myeloma. Front. Med. 2021, 8, 763773. [Google Scholar] [CrossRef]
- Peters, C.; Oberg, H.-H.; Kabelitz, D.; Wesch, D. Phenotype and regulation of immunosuppressive Vδ2-expressing γδ T cells. Cell Mol. Life Sci. 2014, 71, 1943–1960. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, J.; Motai, Y.; Yamagami, S.; Win, S.Y.; Horio, F.; Saeki, H.; Maekawa, N.; Okagawa, T.; Konnai, S.; Ohashi, K.; et al. Programmed Cell Death-1 Expression in T-Cell Subsets in Chickens Infected with Marek’s Disease Virus. Pathogens 2025, 14, 431. https://doi.org/10.3390/pathogens14050431
Sato J, Motai Y, Yamagami S, Win SY, Horio F, Saeki H, Maekawa N, Okagawa T, Konnai S, Ohashi K, et al. Programmed Cell Death-1 Expression in T-Cell Subsets in Chickens Infected with Marek’s Disease Virus. Pathogens. 2025; 14(5):431. https://doi.org/10.3390/pathogens14050431
Chicago/Turabian StyleSato, Jumpei, Yoshinosuke Motai, Shunsuke Yamagami, Shwe Yee Win, Fumiya Horio, Hikaru Saeki, Naoya Maekawa, Tomohiro Okagawa, Satoru Konnai, Kazuhiko Ohashi, and et al. 2025. "Programmed Cell Death-1 Expression in T-Cell Subsets in Chickens Infected with Marek’s Disease Virus" Pathogens 14, no. 5: 431. https://doi.org/10.3390/pathogens14050431
APA StyleSato, J., Motai, Y., Yamagami, S., Win, S. Y., Horio, F., Saeki, H., Maekawa, N., Okagawa, T., Konnai, S., Ohashi, K., & Murata, S. (2025). Programmed Cell Death-1 Expression in T-Cell Subsets in Chickens Infected with Marek’s Disease Virus. Pathogens, 14(5), 431. https://doi.org/10.3390/pathogens14050431





