Kalmusia variispora (Didymosphaeriaceae, Dothideomycetes) Associated with the Grapevine Trunk Disease Complex in Cyprus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Fungal Isolation
2.2. Genomic DNA Extraction, PCR, and Sequencing
2.3. Phylogenetic Analyses
2.4. Morphological Characterization
2.5. Effect of Temperature on Mycelial Growth
2.6. Exoenzyme Production
2.7. Pathogenicity
2.8. Statistical Analyses
3. Results
3.1. Phylogenetic Analyses
3.2. Morphological Characterization
3.3. Effect of Temperature on Mycelial Growth
3.4. Exoenzyme Production
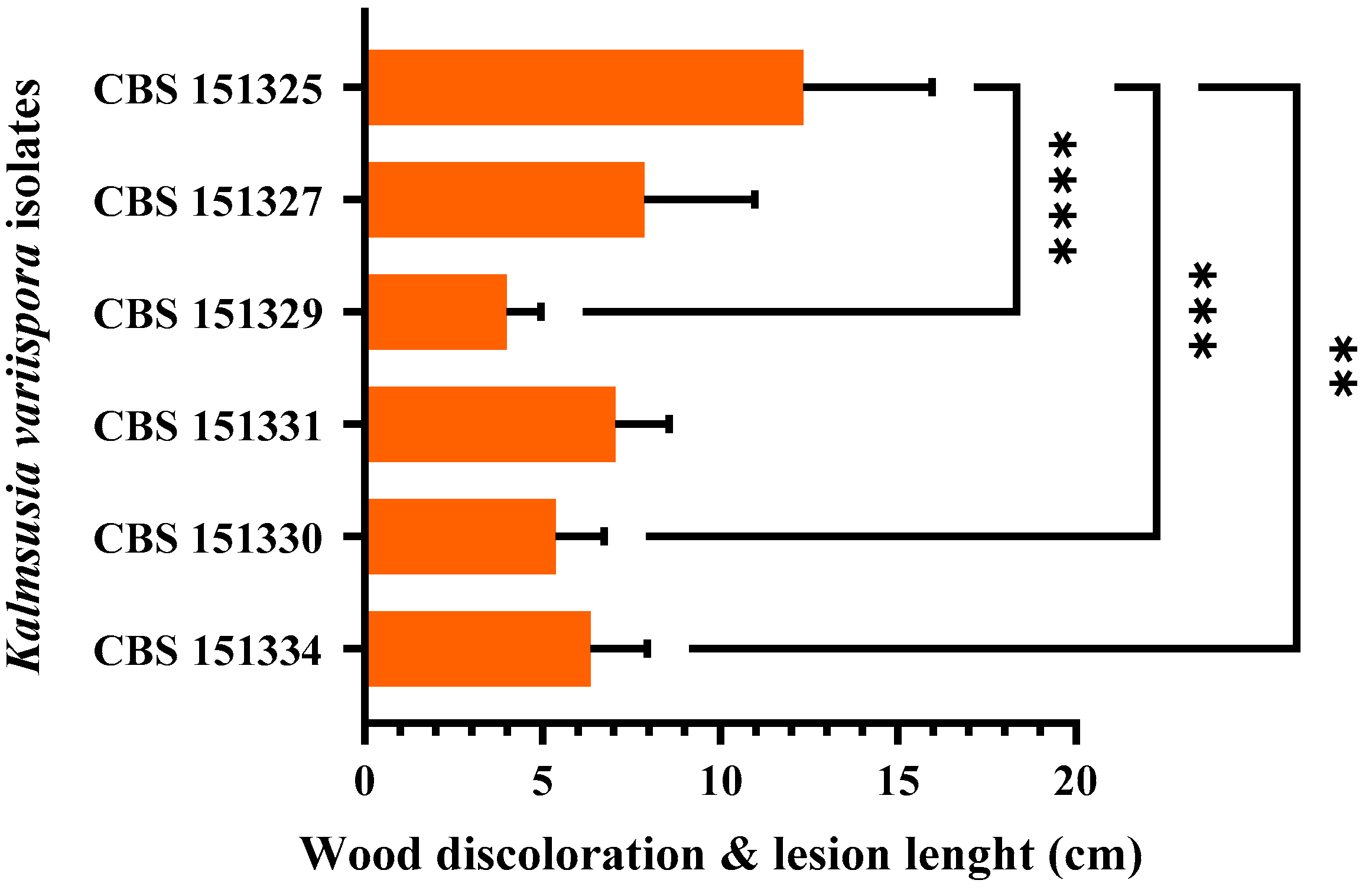
3.5. Pathogenicity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Bruez, E.; Lecomte, P.; Grosman, J.; Doublet, B.; Bertsch, C.; Fontaine, F.; Ugaglia, A.; Teissedre, P.L.; Da Costa, J.P.; Guerin-Dubrana, L.; et al. Overview of grapevine trunk diseases in France in the 2000s. Phytopathol. Mediterr. 2013, 52, 262–275. [Google Scholar]
- Kenfaoui, J.; Radouane, N.; Mennani, M.; Tahiri, A.; El Ghadraoui, L.; Belabess, Z.; Fontaine, F.; El Hamss, H.; Amiri, S.; Lahlali, R.; et al. A Panoramic view on grapevine trunk diseases threats: Case of Eutypa dieback, Botryosphaeria dieback, and Esca disease. J. Fungi 2022, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Úrbez-Torres, J.R.; Peduto, F.; Striegler, R.K.; Urrea-Romero, K.E.; Rupe, J.C.; Cartwright, R.D.; Gubler, W.D. Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers. 2012, 52, 169–189. [Google Scholar] [CrossRef]
- Larignon, P.; Dubos, B. Fungi associated with esca disease in grapevine. Eur. J. Plant Pathol. 1997, 103, 147–157. [Google Scholar] [CrossRef]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef]
- Claverie, M.; Notaro, M.; Fontaine, F.; Wery, J. Current knowledge on grapevine trunk diseases with complex etiology: A systemic approach. Phytopathol. Mediterr. 2020, 59, 29–53. [Google Scholar] [CrossRef]
- Guerin-Dubrana, L.; Fontaine, F.; Mugnai, L. Grapevine trunk disease in European and Mediterranean vineyards: Occurrence, distribution, and associated disease-affecting cultural factors. Phytopathol. Mediterr. 2019, 58, 49–71. [Google Scholar]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Kanetis, L.I.; Taliadoros, D.; Makris, G.; Christoforou, M. A novel Seimatosporium and other Sporocadaceae species associated with Grapevine Trunk Diseases in Cyprus. Plants 2022, 11, 2733. [Google Scholar] [CrossRef]
- Raimondo, M.L.; Carlucci, A.; Ciccarone, C.; Sadallah, A.; Lops, F. Identification and pathogenicity of lignicolous fungi associated with grapevine trunk diseases in southern Italy. Phytopathol. Mediterr. 2019, 58, 639–662. [Google Scholar]
- Armengol, J.; Vicent, A.; Torné, L.; García-Figueres, F.; García-Jiménez, J. Fungi associated with esca and grapevine declines in Spain: A three-year survey. Phytopathol. Mediterr. 2001, 40, 325–329. [Google Scholar]
- Bekris, F.; Vasileiadis, S.; Papadopoulou, E.; Samaras, A.; Testempasis, S.; Gkizi, D.; Tavlaki, G.; Tzima, A.; Paplomatas, E.; Markakis, E.; et al. Grapevine wood microbiome analysis identifies key fungal pathogens and potential interactions with the bacterial community implicated in grapevine trunk disease appearance. Environ. Microbiome 2021, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, A.; Lops, F.; Mostert, L.; Halleen, F.; Raimondo, M.L. Occurrence fungi causing black foot on young grapevines and nursery rootstock plants in Italy. Phytopathol. Mediterr. 2017, 56, 10–39. [Google Scholar]
- Halleen, F.; Crous, P.W.; Petrini, O. Fungi associated with healthy grapevine cuttings in nurseries, with special reference to pathogens involved in the decline of young vines. Australas. Plant Pathol. 2003, 32, 47–52. [Google Scholar] [CrossRef]
- Elena, G.; Bruez, E.; Rey, P.; Luque, J. Microbiota of grapevine woody tissues with or without esca-foliar symptoms in northeast Spain. Phytopathol. Mediterr. 2018, 57, 425–438. [Google Scholar]
- Geiger, A.; Karácsony, Z.; Golen, R.; Váczy, K.Z.; Geml, J. The compositional turnover of grapevine-associated plant pathogenic fungal communities is greater among intraindividual microhabitats and terroirs than among healthy and esca-diseased plants. Phytopathology 2022, 112, 1029–1035. [Google Scholar] [CrossRef]
- Bahmani, Z.; Abdollahzadeh, J.; Amini, J.; Evidente, A. Biscogniauxia rosacearum the charcoal canker agent as a pathogen associated with grapevine trunk diseases in Zagros region of Iran. Sci. Rep. 2021, 11, 14098. [Google Scholar] [CrossRef]
- Abed-Ashtiani, F.; Narmani, A.; Arzanlou, M. Macrophomina phaseolina associated with grapevine decline in Iran. Phytopathol. Mediterr. 2018, 57, 107–111. [Google Scholar]
- Nouri, M.T.; Zhuang, G.; Culumber, C.M.; Trouillas, F.P. First report of Macrophomina phaseolina causing trunk and cordon canker disease of grapevine in the United States. Plant Dis. 2018, 103, 579. [Google Scholar] [CrossRef]
- Arzanlou, M.; Narmani, A.; Moshari, S.; Khodaei, S.; Babai-Ahari, A. Truncatella angustata associated with grapevine trunk disease in northern Iran. Arch. Phytopathol. Plant Prot. 2013, 46, 1168–1181. [Google Scholar] [CrossRef]
- Lengyel, S.; Knapp, D.G.; Karácsony, Z.; Geml, J.; Tempfli, B.; Kovács, G.M.; Váczy, K.Z. Neofabraea kienholzii, a novel causal agent of grapevine trunk diseases in Hungary. Eur. J. Plant Pathol. 2020, 157, 975–984. [Google Scholar] [CrossRef]
- Testempasis, S.I.; Markakis, E.A.; Tavlaki, G.I.; Soultatos, S.K.; Tsoukas, C.; Gkizi, D.; Tzima, A.K.; Paplomatas, E.; Karaoglanidis, G.S. Grapevine trunk diseases in Greece: Disease incidence and fungi involved in discrete geographical zones and varieties. J. Fungi 2024, 10, 2. [Google Scholar] [CrossRef]
- Arzanlou, M.; Narmani, A. ITS sequence data and morphology differentiate Cytospora chrysosperma associated with trunk disease of grapevine in northern Iran. J. Plant Prot. Res. 2015, 55, 117–125. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Pouzoulet, J.; Rolshausen, P.E.; Wilcox, W.F.; Baumgartner, K. Characterization of Cytospora isolates from wood cankers of declining grapevine in north America, with the descriptions of two new Cytospora species. Plant Pathol. 2017, 66, 713–725. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Baumgartner, K. Novel Seimatosporium species from grapevine in northern California and their interactions with fungal pathogens involved in the trunk-disease complex. Plant Dis. 2018, 102, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, J.N.; Khaledi, E.; Abdollahzadeh, J.; Amini, J. Seimatosporium marivanicum, Sporocadus kurdistanicus, and Xenoseimatosporium kurdistanicum: Three new pestalotioid species associated with grapevine trunk diseases from the Kurdistan province, Iran. Mycol. Prog. 2022, 21, 427–446. [Google Scholar] [CrossRef]
- Abed-Ashtiani, F.; Narmani, A.; Arzanlou, M. Analysis of Kalmusia variispora associated with grapevine decline in Iran. Eur. J. Plant Pathol. 2019, 154, 787–799. [Google Scholar] [CrossRef]
- Karácsony, Z.; Knapp, D.G.; Lengyel, S.; Kovács, G.M.; Váczy, K.Z. The fungus Kalmusia longispora is able to cause vascular necrosis on Vitis vinifera. PLoS ONE 2021, 16, e0258043. [Google Scholar] [CrossRef]
- DeKrey, D.H.; Klodd, A.E.; Clark, M.D.; Blanchette, R.A. Grapevine trunk diseases of cold-hardy varieties grown in northern midwest vineyards coincide with canker fungi and winter injury. PLoS ONE 2022, 17, e0269555. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Zhang, W.; Liu, M.; Maharachchikumbura, S.S.N.; Zhou, Y.; Huang, J.B.; Nilthong, S.; Wang, Z.Y.; Li, X.H.; Yan, J.; et al. Identification and characterization of Pestalotiopsis-like fungi related to grapevine diseases in China. Fungal Biol. 2015, 119, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Bhat, J.D.; Dayarathne, M.C.; Huang, S.K.; Norphanphoun, C.; Senanayake, I.C.; Perera, R.H.; et al. Families of Sordariomycetes. Fungal Divers. 2016, 79, 1–317. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Adams, P.; Kamas, J.; Gubler, W.D. Identification, incidence, and pathogenicity of fungal species associated with grapevine dieback in Texas. Am. J. Enol. Vitic. 2009, 60, 497–507. [Google Scholar] [CrossRef]
- Bustamante, M.I.; Todd, C.; Elfar, K.; Hamid, M.I.; Garcia, J.F.; Cantu, D.; Rolshausen, P.E.; Eskalen, A. Identification and pathogenicity of Fusarium species associated with young vine decline in California. Plant Dis. 2019, 108, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Ariyawansa, H.A.; Tanaka, K.; Thambugala, K.M.; Phookamsak, R.; Tian, Q.; Camporesi, E.; Hongsanan, S.; Monkai, J.; Wanasinghe, D.N.; Mapook, A.; et al. A molecular phylogenetic reappraisal of the Didymosphaeriaceae (=Montagnulaceae). Fungal Divers. 2014, 68, 69–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wang, Z.; Fournier, J.; Crous, P.W.; Zhang, X.; Li, W.; Ariyawansa, H.A.; Hyde, K.D. Neotypification and phylogeny of Kalmusia. Phytotataxa 2014, 176, 164–173. [Google Scholar] [CrossRef]
- Verkley, G.J.M.; Dukik, K.; Renfurm, R.; Göker, M.; Stielow, J.B. Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota). Persoonia 2014, 32, 25–51. [Google Scholar] [CrossRef]
- Gomzhina, M.M.; Gasich, E.L.; Gagkaeva, T.Y.; Gannibal, P.B. Biodiversity of fungi inhabiting European blueberry in north-western Russia and in Finland. Dokl Biol. Sci. 2022, 507, 441–455. [Google Scholar] [CrossRef]
- Kadkhoda-Hematabadi, S.; Mohammadi, H.; Sohrabi, M. Morphological and molecular identification of plant pathogenic fungi associated with necrotic wood tissues of pomegranate trees in Iran. J. Plant Pathol. 2023, 105, 465–479. [Google Scholar] [CrossRef]
- Khodaei, S.; Arzanlou, M.; Babai-Ahari, A.; Rota-Stabelli, O.; Pertot, I. Phylogeny and evolution of Didymosphaeriaceae (Pleosporales): New Iranian samples and hosts, first divergence estimates, and multiple evidences of species misidentifications. Phytotaxa 2019, 424, 131–146. [Google Scholar] [CrossRef]
- Liu, J.K.; Hyde, K.D.; Jones, E.B.G.; Ariyawansa, H.A.; Bhat, D.J.; Boonmee, S.; Maharachchikumbura, S.S.N.; McKenzie, E.H.C.; Phookamsak, R.; Phukhamsakda, C.; et al. Fungal diversity notes 1-110: Taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 2015, 72, 1–197. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Calabon, M.S.; Jones, E.B.G.; Zhang, Y.X.; Liao, C.F.; Xiong, Y.R.; Chaiwan, N.; Kularathnage, N.D.; Liu, N.G.; Tang, S.M.; et al. Mycosphere notes 345–386. Mycosphere 2022, 13, 454–557. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Schumacher, R.K.; Akulov, A.; Bulgakov, T.S.; Carnegie, A.J.; Jurjević, Ž.; Decock, C.; Denman, S.; Lombard, L.; et al. New and interesting fungi. 3. Fungal Syst. Evol. 2020, 6, 157–231. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, C.; Quill, Z. Fungal endophyte diversity in table grapes. Can. J. Microbiol. 2021, 67, 29–36. [Google Scholar] [CrossRef]
- Bashiri, S.; Abdollahzadeh, J. Taxonomy and pathogenicity of fungi associated with oak decline in northern and central Zagros forests of Iran with emphasis on coelomycetous species. Front. Plant Sci. 2024, 15, 1377441. [Google Scholar] [CrossRef]
- Martino, I.; Agustí-Brisach, C.; Nari, L.; Gullino, M.L.; Guarnaccia, V. Characterization and pathogenicity of fungal species associated with dieback of apple trees in northern Italy. Plant Dis. 2024, 108, 311–331. [Google Scholar] [CrossRef]
- Travadon, R.; Lawrence, D.P.; Moyer, M.M.; Fujiyoshi, P.T.; Baumgartner, K. Fungal species associated with grapevine trunk diseases in Washington wine grapes and California table grapes, with novelties in the genera Cadophora, Cytospora, and Sporocadus. Front. Fungal Bio. 2022, 3, 1018140. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Garcia, J.F.; Morales-Cruz, A.; Cochetel, N.; Minio, A.; Figueroa-Balderas, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Comparative pangenomic insights into the distinct evolution of virulence factors among grapevine trunk pathogens. Mol. Plant Microbe Interact. 2024, 37, 127–142. [Google Scholar] [CrossRef]
- Stempien, E.; Goddard, M.L.; Wilhelm, K.; Tarnus, C.; Bertsch, C.; Chong, J. Grapevine Botryosphaeria dieback fungi have specific aggressiveness factor repertory involved in wood decay and stilbene metabolization. PLoS ONE 2017, 12, e0188766. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols. A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among Ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenet. Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: Enabling high-impact science for phylogenetics researchers with limited resources. In Proceedings of the 2012 XSEDE Conference: Bridging from the Extreme to the Campus and Beyond; Stewart, C., Ed.; Association for Computing Machinery: New York, NY, USA, 2012; pp. 1–8. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Rayner, R.W. A Mycological Colour Chart; Commonwealth Mycological Institute: Kew, UK, 1970. [Google Scholar]
- Aigoun-Mouhous, W.; Mahamedi, A.E.; León, M.; Chaouia, C.; Zitouni, A.; Barankova, K.; Eichmeier, A.; Armengol, J.; Gramaje, D.; Berraf-Tebbaf, A. Cadophora sabaouae sp. nov. and Phaeoacremonium species associated with Petri disease on grapevine propagation material and young grapevines in Algeria. Plant Dis. 2021, 105, 3657–3668. [Google Scholar] [CrossRef]
- Naik, P.R.; Raman, G.; Narayanan, K.B.; Sakthivel, N. Assessment of genetic and functional diversity of phosphate solubilizing fluorescent pseudomonads isolated from rhizospheric soil. BMC Microbiol. 2008, 8, 230. [Google Scholar] [CrossRef]
- Cattelan, A.J.; Hartel, P.G.; Fuhrmann, J.J. Screening for plant growth-promoting rhizobacteria to promote early soybean growth. Soil Sci. Soc. Am. J. 1999, 63, 1670–1680. [Google Scholar] [CrossRef]
- Mohandas, A.; Raveendran, S.; Parameswaran, B.; Abraham, A.; Athira, R.S.R.; Mathew, A.K.; Pandey, A. Production of pectinase from Bacillus sonorensis MPTD1. Food Technol. Biotechnol. 2018, 56, 110–116. [Google Scholar] [CrossRef]
- Kasana, R.C.; Salwan, R.; Dhar, H.; Dutt, S.; Gulati, A. A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Curr. Microbiol. 2008, 57, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.M.C.N.; da Silva, R.; Gomes, E. Screening of bacterial strains for pectinolytic activity: Characterization of the polygalacturonase produced by Bacillus sp. Rev. Microbiol. 1999, 30, 299–303. [Google Scholar] [CrossRef]
- Devasia, S.; Nair, A.J. Screening of potent laccase-producing organisms based on the oxidation pattern of different phenolic substrates. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 127–137. [Google Scholar] [CrossRef]
- Makris, G.; Solonos, S.; Christodoulou, M.; Kanetis, L.I. First report of Diaporthe foeniculina associated with grapevine trunk diseases on Vitis vinifera in Cyprus. Plant Dis. 2022, 106, 1294. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Pinheiro, C.; Duarte, J.; Sumonte, C.; Ferrada, E.E.; Elfar, K.; Eskalen, A.; Lolas, M.; Galdós, L.; Díaz, G.A. Severe outbreak of dry core rot in apple fruits cv. Fuji caused by Kalmusia variispora during preharvest in Maule region, Chile. Plant Dis. 2022, 106, 2750. [Google Scholar]
- Ntasiou, P.; Samaras, A.; Karaoglanidis, G. Apple fruit core rot agents in Greece and control with succinate dehydrogenase inhibitor fungicides. Plant Dis. 2021, 105, 3072–3081. [Google Scholar] [CrossRef]
- Aroca, Á.; Gramaje, D.; Armengol, J.; García-Jiménez, J.; Raposo, R. Evaluation of the grapevine nursery propagation process as a source of Phaeoacremonium spp. and Phaeomoniella chlamydospora and occurrence of trunk disease pathogens in rootstock mother vines in Spain. Eur. J. Plant Pathol. 2010, 126, 165–174. [Google Scholar] [CrossRef]
- Fourie, P.H.; Halleen, F. Occurrence of grapevine trunk disease pathogens in rootstock mother plants in South Africa. Austalas. Plant Pathol. 2004, 33, 313–315. [Google Scholar] [CrossRef]
- Hofstetter, V.; Buyck, B.; Croll, D.; Viret, O.; Couloux, A.; Gindro, K. What if esca disease of grapevine were not a fungal disease? Fungal Divers. 2012, 54, 51–67. [Google Scholar] [CrossRef]
- Hrycan, J.; Hart, M.; Bowen, P.; Forge, T.; Úrbez-Torres, J.R. Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease development and symptom expression. Phytopathol. Mediterr. 2020, 59, 395–424. [Google Scholar]
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Bellincampi, D.; Cervone, F.; Lionetti, V. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front. Plant Sci. 2014, 5, 228. [Google Scholar] [CrossRef] [PubMed]
- Belair, M.; Grau, A.L.; Chong, J.; Tian, X.; Luo, J.; Guan, X.; Pensec, F. Pathogenicity factors of Botryosphaeriaceae associated with grapevine trunk diseases: New developments on their action on grapevine defense responses. Pathogens 2022, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Bahmani, Z.; Masi, M.; Di Lecce, R.; Amini, J.; Abdollahzadeh, J.; Tuzi, A.; Evidente, A. Massarilactones D and H, phytotoxins produced by Kalmusia variispora, associated with grapevine trunk diseases (GTDs) in Iran. Nat. Prod. Res. 2021, 35, 5192–5198. [Google Scholar] [CrossRef]
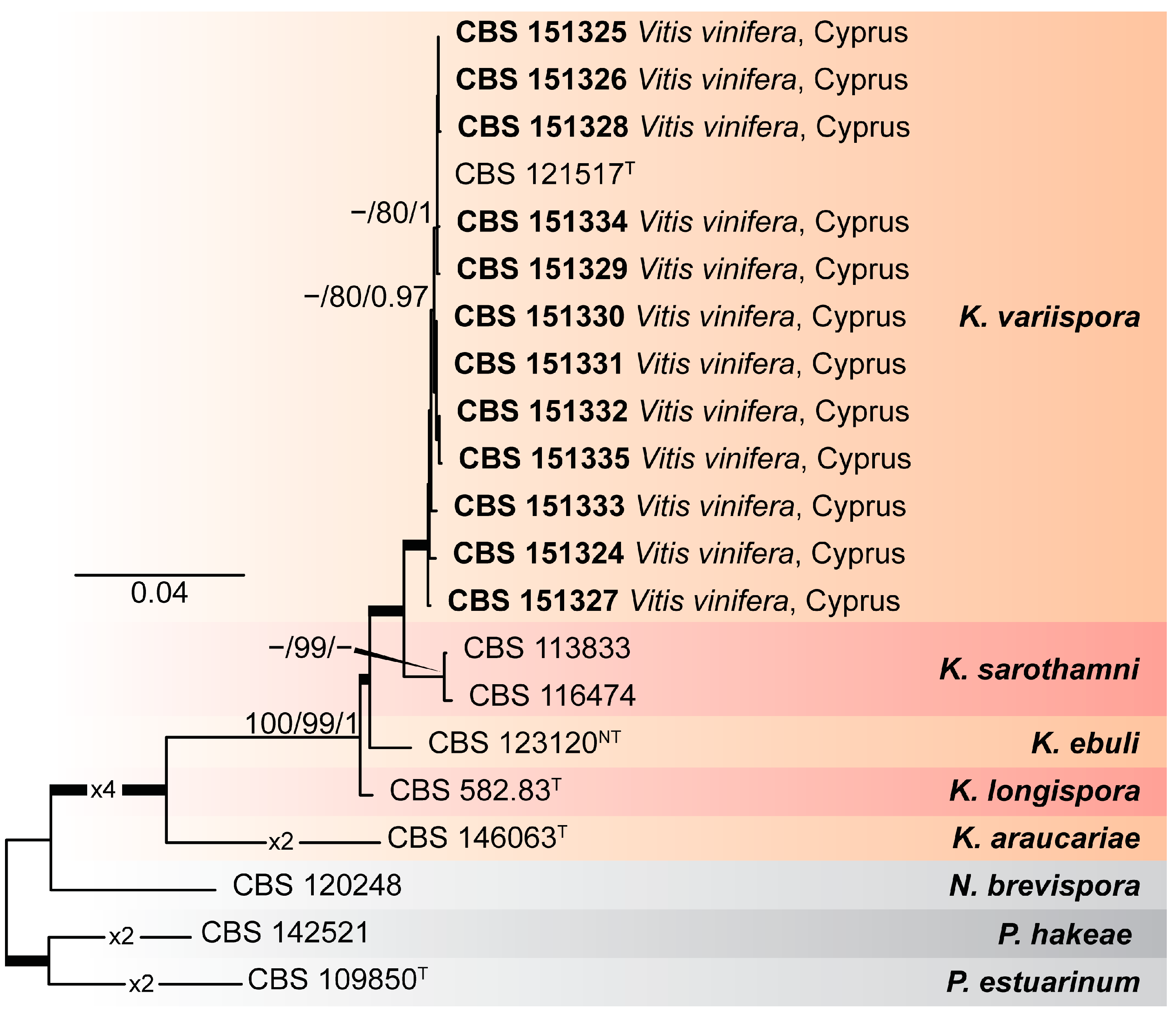
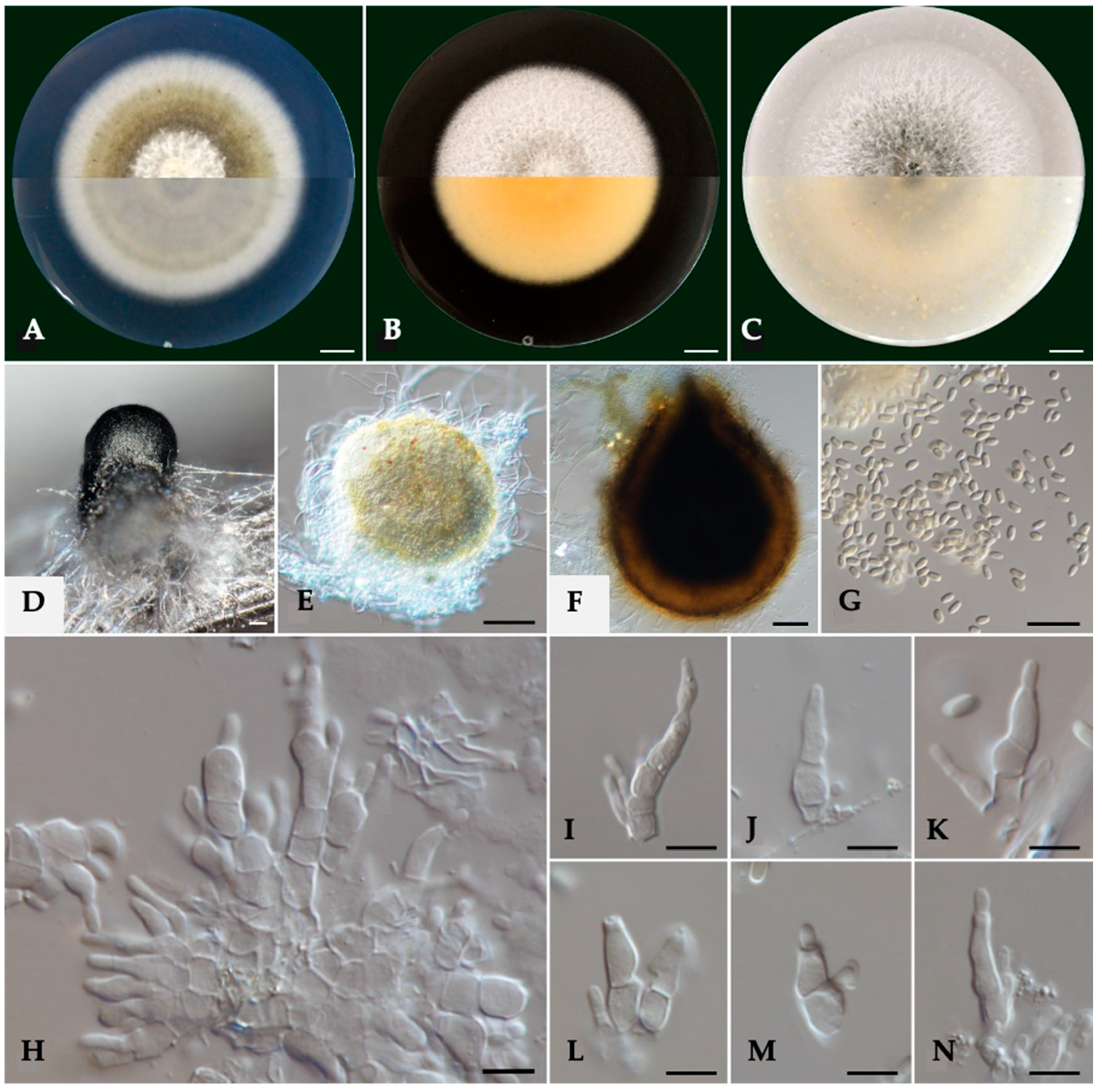
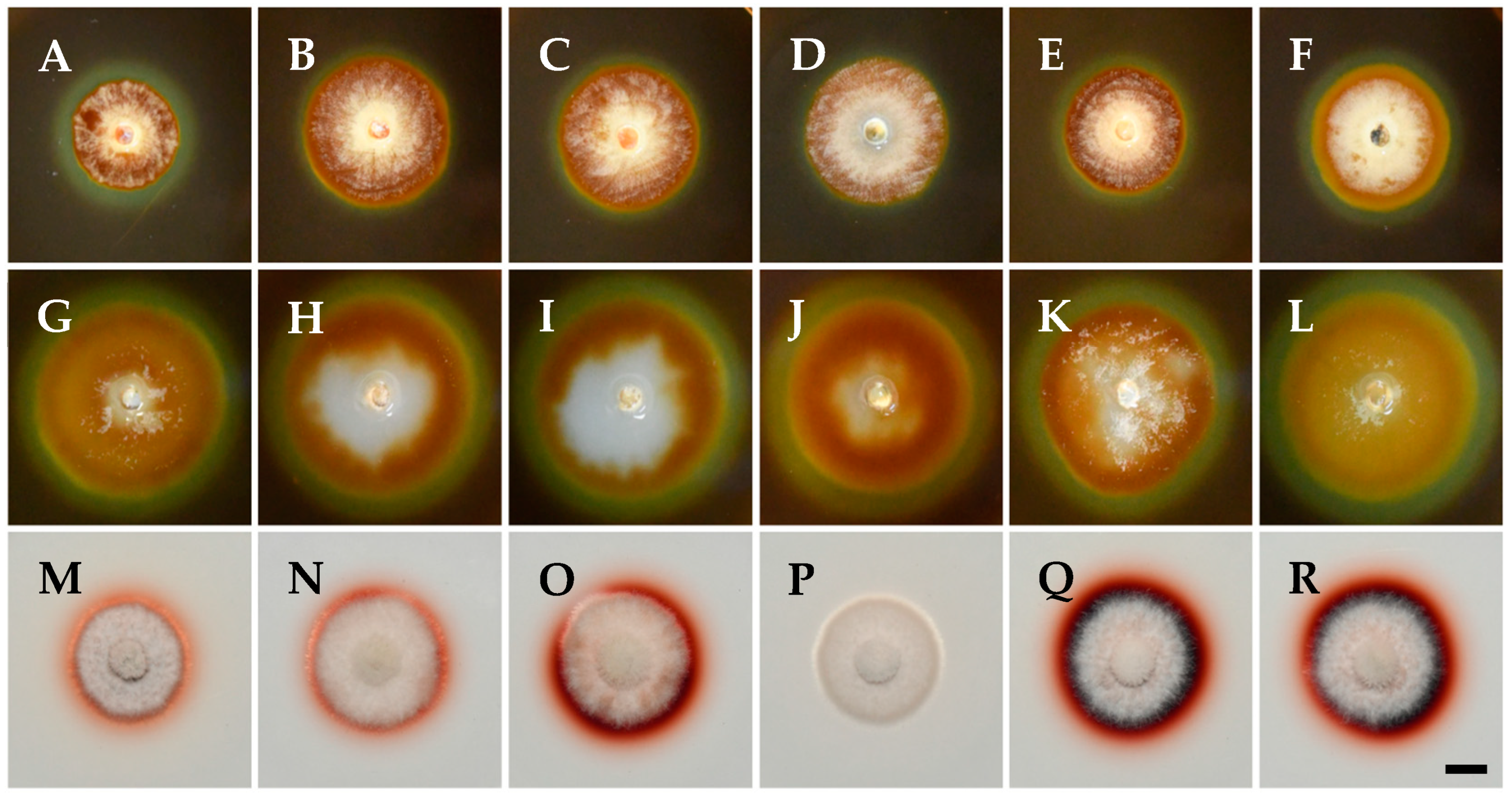
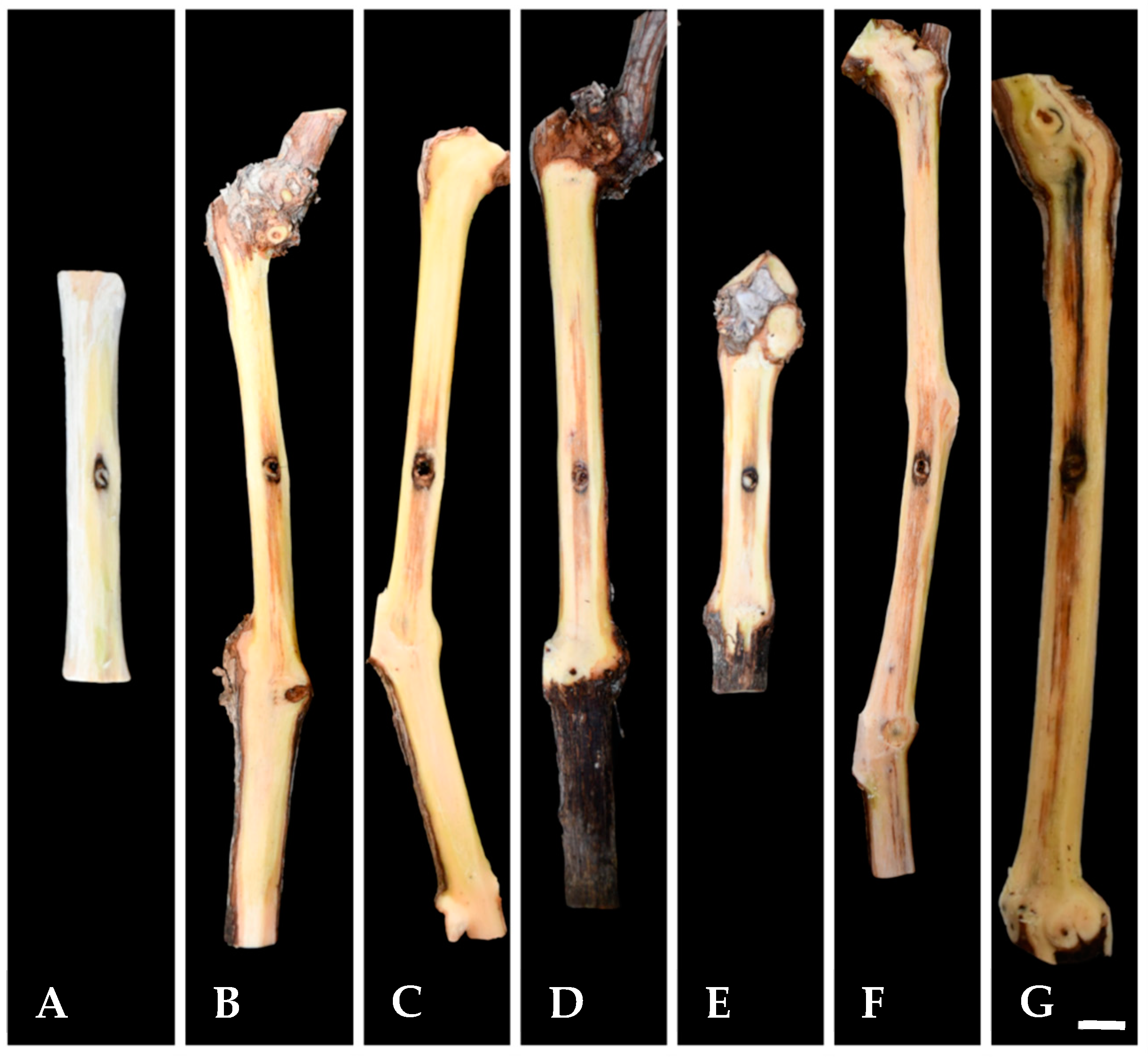
| Species | Strain a,b | Country | Host | GenBank Accession Number c | |||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | SSU | b-tub | rpb2 | tef1-a | ||||
| Kalmusia araucariae | CPC 37475T | USA | Araucaria bidwillii | MT223805 | PP664292 | PP667340 | - | PP715462 | PP715503 |
| K. cordylines | ZHKUCC 21-0092T | China | Cordyline fruticosa | OL352082 | OL818333 | OL818335 | - | - | - |
| K. ebuli | CBS 123120NT | France | Populus tremula | PP667315 | PP664289 | PP667335 | PP715468 | PP715447 | PP715488 |
| K. erioi | MFLUCC 18-0832T | Thailand | - | MN473058 | MN473052 | MN473046 | MN481603 | - | - |
| K. italica | MFLUCC 13-0066T | Italy | Spartium junceum | KP325440 | KP325441 | KP325442 | - | - | - |
| K. longispora | CBS 582.83T | Canada | Arceuthobium pusillum | JX496097 | JX496210 | PP667338 | PP715481 | PP715460 | PP715501 |
| K. sarothamni | CBS 116474 | - | - | PP667313 | PP664288 | PP667332 | PP715466 | PP715444 | PP715485 |
| K. sarothamni | CBS 113833 | - | - | PP667311 | PP664287 | PP667331 | PP715463 | PP715442 | PP715483 |
| K. spartii | MFLUCC 14-0560T | Italy | S. junceum | KP744441 | KP744487 | KP753953 | - | - | - |
| K. variispora | CBS 121517T | Syria | Vitis vinifera | PP667314 | JX496143 | PP667334 | PP715467 | PP715446 | PP715487 |
| K. variispora | CBS 151324 | Cyprus | V. vinifera | PP667316 | PP664290 | PP667336 | PP715469 | PP715448 | PP715489 |
| K. variispora | CBS 151325 | Cyprus | V. vinifera | MZ312148 | MZ312183 | MZ312321 | PP715470 | PP715449 | PP715490 |
| K. variispora | CBS 151326 | Cyprus | V. vinifera | MZ312149 | MZ312184 | MZ312322 | PP715471 | PP715450 | PP715491 |
| K. variispora | CBS 151327 | Cyprus | V. vinifera | MZ312138 | MZ312173 | MZ312311 | PP715472 | PP715451 | PP715492 |
| K. variispora | CBS 151328 | Cyprus | V. vinifera | PP667317 | PP664291 | PP667337 | PP715473 | PP715452 | PP715493 |
| K. variispora | CBS 151329 | Cyprus | V. vinifera | MZ312140 | MZ312175 | MZ312313 | PP715474 | PP715453 | PP715494 |
| K. variispora | CBS 151330 | Cyprus | V. vinifera | MZ312141 | MZ312176 | MZ312314 | PP715475 | PP715454 | PP715495 |
| K. variispora | CBS 151331 | Cyprus | V. vinifera | MZ312143 | MZ312178 | MZ312316 | PP715476 | PP715455 | PP715496 |
| K. variispora | CBS 151332 | Cyprus | V. vinifera | MZ312139 | MZ312174 | MZ312312 | PP715477 | PP715456 | PP715497 |
| K. variispora | CBS 151333 | Cyprus | V. vinifera | MZ312144 | MZ312179 | MZ312317 | PP715478 | PP715457 | PP715498 |
| K. variispora | CBS 151334 | Cyprus | V. vinifera | MZ312142 | MZ312177 | MZ312315 | PP715479 | PP715458 | PP715499 |
| K. variispora | CBS 151335 | Cyprus | V. vinifera | MZ312151 | MZ312186 | MZ312324 | PP715480 | PP715459 | PP715500 |
| Neokalmusia brevispora | CBS 120248 | Japan | Sasa sp. | MH863078 | JX681110 | PP667333 | PP715464 | PP715445 | PP715486 |
| Paraconiothyrium hakae | CBS 142521 | Australia | Hakea sp. | KY979754 | KY979809 | - | KY979920 | KY979847 | KY979892 |
| P. estuarinum | CBS 109850T | Brazil | estuarine sediment | MH862842 | MH874432 | AY642522 | JX496355 | LT854937 | - |
| Isolate | Adjusted Model x | Optimum Temperature (°C) y | Growth Rate (mm/Day) z | |||
|---|---|---|---|---|---|---|
| R2 | a | b | c | |||
| CBS 151327 | 0.89 | −0.017 | 0.7850 | −47.854 | 22.18 a | 3.92 |
| CBS 151329 | 0.90 | −0.019 | 0.8649 | −54.839 | 22.64 a | 4.31 |
| CBS 151331 | 0.90 | −0.020 | 0.9305 | −62.999 | 22.92 a | 4.36 |
| CBS 151334 | 0.93 | −0.018 | 0.8052 | −483.334 | 22.24 a | 4.12 |
| Mean | 22.5 | 4.18 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makris, G.; Sandoval-Denis, M.; Crous, P.W.; Kanetis, L.I. Kalmusia variispora (Didymosphaeriaceae, Dothideomycetes) Associated with the Grapevine Trunk Disease Complex in Cyprus. Pathogens 2025, 14, 428. https://doi.org/10.3390/pathogens14050428
Makris G, Sandoval-Denis M, Crous PW, Kanetis LI. Kalmusia variispora (Didymosphaeriaceae, Dothideomycetes) Associated with the Grapevine Trunk Disease Complex in Cyprus. Pathogens. 2025; 14(5):428. https://doi.org/10.3390/pathogens14050428
Chicago/Turabian StyleMakris, Georgios, Marcelo Sandoval-Denis, Pedro W. Crous, and Loukas I. Kanetis. 2025. "Kalmusia variispora (Didymosphaeriaceae, Dothideomycetes) Associated with the Grapevine Trunk Disease Complex in Cyprus" Pathogens 14, no. 5: 428. https://doi.org/10.3390/pathogens14050428
APA StyleMakris, G., Sandoval-Denis, M., Crous, P. W., & Kanetis, L. I. (2025). Kalmusia variispora (Didymosphaeriaceae, Dothideomycetes) Associated with the Grapevine Trunk Disease Complex in Cyprus. Pathogens, 14(5), 428. https://doi.org/10.3390/pathogens14050428








