Trans-Kingdom sRNA Silencing in Sclerotinia sclerotiorum for Crop Fungal Disease Management
Abstract
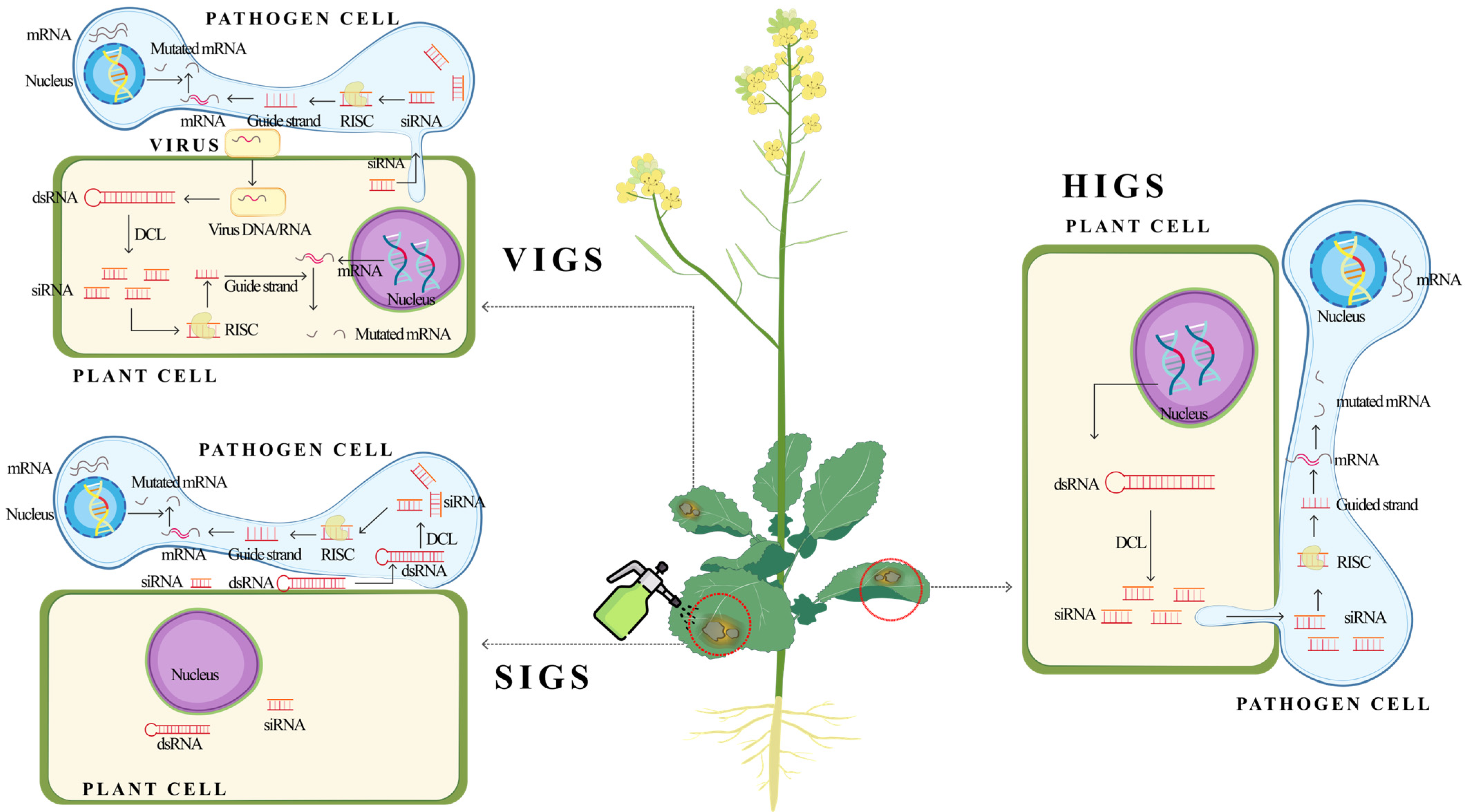
1. Introduction
2. Virulence-Related Genes Newly Identified in S. sclerotiorum
| Coding Gene | Typology | Function | Pathway | Related Gene/Protein | Reference |
|---|---|---|---|---|---|
| SsPEIE1 | Plant early immunosuppressive effector | Positively meditate virulence | Inhibit oligomerization-mediated immune responses | Hypersensitive-induced reaction (HIR) | [39] |
| SsStuA | APSES family transcription factor | Involved in vegetative growth, sclerotia formation, fungicide tolerance, and full virulence | CWI pathway and ROS response | SsSmk3, SsAGM1 | [30] |
| SsZNC1 | Fungal-specific Zn2Cys6 transcription factors | Involved in virulence, sclerotial development, and stress response | Regulate the expressions of genes related to metabolic pathways, biosynthetic pathways, secreted proteins, and autophagy | / | [31] |
| SsFoxE3 | Transcription factor | Contribute to sclerotia, compound appressoria formation, and pathogenicity | Promoter of SsAtg8 | [34] | |
| Sszfh1 | C2H2 transcription factor | Contribute to the proper development and maturation of sclerotia and apothecia, hyphae | ROS production and melanin accumulation | SsNOX1 and SsNOX2 | [53] |
| SsFkh1 | Transcription factor | Hyphal development, virulence, sclerotia formation, and maintenance of the cell wall integrity (CWI) | MAPK signaling pathway | SsMkk1 | [56] |
| SsSnf5 | Transcription factor | Maturation of infection cushions, tolerance of stress, and pathogenicity | SsSnf5-SsHsf1-SsHsp70 module | SsHsf1, SsHsp70 | [50] |
| SsSte12 | Transcription factor | Sclerotia formation, compound appressoria development and sexual reproduction | Pheromone response MAPK cascade | / | [36] |
| SsCTP1 | Effector protein | Inhibit plant immunity and promote pathogen infections | Targeting the chloroplast proteins GmCPX and GmSKL2 | Coproporphyrinogen-III oxidase (GmCPX) and shikimate kinase 2 (GmSKL2) | [40] |
| SsCVNH | Effector protein | Inhibit the plant immune response | Interfere with ROS homeostasis, reduce peroxidase activity, and decrease the activation of defence gene transcription, thereby contributing to the virulence of S. sclerotiorum | Class III peroxidase AtPRX71 | [41] |
| SsINE1/5 | Effector protein | Induce cell death | / | NLR protein | [57] |
| SsYCP1 | Effector protein | Pathogenicity | / | / | [58] |
| SsEmp24/SsErv25 | Secreted protein | Positively regulate vegetative growth and sclerotium formation, cushion formation and virulence | SsEmp24 interacts with SsErv25 to form a p24 protein complex and act as cargo receptors to accept and carry specific cargo proteins between ER and Golgi apparatus, including secreted proteins, GPI-APs, and membrane proteins | Interact with each other | [49] |
| SsERP1 | Secretory protein | Pathogenicity | Plant ethylene signaling pathway | / | [59] |
| SsCut1 | Cutinsae secreted protein | Promote virulence without the influence on growth rate, colony morphology, oxalic acid production, infection cushion formation, and sclerotial development | Enhance the cutinase activity and activate plant immune response | / | [46] |
| SsTrx1: | Substrates for reductive enzymes | Regulate hyphal growth rate, mycelial morphology and sclerotial development, promote pathogenicity and oxidative stress tolerance | Trx system, ROS scavenging pathway | [54] | |
| AGM1 | N-acetylglucosamine-phosphate mutase | Function in vegetative growth, sclerotia production, and infection cushion involved in the response to osmotic stress and inhibitors of cell wall synthesis | Catalyzes intramolecular phosphoryl transfer on the phosphosugar substrate GlcNAc-6P in the biosynthesis of UDP-GlcNAc, therefore meditating the synthesize of chitin, an important component of cell walls | / | [47] |
| SsCak1 | Protein kinase | Positively contribute to the growth, development, and pathogenicity | Effectuating the phosphorylation of CDC28 | / | [51] |
| SsOs4 | MPKKK (phosphotransferase) | Contribute to mycelial growth and differentiation, sclerotia formation, virulence, hyperosmotic adaptation, fungicide sensitivity, and phosphorylation of SsHog1 | The high osmolarity glycerol (HOG) pathway: regulate phosphorylation of SsHog1 and the expression level of Sshog1 | SsglpA and Ssfps1, Sshog1 | [37] |
| SsNR | Nitrate reductase | Contribute to mycelia growth, sclerotia formation, infection cushion formation, cell wall and membrane integrity, OA production, and virulence | Nitrogen metabolism | CYP and infection cushion development-related genes (Ggt1, Sac1, and Smk3) | [48] |
| SsPDE2 | cAMP phosphodiesterase | Sclerotia formation, oxalic acid accumulation, infection cushion functionality, and virulence | cAMP-dependent inhibition of MAPK signaling | / | [60] |
| SsAtg1 | Protein kinase | Nutrient utilization, sclerotia development, cell wall integrity, and pathogenicity | Autophagy pathway | / | [33] |
| SsShy1 | Salicylate hydroxylase | Hyphae growth and sclerotia production, cell wall integrity, and pathogenicity | Tricarboxylic acid cycle of glucose metabolism | / | [42] |
| SsDim5 | Histone methyltransferase | Cell integrity, oxidative stress and osmotic stress response, and pathogenicity | H3K9 trimethylation, mycotoxins biosynthesis | / | [61] |
| SsHog1 | Histidine kinase | Osmotic adaptation, fungicide sensitivity, and virulence | High osmolarity glycerol (Hog1) stress response, MAPK signal transduction pathway | Shk1 | [38] |
| SsXyl2 | Glycosyl hydrolase family 11 xylanase | Host cell physiology, pathogenicity | / | Hypersensitive-induced reaction protein 2 (NbHIR2) | [43] |
| SsCat2 | Catalase | Contribute to the predominant catalase activity, H2O2-induced oxidative stress response, cell integrity of hyphae, and pathogenicity | / | AOX gene | [62] |
| Ssgar1, | Plant cell wall degrading enzymes (CWDEs) | Virulence, tolerance to salt stress., and cell wall stability | D-galacturonic acid catabolism | / | [63] |
| SsGH5 | Cell wall degrading enzymes | Glucan utilization, plant cell death, and pathogenicity | / | / | [64] |
| SsSte11 | MAPKKK | Sclerotia formation, hyphal fusion, vegetative growth, and pathogenicity | SsSte50-SsSte11-SsSte7-Smk1 cascade | SsSte50, SsSte7 | [36] |
| SsSte7 | MAPKK | Hyphal fusion and pathogenicity | SsSte50-SsSte11-SsSte7-Smk1 cascade | SsSte1, Smk1 | [36] |
| Smk1 | MAPK | Hyphal fusion and pathogenicity | SsSte50-SsSte11-SsSte7-Smk1 cascade | SsSte7 | [36] |
| SsSte50 | Adaptor protein | Normal vegetative growth, hyphal fusion, sclerotia formation, compound appressoria formation, and pathogenicity | SsSte50-SsSte11-SsSte7-Smk1 cascade | SsSte11 | [36] |
| SsATG8 | Ubiquitin-like protein | Vegetative growth, sclerotial formation, oxalic acid (OA) production, compound appressoria development, and virulence | Ubiquitin–proteasome and selective autophagy pathways | SsNBR1 | [32] |
| SsGsr1 | Glycosylphosphatidylinositol (GPI)-anchored cell wall protein | Cell wall integrity of hyphae, pathogenicity, and cell death | / | / | [44] |
| SsNEP2 | Necrosis and ethylene-inducible peptide | Involved in fungal virulence by affecting ROS levels and causing cell death | / | / | [45] |
| Sspka2/SspkaR | Catalytic/regulatory | Autophagy regulation and appressorium development | cAMP-dependent protein kinase A (PKA) signaling pathway, carbohydrate metabolism and mobilization | / | [35] |
| SsMRT4 | Ribosome assembly factor | Contribute to mycelia growth, appressorium formation, oxalic acid production, and abilities to ROS elimination and resistance to oxidative and osmotic stresses | / | / | [52] |
| SSA | S. sclerotiorum agglutinin | Positively regulate sclerotial development and resistance to C. minitans mycoparasitism but negatively regulate pathogenicity and resistance to chemical stresses | / | / | [65] |
| SsArf6 | ADP-ribosylation factors | Hyphal growth and development, melanin accumulation, appressorium formation, and fungal virulence | / | / | [55] |
3. Target Gene Selection and Categories of Trans-Kingdom RNAi
3.1. Trans-Kingdom RNAi Target Gene Selection
3.2. Categories of Trans-Kingdom RNAi
4. Application of Trans-Kingdom RNAi in S. sclerotiorum
| Target Gene | Applied Method | Host | Reference |
|---|---|---|---|
| SsSnf5-SsHsf1-SsHsp70 | HIGS | N. benthamiana | [50] |
| SsSte50 | HIGS | N. benthamiana, A. thaliana | [36] |
| SsERP1 | SIGS | Tobacco | [59] |
| SsTrx1 | HIGS | A. thaliana, N. benthamiana | [54] |
| SsCak1 | HIGS | N. benthamiana | [51] |
| SsPDE2 | HIGS | N. benthamiana | [60] |
| ABHYDROLASE-3 | SIGS, HIGS | B. napus; A. thaliana | [102,103,104] |
| SsMNO1 | SIGS | B. napus, A. thaliana | [112] |
| SsGAP1 | HIGS | N. benthamiana, A. thaliana | [100] |
| SsRAS1/SsRAS2 | HIGS | N. benthamiana, A. thaliana | [100] |
| SsOah1 | HIGS | A. thaliana, Soybean | [97,98,113] |
| SsPG1 | HIGS, SIGS | B. napus, N. benthamiana | [113] |
| SsCBH | HIGS, SIGS | B. napus, N. benthamiana | [113] |
| SsCnd1 | HIGS | N. benthamiana, A. thaliana, | [99] |
| SsVPS51/SsDCTN1/SsSAC1 | SIGS | Lactuca sativa var. ramosa Hort, Brassica oleracea var. acephala DC. | [20] |
| SsPac1/SsSmk1 | SIGS | B. juncea, A. thaliana | [101] |
| SsAgo2 | SIGS | N. benthamiana | [114] |
5. Challenges of Trans-Kingdom RNAi in the Control of S. sclerotiorum
6. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Purdy, L.H. Sclerotinia sclerotiorum: History, diseases and symptomatology, host range, geographic distribution, and impact. Phytopathology 1979, 69, 875–880. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, W.; Huang, C. Statistics and analysis of oilseed rape losses caused by main diseases and insect pests in recent 10 years. Plant Prot. 2018, 44, 24–30. [Google Scholar]
- Bandara, A.Y.; Weerasooriya, D.K.; Bradley, C.A.; Allen, T.W.; Esker, P.D. Dissecting the economic impact of soybean diseases in the United States over two decades. PLoS ONE 2020, 15, e0231141. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Belt, K.; Thatcher, L. Tackling control of a cosmopolitan phytopathogen: Sclerotinia. Front. Plant Sci. 2021, 12, 707509. [Google Scholar] [CrossRef]
- Pressete, C.G.; Giannini, L.S.V.; De Paula, D.A.C.; Do Carmo, M.A.V.; Assis, D.M.; Santos, M.F.C.; Machado, J.D.C.; Marques, M.J.; Soares, M.G.; Azevedo, L. Sclerotinia sclerotiorum (white mold): Cytotoxic, mutagenic, and antimalarial effects in vivo and in vitro. J. Food Sci. 2019, 84, 3866–3875. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sultana, F.; Li, W.; Tran, L.-S.P.; Mostofa, M.G. Sclerotinia sclerotiorum (Lib.) de Bary: Insights into the pathogenomic features of a global pathogen. Cells 2023, 12, 1063. [Google Scholar] [CrossRef]
- Hegedus, D.D.; Rimmer, S.R. Sclerotinia sclerotiorum: When “to be or not to be” a pathogen? FEMS Microbiol. Lett. 2005, 251, 177–184. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.-Y.; Cao, J.; Li, Y.-L.; Ding, L.-N.; Zhu, K.-M.; Yang, Y.-H.; Tan, X.-L. Recent advances in mechanisms of plant defense to Sclerotinia sclerotiorum. Front. Plant Sci. 2019, 10, 1314. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Baulcombe, D. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef]
- Knip, M.; Constantin, M.E.; Thordal-Christensen, H. Trans-kingdom cross-talk: Small RNAs on the move. PLoS Genet. 2014, 10, e1004602. [Google Scholar] [CrossRef]
- Liu, S.; Da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The host shapes the gut microbiota via fecal microRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs–big players in plant-microbe interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.C.; Dean, R.A. Host-induced gene silencing: A tool for understanding fungal host interaction and for developing novel disease control strategies. Mol. Plant Pathol. 2012, 13, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Martin-Hernandez, A.M.; Peart, J.R.; Malcuit, I.; Baulcombe, D.C. Virus-induced gene silencing in plants. Methods Protoc. 2003, 30, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Rössner, C.; Lotz, D.; Becker, A. VIGS goes viral: How VIGS transforms our understanding of plant science. Annu. Rev. Plant Biol. 2022, 73, 703–728. [Google Scholar] [CrossRef]
- Whangbo, J.S.; Hunter, C.P. Environmental RNA interference. Trends Genet. 2008, 24, 297–305. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Lin, F.-M.; Thomma, B.P.; Huang, H.-D.; Jin, H. Bidirectional trans-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H. Spray-induced gene silencing: A powerful innovative strategy for crop protection. Trends Microbiol. 2017, 25, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; He, B.; Weiberg, A.; Buck, A.H.; Jin, H. Small RNAs and extracellular vesicles: New mechanisms of cross-species communication and innovative tools for disease control. PLoS Pathog. 2019, 15, e1008090. [Google Scholar] [CrossRef] [PubMed]
- Kabbage, M.; Yarden, O.; Dickman, M.B. Pathogenic attributes of Sclerotinia sclerotiorum: Switching from a biotrophic to necrotrophic lifestyle. Plant Sci. 2015, 233, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Niu, X.; Chen, H.; Xu, L.; Qi, C. Characterization of a canola C2 domain gene that interacts with PG, an effector of the necrotrophic fungus Sclerotinia sclerotiorum. J. Exp. Bot. 2009, 60, 2613–2620. [Google Scholar] [CrossRef][Green Version]
- Dallal Bashi, Z.; Hegedus, D.D.; Buchwaldt, L.; Rimmer, S.R.; Borhan, M.H. Expression and regulation of Sclerotinia sclerotiorum necrosis and ethylene-inducing peptides (NEPs). Mol. Plant Pathol. 2010, 11, 43–53. [Google Scholar] [CrossRef]
- Liang, X.; Liberti, D.; Li, M.; Kim, Y.T.; Hutchens, A.; Wilson, R.; Rollins, J.A. Oxaloacetate acetylhydrolase gene mutants of Sclerotinia sclerotiorum do not accumulate oxalic acid, but do produce limited lesions on host plants. Mol. Plant Pathol. 2015, 16, 559–571. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Newman, T.E.; Khentry, Y.; Owolabi Taiwo, A. The evolutionary and molecular features of the broad-host-range plant pathogen Sclerotinia sclerotiorum. Mol. Plant Pathol. 2022, 23, 1075–1090. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, C.; Deng, Y.; Yuan, W.; Zhang, T.; Lu, J. Recent advances in virulence of a broad host range plant pathogen Sclerotinia sclerotiorum: A mini-review. Front. Microbiol. 2024, 15, 1424130. [Google Scholar] [CrossRef]
- Jiao, W.; Li, M.; Lei, T.; Liu, X.; Zhang, J.; Hu, J.; Zhang, X.; Liu, J.; Shi, S.; Pan, H. The APSES Transcription Factor SsStuA Regulating Cell Wall Integrity Is Essential for Sclerotia Formation and Pathogenicity in Sclerotinia sclerotiorum. J. Fungi 2024, 10, 238. [Google Scholar] [CrossRef]
- Huang, Y.; Zhaxi, Z.; Fu, Y.; Xie, J.; Chen, T.; Li, B.; Yu, X.; Lin, Y.; Jiang, D.; Cheng, J. The Transcription Factor SsZNC1 Mediates Virulence, Sclerotial Development, and Osmotic Stress Response in Sclerotinia sclerotiorum. J. Fungi 2024, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Lai, W.; Huang, K.; Li, Y.; Wang, Z.; Chen, X.; Wang, A. SsATG8 and SsNBR1 mediated-autophagy is required for fungal development, proteasomal stress response and virulence in Sclerotinia sclerotiorum. Fungal Genet. Biol. 2021, 157, 103632. [Google Scholar] [CrossRef]
- Jiao, W.; Yu, H.; Chen, X.; Xiao, K.; Jia, D.; Wang, F.; Zhang, Y.; Pan, H. The SsAtg1 activating autophagy is required for sclerotia formation and pathogenicity in Sclerotinia sclerotiorum. J. Fungi 2022, 8, 1314. [Google Scholar] [CrossRef]
- Jiao, W.; Yu, H.; Cong, J.; Xiao, K.; Zhang, X.; Liu, J.; Zhang, Y.; Pan, H. Transcription factor SsFoxE3 activating SsAtg8 is critical for sclerotia, compound appressoria formation, and pathogenicity in Sclerotinia sclerotiorum. Mol. Plant Pathol. 2022, 23, 204–217. [Google Scholar] [CrossRef]
- Yu, P.-L.; Rollins, J.A. The cAMP-dependent protein kinase A pathway perturbs autophagy and plays important roles in development and virulence of Sclerotinia sclerotiorum. Fungal Biol. 2022, 126, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, J.; Xu, Y.; Qiu, Y.; Zhang, Y.; Li, X. A MAP kinase cascade broadly regulates the lifestyle of Sclerotinia sclerotiorum and can be targeted by HIGS for disease control. Plant J. 2024, 118, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xiu, Q.; Wang, J.; Duan, Y.; Zhou, M. A putative MAPK kinase kinase gene Ssos4 is involved in mycelial growth, virulence, osmotic adaptation, and sensitivity to fludioxonil and is essential for SsHog1 phosphorylation in Sclerotinia sclerotiorum. Phytopathology 2021, 111, 521–530. [Google Scholar] [CrossRef]
- Ma, J.; Park, S.-W.; Kim, G.; Kim, C.S.; Chang, H.-X.; Chilvers, M.I.; Sang, H. Characterization of SsHog1 and Shk1 Using Efficient Gene Knockout Systems through Repeated Protoplasting and CRISPR/Cas9 Ribonucleoprotein Approaches in Sclerotinia sclerotiorum. J. Agric. Food Chem. 2024, 72, 4237–4245. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, H.; Yuan, M.; Li, P.; Xie, J.; Fu, Y.; Li, B.; Yu, X.; Chen, T.; Lin, Y. An effector essential for virulence of necrotrophic fungi targets plant HIRs to inhibit host immunity. Nat. Commun. 2024, 15, 9391. [Google Scholar] [CrossRef]
- Cui, W.; Xiao, K.; Yang, F.; Qiao, K.; Xu, X.; Gu, S.; Guo, J.; Song, Z.; Pan, H.; Wang, F. A Virulence Factor from Sclerotinia sclerotiorum Targets the Host Chloroplast Proteins to Promote Infection. Plants 2024, 13, 3430. [Google Scholar] [CrossRef]
- Ma, M.; Tang, L.; Sun, R.; Lyu, X.; Xie, J.; Fu, Y.; Li, B.; Chen, T.; Lin, Y.; Yu, X. An effector SsCVNH promotes the virulence of Sclerotinia sclerotiorum through targeting class III peroxidase AtPRX71. Mol. Plant Pathol. 2024, 25, e13464. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Huang, K.; Li, B.; Lu, G.; Wang, A. Functional Analysis of a Salicylate Hydroxylase in Sclerotinia sclerotiorum. J. Fungi 2023, 9, 1169. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Hu, Y.; Chen, Z.; Han, L.; Zhu, W.; Tian, B.; Fang, A.; Yang, Y.; Bi, C. Plant hypersensitive induced reaction protein facilitates cell death induced by secreted xylanase associated with the pathogenicity of Sclerotinia sclerotiorum. Plant J. 2024, 118, 90–105. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, H.; Lu, Z.; Zhang, P.; Zheng, S.; Wang, J.; Tian, B.; Fang, A.; Yang, Y.; Bi, C. Variable tandem glycine-rich repeats contribute to cell death-inducing activity of a glycosylphosphatidylinositol-anchored cell wall protein that is associated with the pathogenicity of Sclerotinia sclerotiorum. Microbiol. Spectr. 2023, 11, e00986-23. [Google Scholar] [CrossRef]
- Yang, C.; Li, W.; Huang, X.; Tang, X.; Qin, L.; Liu, Y.; Xia, Y.; Peng, Z.; Xia, S. SsNEP2 Contributes to the Virulence of Sclerotinia sclerotiorum. Pathogens 2022, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fu, Y.; Xie, J.; Li, B.; Chen, T.; Lin, Y.; Chen, W.; Jiang, D.; Cheng, J. Sclerotinia sclerotiorum SsCut1 modulates virulence and cutinase activity. J. Fungi 2022, 8, 526. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, K.; Li, M.; Hu, H.; Zhang, X.; Liu, J.; Pan, H.; Zhang, Y. SsAGM1-mediated uridine diphosphate-N-acetylglucosamine synthesis is essential for development, stress response, and pathogenicity of Sclerotinia sclerotiorum. Front. Microbiol. 2022, 13, 938784. [Google Scholar] [CrossRef]
- Wei, J.; Yao, C.; Zhu, Z.; Gao, Z.; Yang, G.; Pan, Y. Nitrate reductase is required for sclerotial development and virulence of Sclerotinia sclerotiorum. Front. Plant Sci. 2023, 14, 1096831. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Shang, Q.; Mo, C.; Xiao, Y.; Wang, G.; Xie, J.; Jiang, D.; Xiao, X. Early secretory pathway-associated proteins SsEmp24 and SsErv25 are involved in morphogenesis and pathogenicity in a filamentous phytopathogenic fungus. mBio 2021, 12, e03173-21. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, L.; He, R.; Rollins, J.A.; Li, A.; Zhang, G.; He, X.; Wang, R.; Liu, J.; Zhang, X. The Snf5-Hsf1 transcription module synergistically regulates stress responses and pathogenicity by maintaining ROS homeostasis in Sclerotinia sclerotiorum. New Phytol. 2024, 241, 1794–1812. [Google Scholar] [CrossRef]
- Qin, L.; Nong, J.; Cui, K.; Tang, X.; Gong, X.; Xia, Y.; Xu, Y.; Qiu, Y.; Li, X.; Xia, S. SsCak1 Regulates Growth and Pathogenicity in Sclerotinia sclerotiorum. Int. J. Mol. Sci. 2023, 24, 12610. [Google Scholar] [CrossRef]
- Yang, C.; Tang, L.; Qin, L.; Zhong, W.; Tang, X.; Gong, X.; Xie, W.; Li, Y.; Xia, S. mRNA turnover protein 4 is vital for fungal pathogenicity and response to oxidative stress in Sclerotinia sclerotiorum. Pathogens 2023, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lyu, X.; Pan, Z.; Wang, Q.; Mu, W.; Benny, U.; Rollins, J.A.; Pan, H. The C2H2 transcription factor SsZFH1 regulates the size, number, and development of apothecia in Sclerotinia sclerotiorum. Phytopathology 2022, 112, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Ding, Y.; Banga, S.S.; Liao, H.; Zhao, S.; Yu, Y.; Qian, W. Sclerotinia sclerotiorum Thioredoxin1 (SsTrx1) is required for pathogenicity and oxidative stress tolerance. Mol. Plant Pathol. 2021, 22, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, S.; Wang, T.; Xia, Q.; Xia, S. The Sclerotinia sclerotiorum ADP-Ribosylation Factor 6 Plays an Essential Role in Abiotic Stress Response and Fungal Virulence to Host Plants. J. Fungi 2023, 10, 12. [Google Scholar] [CrossRef]
- Cong, J.; Xiao, K.; Jiao, W.; Zhang, C.; Zhang, X.; Liu, J.; Zhang, Y.; Pan, H. The coupling between cell wall integrity mediated by MAPK kinases and SsFkh1 is involved in sclerotia formation and pathogenicity of Sclerotinia sclerotiorum. Front. Microbiol. 2022, 13, 816091. [Google Scholar] [CrossRef]
- Newman, T.E.; Kim, H.; Khentry, Y.; Sohn, K.H.; Derbyshire, M.C.; Kamphuis, L.G. The broad host range pathogen Sclerotinia sclerotiorum produces multiple effector proteins that induce host cell death intracellularly. Mol. Plant Pathol. 2023, 24, 866–881. [Google Scholar] [CrossRef]
- Fan, H.; Yang, W.; Nie, J.; Lin, C.; Wu, J.; Wu, D.; Wang, Y. Characterization of a secretory YML079-like cupin protein that contributes to Sclerotinia sclerotiorum pathogenicity. Microorganisms 2021, 9, 2519. [Google Scholar] [CrossRef]
- Fan, H.; Yang, W.; Nie, J.; Zhang, W.; Wu, J.; Wu, D.; Wang, Y. A novel effector protein SsERP1 inhibits plant ethylene signaling to promote Sclerotinia sclerotiorum infection. J. Fungi 2021, 7, 825. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, Y.; Zhang, Y.; Li, X. A cAMP phosphodiesterase is essential for sclerotia formation and virulence in Sclerotinia sclerotiorum. Front. Plant Sci. 2023, 14, 1175552. [Google Scholar] [CrossRef]
- Qin, L.; Gong, X.; Nong, J.; Tang, X.; Cui, K.; Zhao, Y.; Xia, S. Histone Methyltransferase SsDim5 Regulates Fungal Virulence through H3K9 Trimethylation in Sclerotinia sclerotiorum. J. Fungi 2024, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lu, J.; Liu, R.; Wang, P.; Hu, Y.; Fang, A.; Yang, Y.; Qing, L.; Bi, C.; Yu, Y. SsCat2 encodes a catalase that is critical for the antioxidant response, QoI fungicide sensitivity, and pathogenicity of Sclerotinia sclerotiorum. Fungal Genet. Biol. 2021, 149, 103530. [Google Scholar] [CrossRef]
- Wei, W.; Pierre-Pierre, N.; Peng, H.; Ellur, V.; Vandemark, G.J.; Chen, W. The D-galacturonic acid catabolic pathway genes differentially regulate virulence and salinity response in Sclerotinia sclerotiorum. Fungal Genet. Biol. 2020, 145, 103482. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, H.; Xie, J.; Fu, Y.; Li, B.; Yu, X.; Chen, T.; Lin, Y.; Jiang, D.; Cheng, J. A Glycosyl Hydrolase 5 Family Protein Is Essential for Virulence of Necrotrophic Fungi and Can Suppress Plant Immunity. Int. J. Mol. Sci. 2024, 25, 2693. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Wei, J.; Zhang, J.; Wu, M.; Li, G.; Yang, L. Sclerotinia sclerotiorum agglutinin modulates sclerotial development, pathogenicity and response to abiotic and biotic stresses in different manners. J. Fungi 2023, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Kogel, K.H. New wind in the sails: Improving the agronomic value of crop plants through RNA i-mediated gene silencing. Plant Biotechnol. J. 2014, 12, 821–831. [Google Scholar] [CrossRef]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.-H. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase–encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Luo, Y.; Zhou, S.; An, L.; Wang, C.; Jin, Q.; Zhou, M.; Xu, J.-R. Molecular evolution and functional divergence of tubulin superfamily in the fungal tree of life. Sci. Rep. 2014, 4, 6746. [Google Scholar] [CrossRef]
- Gu, K.-X.; Song, X.-S.; Xiao, X.-M.; Duan, X.-X.; Wang, J.-X.; Duan, Y.-B.; Hou, Y.-P.; Zhou, M.-G. A β2-tubulin dsRNA derived from Fusarium asiaticum confers plant resistance to multiple phytopathogens and reduces fungicide resistance. Pestic. Biochem. Physiol. 2019, 153, 36–46. [Google Scholar] [CrossRef]
- Yin, C.; Jurgenson, J.E.; Hulbert, S.H. Development of a host-induced RNAi system in the wheat stripe rust fungus Puccinia striiformis f. sp. tritici. Mol. Plant-Microbe Interact. 2011, 24, 554–561. [Google Scholar] [CrossRef]
- Yin, C.; Downey, S.I.; Klages-Mundt, N.L.; Ramachandran, S.; Chen, X.; Szabo, L.J.; Pumphrey, M.; Hulbert, S.H. Identification of promising host-induced silencing targets among genes preferentially transcribed in haustoria of Puccinia. BMC Genom. 2015, 16, 579. [Google Scholar] [CrossRef]
- Qi, T.; Zhu, X.; Tan, C.; Liu, P.; Guo, J.; Kang, Z.; Guo, J. Host-induced gene silencing of an important pathogenicity factor PsCPK 1 in Puccinia striiformis f. sp. tritici enhances resistance of wheat to stripe rust. Plant Biotechnol. J. 2018, 16, 797–807. [Google Scholar] [CrossRef]
- Schaefer, L.K.; Parlange, F.; Buchmann, G.; Jung, E.; Wehrli, A.; Herren, G.; Müller, M.C.; Stehlin, J.; Schmid, R.; Wicker, T. Trans-kingdom RNAi of pathogen effectors leads to quantitative adult plant resistance in wheat. Front. Plant Sci. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Faris, J.; Muthukrishnan, S.; Liu, D.; Chen, P.; Gill, B. Isolation and characterization of novel cDNA clones of acidic chitinases and β-1, 3-glucanases from wheat spikes infected by Fusarium graminearum. Theor. Appl. Genet. 2001, 102, 353–362. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Song, N.; Zhao, M.; Liu, R.; Feng, H.; Wang, X.; Kang, Z. Puccinia striiformis f. sp. tritici microRNA-like RNA 1 (Pst-milR1), an important pathogenicity factor of Pst, impairs wheat resistance to Pst by suppressing the wheat pathogenesis-related 2 gene. New Phytol. 2017, 215, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Amselem, J.; Cuomo, C.A.; Van Kan, J.A.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; De Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.C.; Yadav, S.; Arora, S.; Mishra, D.C.; Budhlakoti, N.; Gaikwad, K.; Rao, M.; Prasad, L.; Rai, P.K.; Sharma, P. Draft genome sequencing and secretome profiling of Sclerotinia sclerotiorum revealed effector repertoire diversity and allied broad-host range necrotrophy. Sci. Rep. 2022, 12, 21855. [Google Scholar] [CrossRef]
- Mcloughlin, A.G.; Wytinck, N.; Walker, P.L.; Girard, I.J.; Rashid, K.Y.; De Kievit, T.; Fernando, W.G.D.; Whyard, S.; Belmonte, M.F. Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep. 2018, 8, 7320. [Google Scholar] [CrossRef]
- Gebremichael, D.E.; Haile, Z.M.; Negrini, F.; Sabbadini, S.; Capriotti, L.; Mezzetti, B.; Baraldi, E. RNA interference strategies for future management of plant pathogenic fungi: Prospects and challenges. Plants 2021, 10, 650. [Google Scholar] [CrossRef]
- Hannon, G. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Meister, G.; Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pyott, D.E.; Molnar, A. Going mobile: Non-cell-autonomous small RNAs shape the genetic landscape of plants. Plant Biotechnol. J. 2015, 13, 306–318. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Bellinger, M.; Jin, H. Small RNAs: A new paradigm in plant-microbe interactions. Annu. Rev. Phytopathol. 2014, 52, 495–516. [Google Scholar] [CrossRef]
- Choudry, M.W.; Nawaz, P.; Jahan, N.; Riaz, R.; Ahmad, B.; Raza, M.H.; Fayaz, Z.; Malik, K.; Afzal, S. RNA based gene silencing modalities to control insect and fungal plant pests–Challenges and future prospects. Physiol. Mol. Plant Pathol. 2024, 130, 102241. [Google Scholar] [CrossRef]
- Zand Karimi, H.; Innes, R.W. Molecular mechanisms underlying host-induced gene silencing. Plant Cell 2022, 34, 3183–3199. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.; Kim, J.-I. Advanced strategies to control plant pathogenic fungi by host-induced gene silencing (HIGS) and spray-induced gene silencing (SIGS). Plant Biotechnol. Rep. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Nowara, D.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J.; Schweizer, P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 2010, 22, 3130–3141. [Google Scholar] [CrossRef]
- Su, X.; Wang, Q.; Zhang, T.; Ge, X.; Liu, W.; Guo, H.; Wang, X.; Sun, Z.; Li, Z.; Cheng, H. A Verticillium dahliae exoglucanase as potential HIGS target interacts with a cotton cysteine protease to confer resistance to cotton Verticillium wilt. Plant Biotechnol. J. 2024, 22, 2107–2109. [Google Scholar] [CrossRef]
- Wang, M.; Dean, R.A. Host induced gene silencing of Magnaporthe oryzae by targeting pathogenicity and development genes to control rice blast disease. Front. Plant Sci. 2022, 13, 959641. [Google Scholar] [CrossRef]
- Hu, D.; Chen, Z.Y.; Zhang, C.; Ganiger, M. Reduction of Phakopsora pachyrhizi infection on soybean through host-and spray-induced gene silencing. Mol. Plant Pathol. 2020, 21, 794–807. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Farooq, M.A.; Zhao, T.; Wang, P.; Tabusam, J.; Wang, Y.; Xuan, S.; Zhao, J.; Chen, X.; Shen, S. Virus-induced gene silencing (VIGS): A powerful tool for crop improvement and its advancement towards epigenetics. Int. J. Mol. Sci. 2023, 24, 5608. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Dong, L.; Jiang, T.; Fan, Z. Silencing specific genes in plants using virus-induced gene silencing (VIGS) vectors. In Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 149–161. [Google Scholar]
- Xu, J.; Zhang, N.; Wang, K.; Xian, Q.; Dong, J.; Qi, X.; Chen, X. Chitinase chi 2 positively regulates cucumber resistance against Fusarium oxysporum f. sp. cucumerinum. Genes 2021, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; San-Blas, G. Chitin synthesis as a target for antifungal drugs. Curr. Drug Targets-Infect. Disord. 2003, 3, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Tinoco, M.L.; Rieth, A.; Maia, F.; Aragão, F. Host-induced gene silencing in the necrotrophic fungal pathogen Sclerotinia sclerotiorum. Plant Pathol. 2016, 65, 626–632. [Google Scholar] [CrossRef]
- Rana, K.; Yuan, J.; Liao, H.; Banga, S.S.; Kumar, R.; Qian, W.; Ding, Y. Host-induced gene silencing reveals the role of Sclerotinia sclerotiorum oxaloacetate acetylhydrolase gene in fungal oxalic acid accumulation and virulence. Microbiol. Res. 2022, 258, 126981. [Google Scholar] [CrossRef]
- Mccaghey, M.; Shao, D.; Kurcezewski, J.; Lindstrom, A.; Ranjan, A.; Whitham, S.A.; Conley, S.P.; Williams, B.; Smith, D.L.; Kabbage, M. Host-Induced Gene Silencing of a Sclerotinia sclerotiorum oxaloacetate acetylhydrolase Using Bean Pod Mottle Virus as a Vehicle Reduces Disease on Soybean. Front. Plant Sci. 2021, 12, 677631. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Yan, B.; Liao, H.; Dong, M.; Meng, X.; Wan, H.; Qian, W. Host-induced gene silencing of a multifunction gene SsCnd1 enhances plant resistance against Sclerotinia sclerotiorum. Front. Microbiol. 2021, 12, 693334. [Google Scholar] [CrossRef]
- Xu, Y.; Tan, J.; Lu, J.; Zhang, Y.; Li, X. RAS signalling genes can be used as host-induced gene silencing targets to control fungal diseases caused by Sclerotinia sclerotiorum and Botrytis cinerea. Plant Biotechnol. J. 2024, 22, 262–277. [Google Scholar] [CrossRef]
- Pant, P.; Kaur, J. Control of Sclerotinia sclerotiorum via an RNA interference (RNAi)-mediated targeting of SsPac1 and SsSmk1. Planta 2024, 259, 153. [Google Scholar] [CrossRef]
- Walker, P.L.; Ziegler, D.J.; Giesbrecht, S.; Mcloughlin, A.; Wan, J.; Khan, D.; Hoi, V.; Whyard, S.; Belmonte, M.F. Control of white mold (Sclerotinia sclerotiorum) through plant-mediated RNA interference. Sci. Rep. 2023, 13, 6477. [Google Scholar] [CrossRef]
- Wytinck, N.; Ziegler, D.J.; Walker, P.L.; Sullivan, D.S.; Biggar, K.T.; Khan, D.; Sakariyahu, S.K.; Wilkins, O.; Whyard, S.; Belmonte, M.F. Host induced gene silencing of the Sclerotinia sclerotiorum ABHYDROLASE-3 gene reduces disease severity in Brassica napus. PLoS ONE 2022, 17, e0261102. [Google Scholar] [CrossRef]
- Azizi, A.; Del Río Mendoza, L.E. Effective Control of Sclerotinia Stem Rot in Canola Plants Through Application of Exogenous Hairpin RNA of Multiple Sclerotinia sclerotiorum Genes. Phytopathology 2024, 114, 1000–1010. [Google Scholar] [CrossRef]
- Ranjan, A.; Jayaraman, D.; Grau, C.; Hill, J.H.; Whitham, S.A.; Ané, J.M.; Smith, D.L.; Kabbage, M. The pathogenic development of Sclerotinia sclerotiorum in soybean requires specific host NADPH oxidases. Mol. Plant Pathol. 2018, 19, 700–714. [Google Scholar] [CrossRef]
- Wang, K.; Uppalapati, S.R.; Zhu, X.; Dinesh-Kumar, S.P.; Mysore, K.S. SGT1 positively regulates the process of plant cell death during both compatible and incompatible plant-pathogen interactions. Mol. Plant Pathol. 2010, 11, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.; Xia, J.; Jia, J.; Jiang, D.; Zhang, B.; Fu, Y.; Cheng, J.; Xie, J. Exploring the interaction between endornavirus and Sclerotinia sclerotiorum: Mechanisms of phytopathogenic fungal virulence and antivirus. mBio 2025, 16, e0336524. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.; Li, B.; Cheng, S.; Jia, J.; Jiang, D.; Fu, Y.; Cheng, J.; Lin, Y.; Chen, T.; Xie, J. Nine viruses from eight lineages exhibiting new evolutionary modes that co-infect a hypovirulent phytopathogenic fungus. PLoS Pathog. 2021, 17, e1009823. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Qu, Z.; Pierre-Pierre, N.; Jiang, D.; Souza, F.L.; Miklas, P.N.; Porter, L.D.; Vandemark, G.J.; Chen, W. Exploring the Mycovirus Sclerotinia sclerotiorum Hypovirulence-Associated DNA Virus 1 as a Biocontrol Agent of White Mold Caused by Sclerotinia sclerotiorum. Plant Dis. 2024, 108, 624–634. [Google Scholar] [CrossRef]
- Jia, J.; Jiang, D.; Xie, J. Viruses shuttle between fungi and plants. Trends Microbiol. 2024, 32, 620–621. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Pearson, M.N. Molecular characterisation of an endornavirus infecting the phytopathogen Sclerotinia sclerotiorum. Virus Res. 2014, 189, 303–309. [Google Scholar] [CrossRef]
- Han, L.; Li, Y.; Yuan, Z.; Wang, J.; Tian, B.; Fang, A.; Yang, Y.; Bi, C.; Yu, Y. RNA interference-mediated targeting of monooxygenase SsMNO1 for controlling Sclerotinia stem rot caused by Sclerotinia sclerotiorum. Pest Manag. Sci. 2024, 81, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yin, S.; Lin, L.; Liu, D.; Ren, S.; Zhang, W.; Meng, W.; Chen, P.; Sun, Q.; Fang, Y. Host-induced gene silencing of multiple pathogenic factors of Sclerotinia sclerotiorum confers resistance to Sclerotinia rot in Brassica napus. Crop J. 2022, 10, 661–671. [Google Scholar] [CrossRef]
- Mukherjee, S.; Beligala, G.; Feng, C.; Marzano, S.-Y. Double-stranded RNA targeting white mold Sclerotinia sclerotiorum argonaute 2 for disease control via spray-induced gene silencing. Phytopathology 2024, 114, 1253–1262. [Google Scholar] [CrossRef]
- Santala, J.; Valkonen, J.P. Sensitivity of small RNA-based detection of plant viruses. Front. Microbiol. 2018, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: Efficacy and resistance management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- Sidorova, T.; Miroshnichenko, D.; Kirov, I.; Pushin, A.; Dolgov, S. Effect of grafting on viral resistance of non-transgenic plum scion combined with transgenic PPV-resistant rootstock. Front. Plant Sci. 2021, 12, 621954. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, S.; Liu, Y.; Wang, L.; Jiang, J.; Zhao, S.; Fang, W.; Chen, F.; Guan, Z. Long-distance transport RNAs between rootstocks and scions and graft hybridization. Planta 2022, 255, 96. [Google Scholar] [CrossRef]
- Zhao, D.; Zhong, G.Y.; Song, G.Q. Transfer of endogenous small RNAs between branches of scions and rootstocks in grafted sweet cherry trees. PLoS ONE 2020, 15, e0236376. [Google Scholar] [CrossRef]
- Zhao, D.; Song, G.Q. Rootstock-to-scion transfer of transgene-derived small interfering RNA s and their effect on virus resistance in nontransgenic sweet cherry. Plant Biotechnol. J. 2014, 12, 1319–1328. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Y.; Kang, L.; Roossinck, M.J.; Mysore, K.S. Computational estimation and experimental verification of off-target silencing during posttranscriptional gene silencing in plants. Plant Physiol. 2006, 142, 429–440. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.-M.; Palmquist, J.; Huang, S.-D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Höfle, L.; Biedenkopf, D.; Werner, B.; Shrestha, A.; Jelonek, L.; Koch, A. Study on the efficiency of dsRNAs with increasing length in RNA-based silencing of the Fusarium CYP51 genes. RNA Biol. 2020, 17, 463–473. [Google Scholar] [CrossRef]
- Arjunan, N.; Thiruvengadam, V.; Sushil, S. Nanoparticle-mediated dsRNA delivery for precision insect pest control: A comprehensive review. Mol. Biol. Rep. 2024, 51, 355. [Google Scholar] [CrossRef]
- Yong, J.; Wu, M.; Zhang, R.; Bi, S.; Mann, C.W.; Mitter, N.; Carroll, B.J.; Xu, Z.P. Clay nanoparticles efficiently deliver small interfering RNA to intact plant leaf cells. Plant Physiol. 2022, 190, 2187–2202. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.G.; Zhao, J.H.; Zhang, B.S.; Gao, F.; Wu, X.M.; Yan, Y.S.; Zhang, J.; Guo, H.S. Microbe-induced gene silencing boosts crop protection against soil-borne fungal pathogens. Nat. Plants 2023, 9, 1409–1418. [Google Scholar] [CrossRef]
- Fang, R. Microbe-induced gene silencing explores interspecies RNAi and opens up possibilities of crop protection. Sci. China Life Sci. 2024, 67, 626–628. [Google Scholar] [CrossRef]
- Zhao, J.H.; Liu, Q.Y.; Xie, Z.M.; Guo, H.S. Exploring the challenges of RNAi-based strategies for crop protection. Adv. Biotechnol. 2024, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. dsRNA uptake in plant pests and pathogens: Insights into RNAi-based insect and fungal control technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Wytinck, N.; Sullivan, D.S.; Biggar, K.T.; Crisostomo, L.; Pelka, P.; Belmonte, M.F.; Whyard, S. Clathrin mediated endocytosis is involved in the uptake of exogenous double-stranded RNA in the white mold phytopathogen Sclerotinia sclerotiorum. Sci. Rep. 2020, 10, 12773. [Google Scholar] [CrossRef]
- Liu, C.; Cui, C.; Zhou, G.; Gao, F.; Zhao, J.; Guo, H.; Jin, Y. The endocytic pathway for absorption of exogenous RNAs in Verticillium dahliae. mLife 2025, 4, 45–54. [Google Scholar] [CrossRef]
- Xiao, D.; Gao, X.; Xu, J.; Liang, X.; Li, Q.; Yao, J.; Zhu, K.Y. Clathrin-dependent endocytosis plays a predominant role in cellular uptake of double-stranded RNA in the red flour beetle. Insect Biochem. Mol. Biol. 2015, 60, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, E.H.; Hunter, C.P. Transport of dsRNA into cells by the transmembrane protein SID-1. Science 2003, 301, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, M.; Pan, R.; Zhang, T.; Lu, X.; Zhen, X.; Che, Y.; Li, R.; Liu, J.; Chen, Y.; et al. Editing of the MeSWEET10a promoter yields bacterial blight resistance in cassava cultivar SC8. Mol. Plant Pathol. 2024, 25, e70010. [Google Scholar] [CrossRef] [PubMed]
- Langner, T.; Kamoun, S.; Belhaj, K. CRISPR crops: Plant genome editing toward disease resistance. Annu. Rev. Phytopathol. 2018, 56, 479–512. [Google Scholar] [CrossRef]
- Cox, D.B.T.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA editing with CRISPR-Cas13. Science 2017, 358, 1019–1027. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Y.; Xia, Y.; Tang, X.; Qin, L.; Xia, S. Trans-Kingdom sRNA Silencing in Sclerotinia sclerotiorum for Crop Fungal Disease Management. Pathogens 2025, 14, 398. https://doi.org/10.3390/pathogens14040398
Ouyang Y, Xia Y, Tang X, Qin L, Xia S. Trans-Kingdom sRNA Silencing in Sclerotinia sclerotiorum for Crop Fungal Disease Management. Pathogens. 2025; 14(4):398. https://doi.org/10.3390/pathogens14040398
Chicago/Turabian StyleOuyang, Yuqing, Yunong Xia, Xianyu Tang, Lei Qin, and Shitou Xia. 2025. "Trans-Kingdom sRNA Silencing in Sclerotinia sclerotiorum for Crop Fungal Disease Management" Pathogens 14, no. 4: 398. https://doi.org/10.3390/pathogens14040398
APA StyleOuyang, Y., Xia, Y., Tang, X., Qin, L., & Xia, S. (2025). Trans-Kingdom sRNA Silencing in Sclerotinia sclerotiorum for Crop Fungal Disease Management. Pathogens, 14(4), 398. https://doi.org/10.3390/pathogens14040398






