Antiviral Effect of Erdosteine in Cells Infected with Human Respiratory Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Virus, and Reagents
2.2. Cell Viability Assay
2.3. In Vitro Viral Infection Assay and Treatment Protocols
2.4. Antiviral Effect of Erdosteine
2.5. Transcriptome Analysis by RT-PCR Custom-Array
2.6. Cytokine Quantification by Multiplex Immunoassay
2.7. Statistical Analyses
3. Results
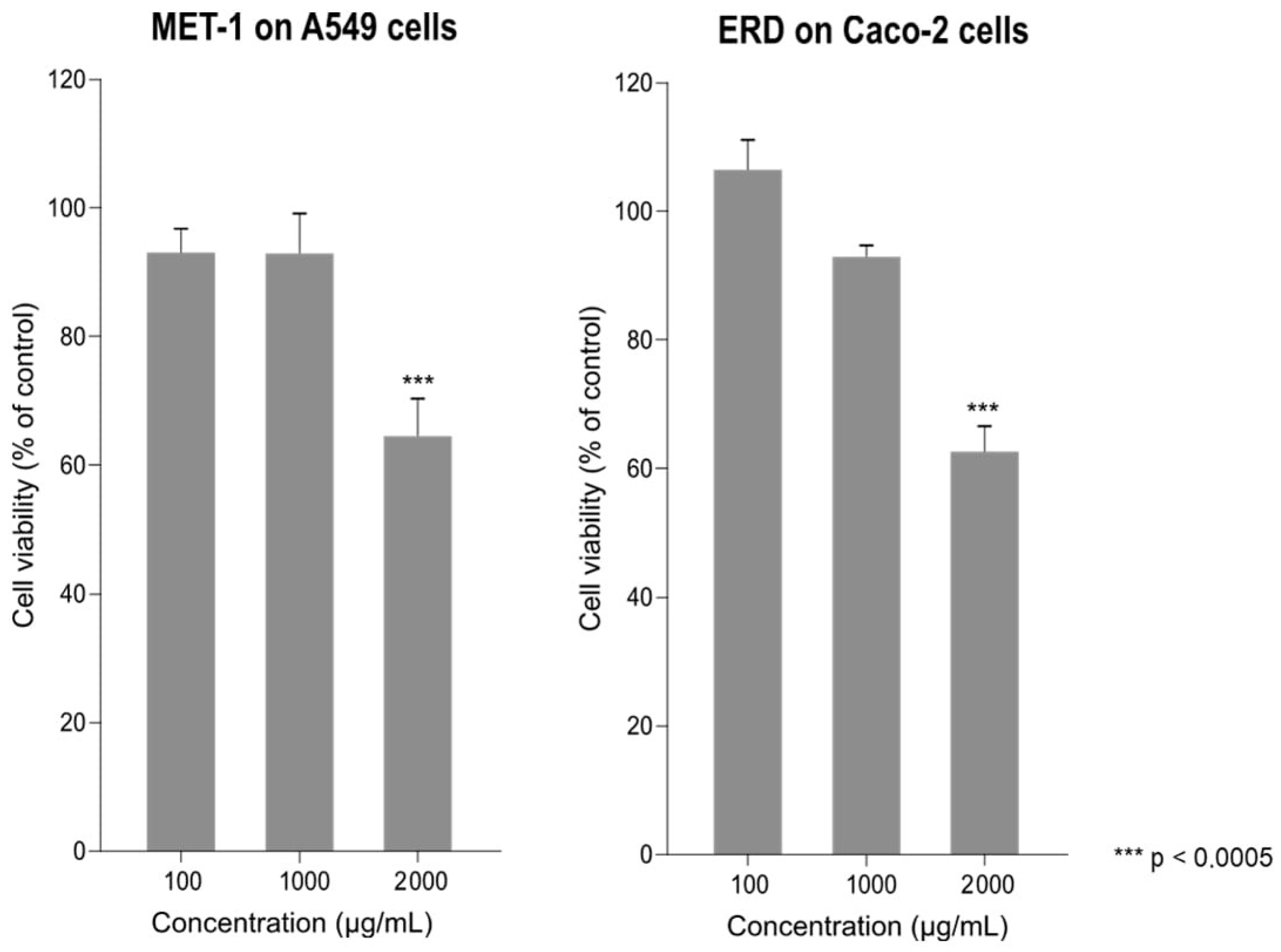
3.1. Effect of Erdosteine and MET-1 on Cell Viability
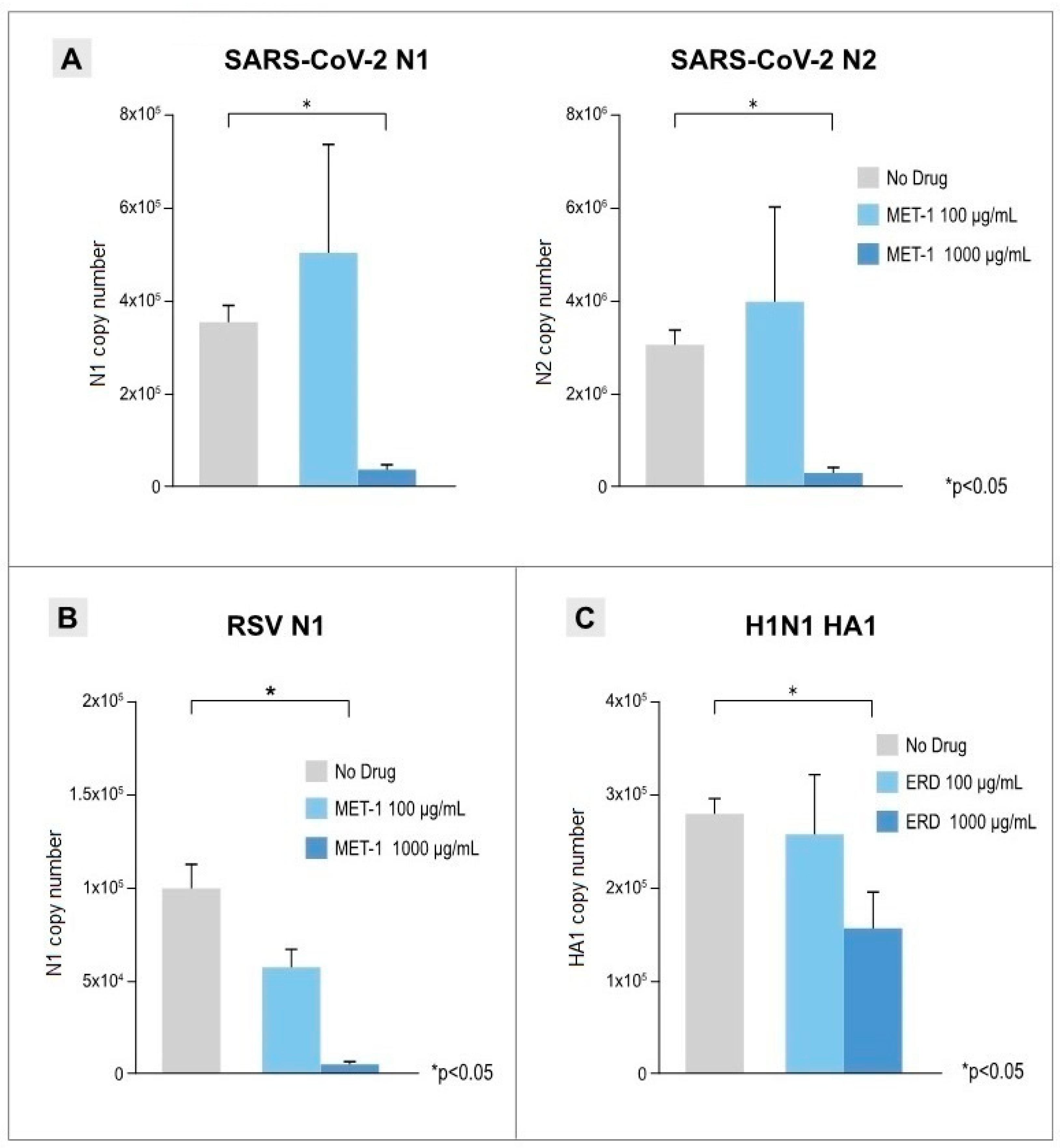
3.2. Antiviral Effects of Erdosteine and MET-1 on Virus-Infected Cells
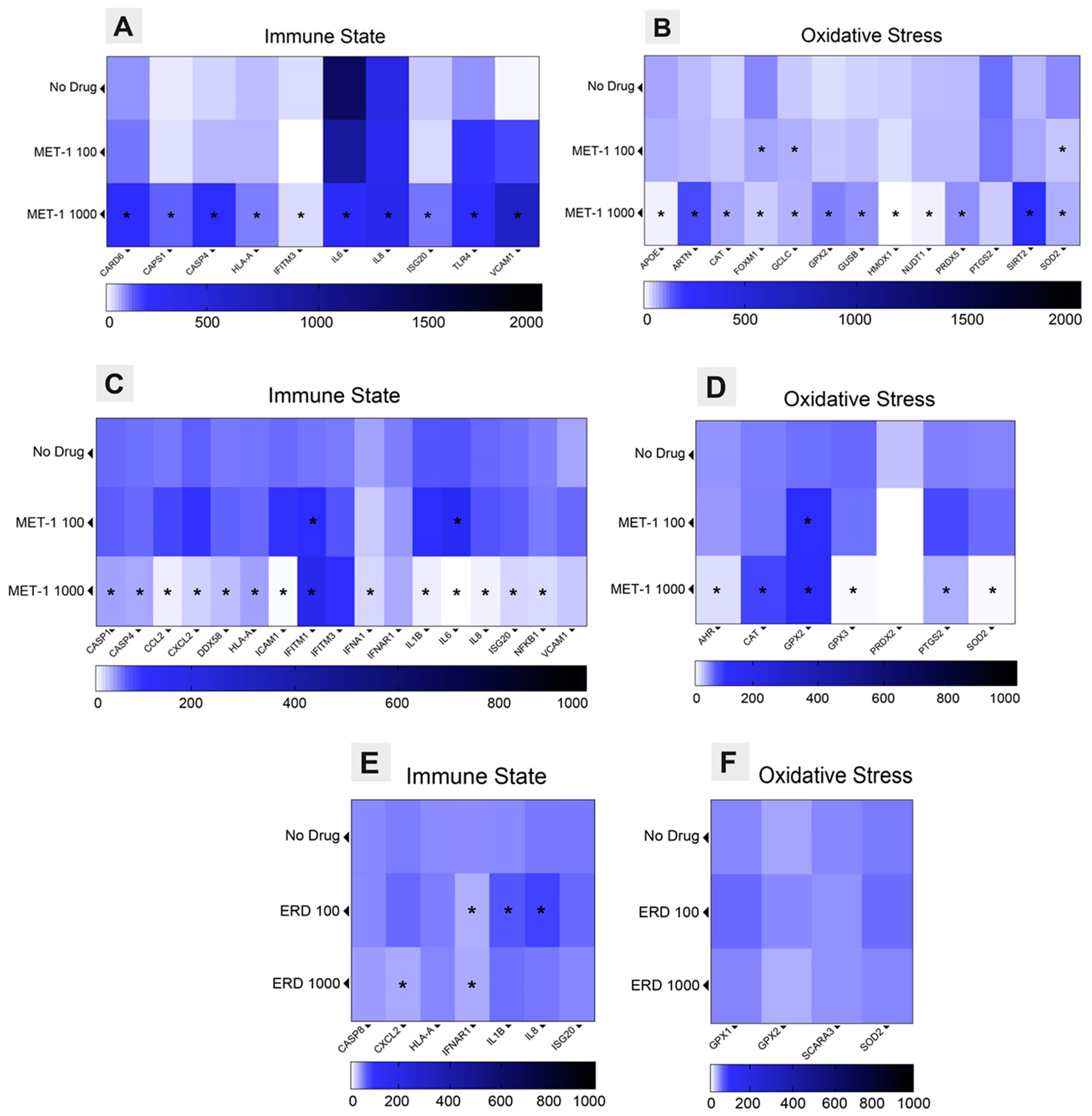
3.3. Immune State and Oxidative Stress Modulation Triggered by Erdosteine
3.4. Anti-Inflammatory Effects of Erdosteine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Belongia, E.A.; King, J.P.; Kieke, B.A.; Pluta, J.; Al-Hilli, A.; Meece, J.K.; Shinde, V. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect. Dis. 2018, 5, ofy316. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Welch, V.L.; Moran, M.M.; Cane, A.; Lopez, S.M.C.; Srivastava, A.; Enstone, A.L.; Sears, A.; Markus, K.J.; Heuser, M.; et al. High clinical burden of influenza disease in adults aged ≥ 65 years: Can we do better? A systematic literature review. Adv. Ther. 2023, 40, 1601–1627. [Google Scholar] [CrossRef] [PubMed]
- Macias, A.E.; McElhaney, J.E.; Chaves, S.S.; Nealon, J.; Nunes, M.C.; Samson, S.I.; Seet, B.T.; Weinke, T.; Yu, H. The disease burden of influenza beyond respiratory illness. Vaccine 2021, 39 (Suppl. 1), A6–A14. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Saiman, L.; Walsh, E.E.; Falsey, A.R.; Sieling, W.D.; Greendyke, W.; Peterson, D.R.; Vargas, C.Y.; Phillips, M.; Finelli, L. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin. Infect. Dis. 2022, 74, 1004–1011. [Google Scholar] [CrossRef]
- Maggi, S.; Veronese, N.; Burgio, M.; Cammarata, G.; Ciuppa, M.E.; Ciriminna, S.; Di Gennaro, F.; Smith, L.; Trott, M.; Dominguez, L.J.; et al. Rate of hospitalizations and mortality of respiratory syncytial virus infection compared to influenza in older people: A systematic review and meta-analysis. Vaccines 2022, 10, 2092. [Google Scholar] [CrossRef]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir. Viruses 2023, 17, e13031. [Google Scholar] [CrossRef]
- Santus, P.; Radovanovic, D.; Gismondo, M.R.; Rimoldi, S.G.; Lombardi, A.; Danzo, F.; Gori, A.; Antinori, S.; Rizzardini, G. Respiratory syncytial virus burden and risk factors for severe disease in patients presenting to the emergency department with flu-like symptoms or acute respiratory failure. Respir. Med. 2023, 218, 107404. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. 2024 GOLD Report. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2023. Available online: https://goldcopd.org/2024-gold-report/ (accessed on 31 January 2024).
- Global Initiative for Asthma, Global Strategy for Asthma Management and Prevention. 2024. Available online: https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Report-24_05_22_WMS.pdf (accessed on 7 May 2024).
- Zwaans, W.A.; Mallia, P.; van Winden, M.E.; Rohde, G.G. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—A systematic review. J. Clin. Virol. 2014, 61, 181–188. [Google Scholar] [CrossRef]
- Jang, J.G.; Ahn, J.H.; Jin, H.J. Incidence and prognostic factors of respiratory viral infections in severe acute exacerbation of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 1265–1273. [Google Scholar] [CrossRef]
- Ascough, S.; Paterson, S.; Chiu, C. Induction and subversion of human protective immunity: Contrasting influenza and respiratory syncytial virus. Front. Immunol. 2018, 9, 323. [Google Scholar] [CrossRef]
- Marinelli, F.; Caporilli, C.; Titolo, A.; Rigante, D.; Esposito, S. Clinical impact and disease evolution of SARS-CoV-2 infection in familial Mediterranean fever. Pharmacol. Res. 2022, 182, 106293. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.; Zhang, R.; Tan, Y.L.; Chai, C.L.L. COVID-19 and the promise of small molecule therapeutics: Are there lessons to be learnt? Pharmacol. Res. 2022, 179, 106201. [Google Scholar] [CrossRef] [PubMed]
- Nakagome, K.; Nagata, M. Innate immune responses by respiratory viruses, including rhinovirus, during asthma exacerbation. Front. Immunol. 2022, 13, 865973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, X.; Luo, H.; Zhi, Y.; Yi, X.; Wu, X.; Duan, W.; Cao, Y.; Pang, J.; Liu, S.; et al. Pharmacological inhibition of Kv1.3 channel impairs TLR3/4 activation and type I IFN response and confers protection against Listeria monocytogenes infection. Pharmacol. Res. 2022, 177, 106112. [Google Scholar] [CrossRef]
- Wu, N.; Zheng, C.; Xu, J.; Ma, S.; Jia, H.; Yan, M.; An, F.; Zhou, Y.; Qi, J.; Bian, H. Race between virus and inflammasomes: Inhibition or escape, intervention and therapy. Front. Cell. Infect. Microbiol. 2023, 13, 1173505. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Cerato, J.A.; da Silva, E.F.; Porto, B.N. Breaking bad: Inflammasome activation by respiratory viruses. Biology 2023, 12, 943. [Google Scholar] [CrossRef]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox biology of respiratory viral infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef]
- Fernandes, I.G.; de Brito, C.A.; Reis, V.M.S.D.; Sato, M.N.; Pereira, N.Z. SARS-CoV-2 and other respiratory viruses: What does oxidative stress have to do with It? Oxid. Med. Cell. Longev. 2020, 2020, 8844280. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Kleniewska, P.; Pawliczak, R. Oxidative stress-related mechanisms in SARS-CoV-2 infections. Oxid. Med. Cell. Longev. 2022, 2022, 5589089. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Nie, Y.; Zhan, F.; Zhu, B. Oxidative stress and ROS-mediated cellular events in RSV infection: Potential protective roles of antioxidants. Virol. J. 2023, 20, 224. [Google Scholar] [CrossRef]
- Bartolini, D.; Stabile, A.M.; Bastianelli, S.; Giustarini, D.; Pierucci, S.; Busti, C.; Vacca, C.; Gidari, A.; Francisci, D.; Castronari, R.; et al. SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione. Redox. Biol. 2021, 45, 102041. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Marcantonini, G.; Giustarini, D.; Albertini, M.C.; Migni, A.; Zatini, L.; Gioiello, A.; Rossi, R.; Bartolini, D. How aging and oxidative stress influence the cytopathic and inflammatory effects of SARS-CoV-2 infection: The role of cellular glutathione and cysteine metabolism. Antioxidants 2022, 11, 1366. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Danzo, F.; Zuffi, A.; Pini, S.; Saad, M.; Visconti, A.; Radovanovic, D. Oxidative stress and viral Infections: Rationale, experiences, and perspectives on N-acetylcysteine. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8582–8590. [Google Scholar] [CrossRef] [PubMed]
- Labarrere, C.A.; Kassab, G.S. Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease. Front. Microbiol. 2022, 13, 979719. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Page, C.; Rogliani, P.; Matera, M.G. Thiol-based drugs in pulmonary medicine: Much more than mucolytics. Trends Pharmacol. Sci. 2019, 40, 452–463. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Salvi, S.S.; Ora, J.; Matera, M.G. Use of thiols in the treatment of COVID-19: Current evidence. Lung 2021, 199, 335–343. [Google Scholar] [CrossRef]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Nencioni, L.; Garaci, E.; Palamara, A.T.; Magnani, M. GSH and analogs in antiviral therapy. Mol. Aspects Med. 2009, 30, 99–110. [Google Scholar] [CrossRef]
- Negro, R.D.; Pozzi, E.; Cella, S.G. Erdosteine: Drug exhibiting polypharmacy for the treatment of respiratory diseases. Pulmon. Pharmacol. Therap. 2018, 53, 80–85. [Google Scholar] [CrossRef]
- Cazzola, M.; Page, C.; Rogliani, P.; Calzetta, L.; Matera, M.G. Multifaceted beneficial effects of erdosteine: More than a mucolytic agent. Drugs 2020, 80, 1799–1809. [Google Scholar] [CrossRef]
- Moretti, M.; Marchioni, C.F. An overview of erdosteine antioxidant activity in experimental research. Pharmacol. Res. 2007, 55, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Tursi, F.; Croce, G.; Di Simone, C.; Frassanito, F.; Gaboardi, P.; Airoldi, A.; Pecis, M.; Negretto, G.; Radovanovic, D. Changes in quality of life and dyspnoea after hospitalization in COVID-19 patients discharged at home. Multidiscip. Respir. Med. 2020, 15, 713. [Google Scholar] [CrossRef]
- Vanetti, C.; Saulle, I.; Artusa, V.; Moscheni, C.; Cappelletti, G.; Zecchini, S.; Strizzi, S.; Garziano, M.; Fenizia, C.; Tosoni, A.; et al. A complex remodeling of cellular homeostasis distinguishes RSV/SARS-CoV-2 co-infected A549-hACE2 expressing cell lines. Microb Cell. 2024, 11, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Valeri, A.; Chiricosta, L.; Gugliandolo, A.; Biasin, M.; Avanzini, M.A.; Calcaterra, V.; Cappelletti, G.; Carelli, S.; Zuccotti, G.V.; Silvestro, S.; et al. SARS-CoV-2 Exposed Mesenchymal Stromal Cell from Congenital Pulmonary Airway Malformations: Transcriptomic Analysis and the Expression of Immunomodulatory Genes. Int. J. Mol. Sci. 2021, 22, 11814. [Google Scholar] [CrossRef] [PubMed]
- Barrow, K.A.; Rich, L.M.; Vanderwall, E.R.; Reeves, S.R.; Rathe, J.A.; White, M.P.; Debley, J.S. Inactivation of material from SARS-CoV-2-infected primary airway epithelial cell cultures. Methods Protoc. 2021, 4, 7. [Google Scholar] [CrossRef]
- Saritas, A.; Kandis, H.; Baltaci, D.; Yildirim, U.; Kaya, H.; Karakus, A.; Colakoglu, S.; Memisogullari, R.; Kara, I.H. N-acetyl cysteine and erdosteine treatment in acetaminophen-induced liver damage. Toxicol. Ind. Health 2014, 30, 670–678. [Google Scholar] [CrossRef]
- Trabattoni, D.; Gnudi, F.; Ibba, S.V.; Saulle, I.; Agostini, S.; Masetti, M.; Biasin, M.; Rossignol, J.F.; Clerici, M. Thiazolides elicit anti-viral innate immunity and reduce HIV replication. Sci. Rep. 2016, 6, 27148. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Islamuddin, M.; Mustfa, S.A.; Ullah, S.N.M.N.; Omer, U.; Kato, K.; Parveen, S. Innate immune response and inflammasome activation during SARS-CoV-2 infection. Inflammation 2022, 45, 1849–1863. [Google Scholar] [CrossRef]
- Yamada, T.; Takaoka, A. Innate immune recognition against SARS-CoV-2. Inflamm. Regener. 2023, 43, 7. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Hansen, M.R.; Tripp, R.A. Interferons-implications in the immune response to respiratory viruses. Microorganisms 2023, 11, 2179. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, P.; Shepard, R.; Uehling, M.; Krenz, M.; Sheikh, F.; Thayer, K.R.; Huang, L.; Yan, L.; Panda, D.; Luongo, C.; et al. Differential responses by human respiratory epithelial cell lines to respiratory syncytial virus reflect distinct patterns of infection control. J. Virol. 2018, 92, e02202-17. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Jin, M.; Chen, H.; Wu, Z.; Yuan, L.; Liang, S.; Wang, X.; Memon, F.U.; Eldemery, F.; Si, H.; et al. Inflammasome activation by viral infection: Mechanisms of activation and regulation. Front. Microbiol. 2023, 14, 1247377. [Google Scholar] [CrossRef]
- Choudhury, S.M.; Ma, X.; Abdullah, S.W.; Zheng, H. Activation and inhibition of the NLRP3 inflammasome by RNA viruses. J. Inflamm. Res. 2021, 14, 1145–1163. [Google Scholar] [CrossRef]
- Deng, C.H.; Li, T.Q.; Zhang, W.; Zhao, Q.; Wang, Y. Targeting inflammasome activation in viral infection: A therapeutic solution? Viruses 2023, 15, 1451. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Chang, W.; Zhang, Y.; Zhang, L.; Li, P. Inflammasomes: A rising star on the horizon of COVID-19 pathophysiology. Front. Immunol. 2023, 14, 1185233. [Google Scholar] [CrossRef]
- Zhang, T.; Tsutsuki, H.; Li, X.; Sawa, T. New insights into the regulatory roles of glutathione in NLRP3-inflammasome-mediated immune and inflammatory responses. J. Biochem. 2022, 171, 367–377. [Google Scholar] [CrossRef]
- Polonikov, Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 2020, 6, 1558–1562. [CrossRef]
- Mokra, M.; Mokry, J.; Barosova, R.; Hanusrichterova, J. Advances in the use of N-acetylcysteine in chronic respiratory diseases. Antioxidants 2023, 12, 1713. [Google Scholar] [CrossRef]
- Milara, J.; Martínez-Expósito, F.; Montero, P.; Roger, I.; Bayarri, M.A.; Ribera, P.; Oishi-Konari, M.N.; Alba-García, J.R.; Zapater, E.; Cortijo, J. N-acetylcysteine reduces inflammasome activation induced by SARS-CoV-2 proteins in vitro. Int. J. Mol. Sci. 2022, 23, 14518. [Google Scholar] [CrossRef] [PubMed]
- Checconi, P.; De Angelis, M.; Marcocci, M.E.; Fraternale, A.; Magnani, M.; Palamara, A.T.; Nencioni, L. Redox-modulating agents in the treatment of viral Infictions. Int. J. Mol. Sci. 2020, 21, 4084. [Google Scholar] [CrossRef] [PubMed]
- Hosakote, Y.M.; Liu, T.; Castro, S.M.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 2009, 41, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Daskou, M.; Abadi, L.F.; Gain, C.; Wong, M.; Sharma, E.; Kombe, A.J.K.; Nanduri, R.; Kelesidis, T. The role of the NRF2 pathway in the pathogenesis of viral respiratory infections. Pathogens 2023, 13, 39. [Google Scholar] [CrossRef]
- Marabini, L.; Calò, R.; Braga, P.C. Protective effect of erdosteine metabolite I against hydrogen peroxide-induced oxidative DNA-damage in lung epithelial cells. Arzneimittelforschung 2011, 61, 700–706. [Google Scholar] [CrossRef]
- Mancini, C.; Silvestro, G.; Savu, S. Pilot study on erdosteine activity on reduced glutathione (GSH) plasmatic levels in correlation with levels of the mother compound and the main metabolite (N-thiodiglycolyl-homocysteine). In Proceedings of the Fourth European Congress of Pharmaceutical Sciences, Milan, Italy, 11–13 September 1998. [Google Scholar]
- Mitrea, M.; Silvestro, L.; Savu, S.; Mancini, C. Reduced (GSH) and oxidized (GSSG) levels in plasma and BAL of chronic bronchitis patients treated with erdosteine or N-acetylcysteine (NAC). In Proceedings of the 6th International Conference on Bronchoalveolar Lavage, Corfu, Greece, 24–27 June 1998. [Google Scholar]
- Papi, A.; Bellettato, C.M.; Braccioni, F.; Romagnoli, M.; Casolari, P.; Caramori, G.; Fabbri, L.M.; Johnston, S.L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006, 173, 1114–1121. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Seemungal, T.A. COPD exacerbations: Defining their cause and prevention. Lancet 2007, 370, 786–796. [Google Scholar] [CrossRef]
- Hewitt, R.; Farne, H.; Ritchie, A.; Luke, E.; Johnston, S.L.; Mallia, P. The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma. Ther. Adv. Respir. Dis. 2016, 10, 158–174. [Google Scholar] [CrossRef]
- Romero-Tapia, S.J.; Priego, C.G.G.; Del-Río-Navarro, B.E.; Sánchez-Solis, M. Advances in the relationship between respiratory viruses and asthma. J. Clin. Med. 2023, 12, 5501. [Google Scholar] [CrossRef]
- Negro, R.W.D.; Wedzicha, J.A.; Iversen, M.; Fontana, G.; Page, C.; Cicero, A.F.; Pozzi, E.; Calverley, P.M.A. Effect of erdosteine on the rate and duration of COPD exacerbations: The RESTORE study. Eur. Respir. J. 2017, 50, 1700711. [Google Scholar] [CrossRef]
- Calverley, P.M.; Page, C.; Negro, R.W.D.; Fontana, G.; Cazzola, M.; Cicero, A.F.; Pozzi, E.; Wedzicha, J.A. Effect of erdosteine on COPD exacerbations in COPD patients with moderate airflow limitation. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2733–2744. [Google Scholar] [CrossRef] [PubMed]
- Calverley, P.M.A.; Papi, A.; Page, C.; Rogliani, P.; Negro, R.W.D.; Cazzola, M.; Cicero, A.F.; Wedzicha, J.A. The effect of maintenance treatment with erdosteine on exacerbation treatment and health status in patients with COPD: A post-hoc analysis of the RESTORE dataset. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 1909–1920, Erratum in Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 2451–2452. [Google Scholar] [CrossRef] [PubMed]
- Sainas, S.; Pippione, A.C.; Giorgis, M.; Lupino, E.; Goyal, P.; Ramondetti, C.; Buccinnà, B.; Piccinini, M.; Braga, R.C.; Andrade, C.H.; et al. Design, synthesis, biological evaluation and X-ray structural studies of potent human dihydroorotate dehydrogenase inhibitors based on hydroxylated azole scaffolds. Eur. J. Med. Chem. 2017, 129, 287–302. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santus, P.; Strizzi, S.; Danzo, F.; Biasin, M.; Saulle, I.; Vanetti, C.; Saad, M.; Radovanovic, D.; Trabattoni, D. Antiviral Effect of Erdosteine in Cells Infected with Human Respiratory Viruses. Pathogens 2025, 14, 388. https://doi.org/10.3390/pathogens14040388
Santus P, Strizzi S, Danzo F, Biasin M, Saulle I, Vanetti C, Saad M, Radovanovic D, Trabattoni D. Antiviral Effect of Erdosteine in Cells Infected with Human Respiratory Viruses. Pathogens. 2025; 14(4):388. https://doi.org/10.3390/pathogens14040388
Chicago/Turabian StyleSantus, Pierachille, Sergio Strizzi, Fiammetta Danzo, Mara Biasin, Irma Saulle, Claudia Vanetti, Marina Saad, Dejan Radovanovic, and Daria Trabattoni. 2025. "Antiviral Effect of Erdosteine in Cells Infected with Human Respiratory Viruses" Pathogens 14, no. 4: 388. https://doi.org/10.3390/pathogens14040388
APA StyleSantus, P., Strizzi, S., Danzo, F., Biasin, M., Saulle, I., Vanetti, C., Saad, M., Radovanovic, D., & Trabattoni, D. (2025). Antiviral Effect of Erdosteine in Cells Infected with Human Respiratory Viruses. Pathogens, 14(4), 388. https://doi.org/10.3390/pathogens14040388







