Long-Term Effects of Vegetative-Propagation-Mediated TuMV-ZR Transmission on Yield, Quality, and Stress Resistance in Pseudostellaria heterophylla
Abstract
1. Introduction
2. Materials and Methods
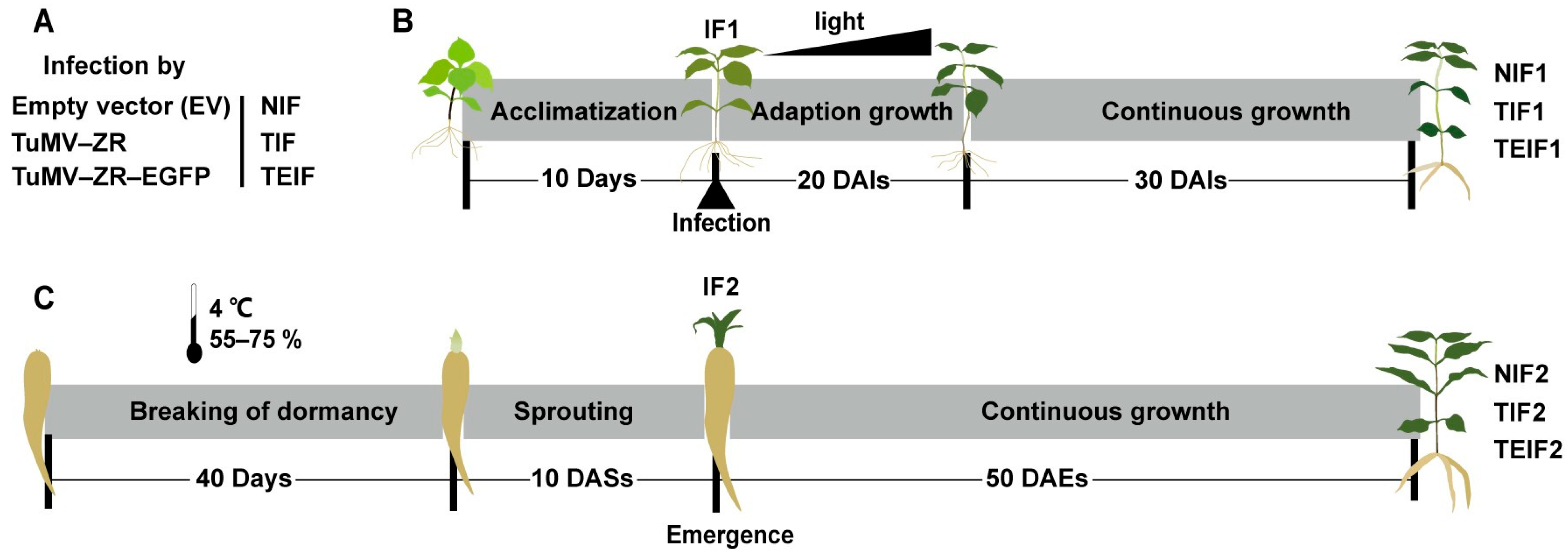
2.1. Planting and Management of Experimental Material
2.2. Preparation of Virus Particles of P. heterophylla TuMV-ZR Virus
2.3. Inoculation of P. heterophylla TuMV-ZR Virus Particles in P. heterophylla
2.4. Validation of Virus Levels and Infection States in Virus-Infected P. heterophylla
2.5. Breaking Dormancy of Tuberous Roots of Virus-Infected P. heterophylla
2.6. Morphological Observation and Biomass Measurement of Virus-Infected P. heterophylla
2.7. Determination of Physiological Indices of Virus Treated P. heterophylla
2.8. Data Statistics and Analysis
3. Results
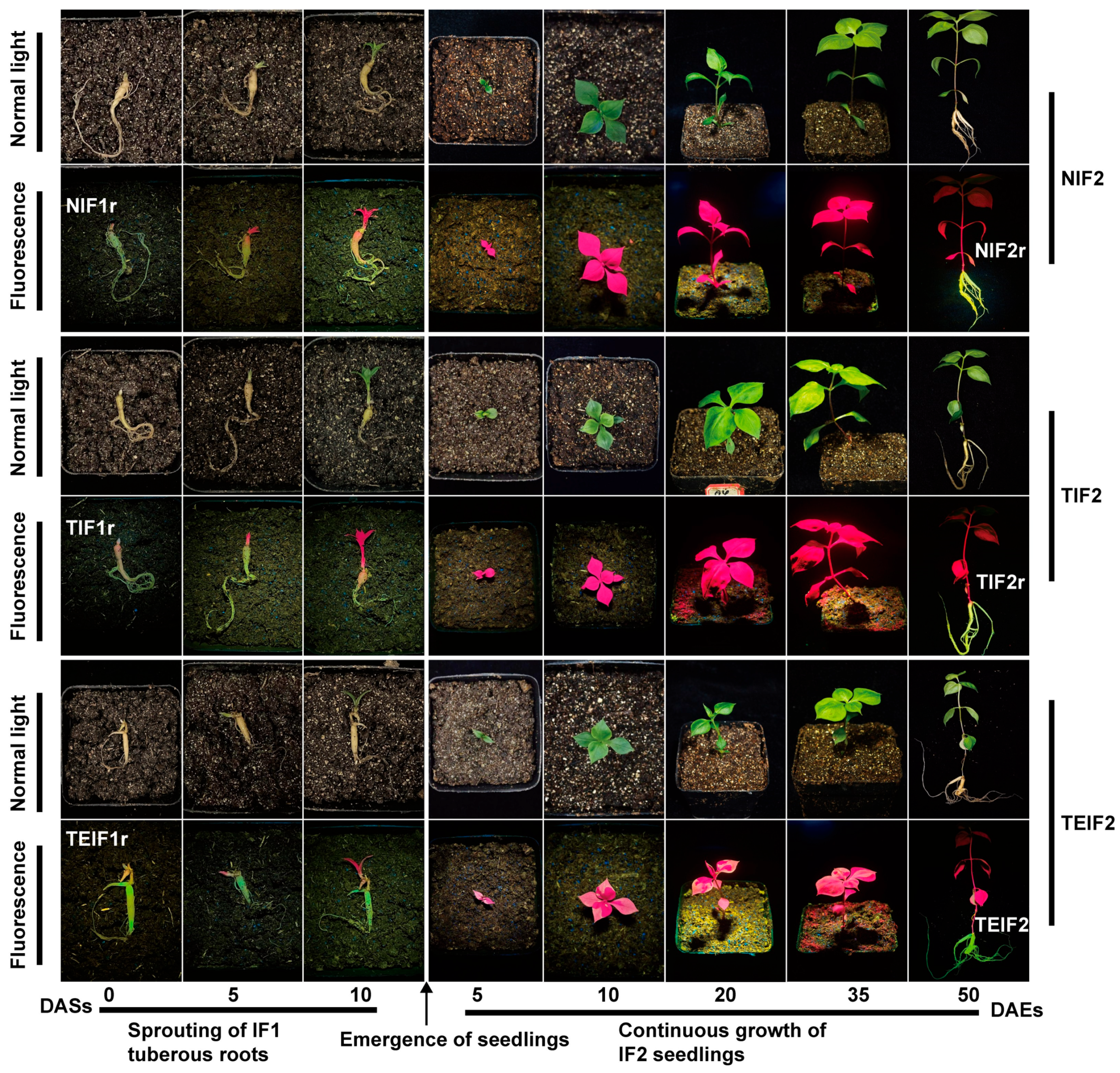
3.1. Tracing of TuMV-ZR Virus Transmission in Initial Virus Infection Cycle of P. heterophylla
3.2. The Transmission of TuMV-ZR Virus in Subsequent Infection Cycle of P. heterophylla and Its Effect on Phenotypic Characteristics of Plants
3.3. The Effects of TuMV-ZR Infection Across Successive Vegetative Propagation Cycles on the Yield and Quality of of P. heterophylla
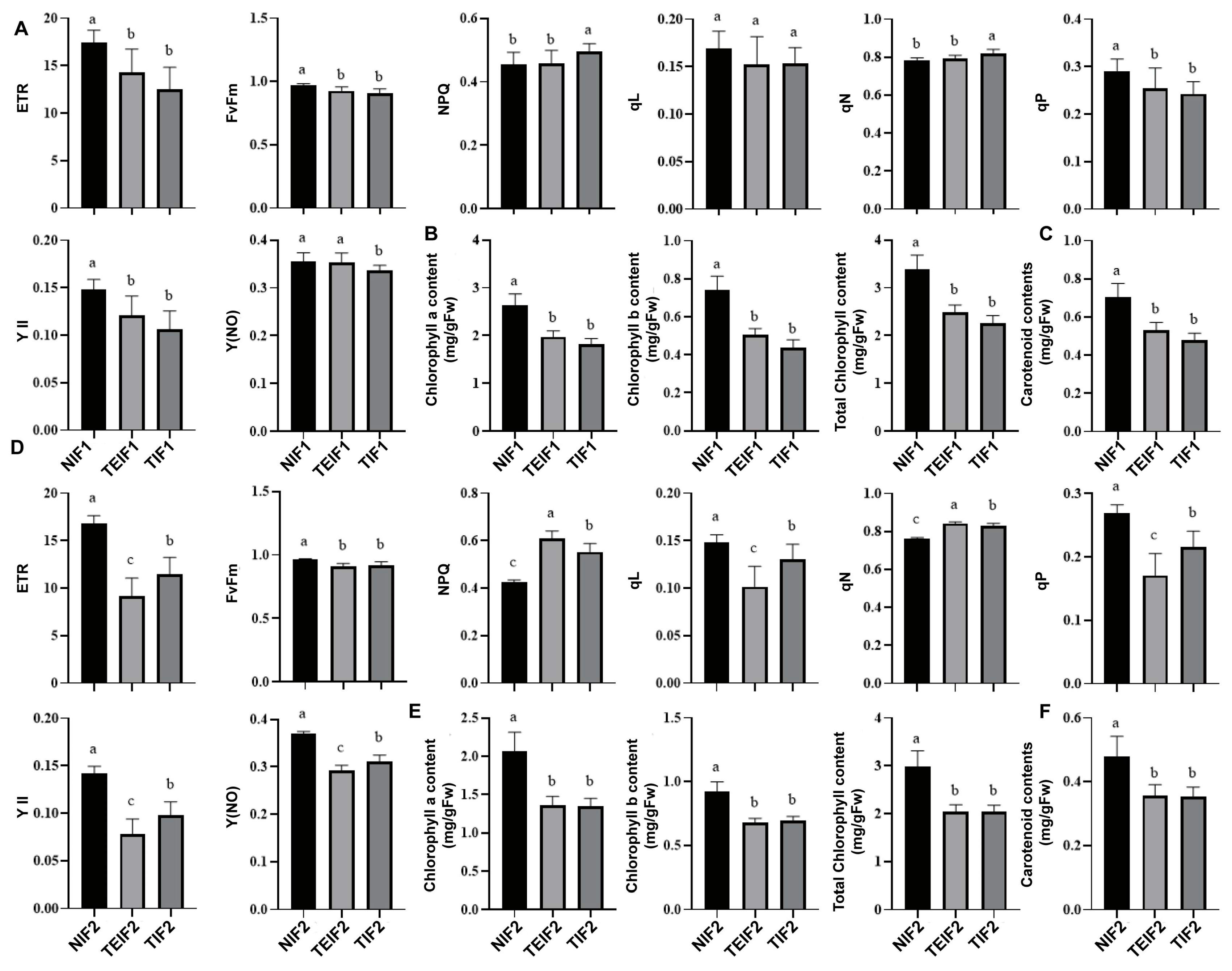
3.4. Effects of TuMV Transmission Across Successive Vegetative Propagation Cycles on the Photosynthetic Characteristics of P. heterophylla
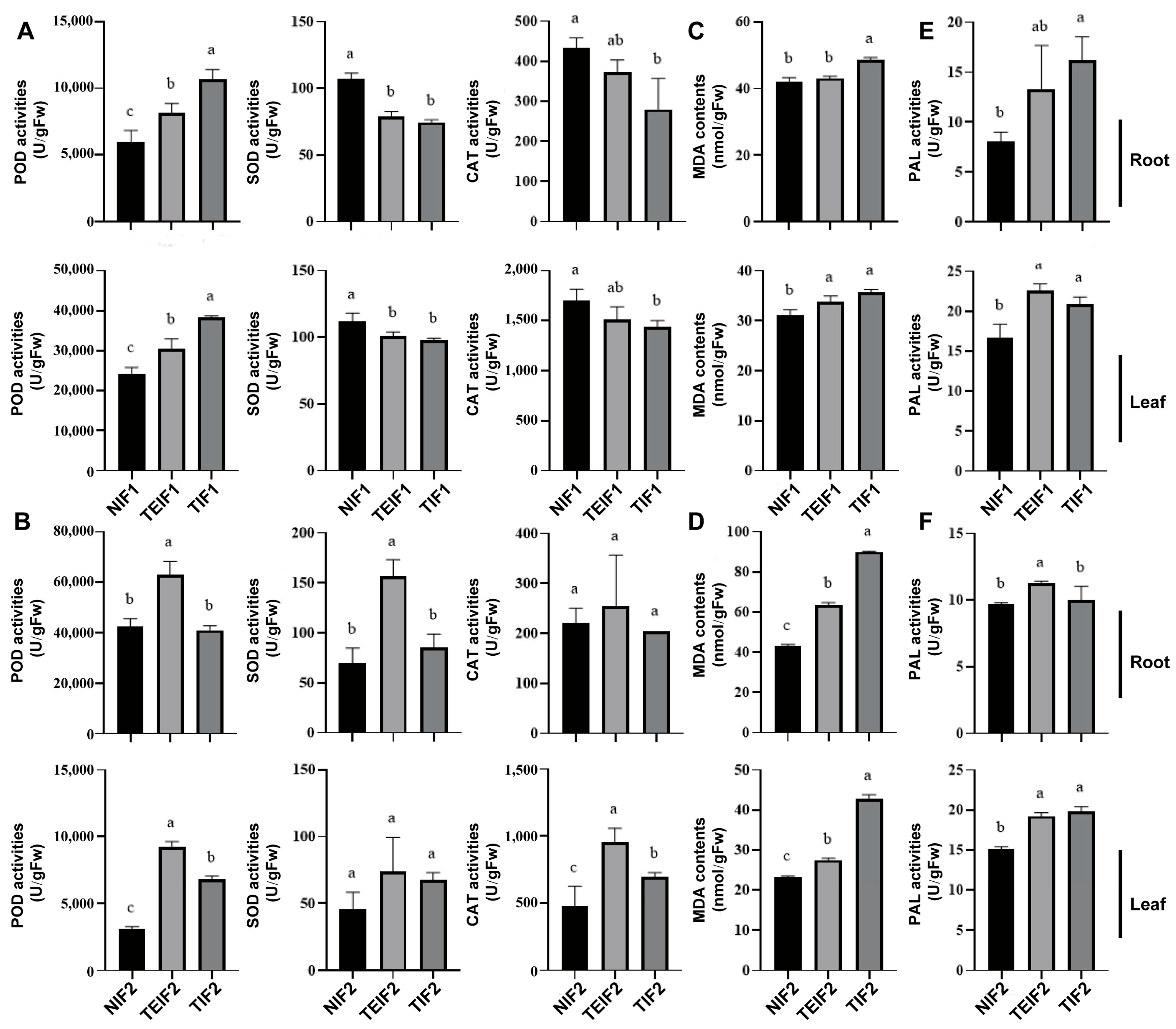
3.5. Effects of TuMV-ZR Infection on the Antioxidant Enzyme of Initial and Subsequent Infection Generation of P. heterophylla
3.6. Effects of TuMV-ZR Infection Across Successive Vegetative Propagation Cycles on MDA Content and PAL Activity of P. heterophylla
3.7. Effect of TuMZ-ZR Infection Across Successive Vegetative Propagation Cycles on the Content of the Osmotic Substances of P. heterophylla
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni, J.C.; Fan, Y.F.; Ye, Z.Y. Research Progress on Chemical Constituents, Pharmacological Effects and Application of Pseudostellariae Radix. Chin. Trad. Herb. Drugs 2023, 54, 1963–1977. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Wei, R.F.; Chen, B.W.; Wang, T.Y.; Ding, W.L. Investigation on the Occurrence of Virus Diseases in the Main Producing Areas of Pseudostellaria heterophylla in China. Plant Prot. 2022, 48, 204–210. [Google Scholar] [CrossRef]
- Gu, L.; Liu, C.S.; Yao, S.T.; Wu, J.X.; Wang, L.H.; Mu, J.; Wang, Y.K.; Wang, J.M.; Zhang, Z.Y.; Li, M.J. Development of a TaqMan qPCR for the Simultaneous Detection of the TuMV and BBWV2 Viruses Responsible for the Viral Disease in Pseudostellaria heterophylla. Microorganisms 2024, 12, 2663. [Google Scholar] [CrossRef]
- Yuan, X.T.; Yuan, J.X. Status and Countermeasures of Heterophylly Faalsestarwort Root (Pseudostellaria heterophylla) Industry in Zherong County. Fujian Agric. Sci. Technol. 2014, 45, 68–70. [Google Scholar] [CrossRef]
- Wu, X.H.; Lou, F.; Li, W.; Li, T.Z.; Liu, H.C. Comparison of Different Propagation Materials of Pseudostellaria heterophylla. J. Zhejiang Agric. Sci. 2021, 62, 530–531. [Google Scholar] [CrossRef]
- Yang, X.W.; Gu, L.; Liu, H.X.; Liu, C.S.; Yuan, J.D.; Qian, S.; Wang, J.M.; Yuan, F.Y.; Zhang, Z.Y.; Mu, J.; et al. Identification of a TuMV Isolate (TuMV-ZR) from Pseudostellaria heterophylla and Its Development into a Viral Expression Vector. Virus Res. 2023, 332, 199127. [Google Scholar] [CrossRef]
- Ohshima, K.; Yamaguchi, Y.; Hirota, R.; Hamamoto, T.; Tomimura, K.; Tan, Z.Y.; Sano, T.; Azuhata, F.; Walsh, J.A.; Fletcher, J.; et al. Molecular Evolution of Turnip Mosaic Virus: Evidence of Host Adaptation, Genetic Recombination and Geographical Spread. J. Gen. Virol. 2002, 83, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- van Regenmortel, M.H.V.; Fauquet, C.M.; Bishop, D.H.L.; Carstens, E.B.; Estes, M.K.; Lemon, S.M.; Maniloff, J.; Mayo, M.A.; McGeoch, D.J.; Pringle, C.R.; et al. (Eds.) Virus Taxonomy: Classification and Nomenclature of Viruses. Seventh Report of the International Committee on Taxonomy of Viruses; Virology Division, International Union of Microbiological Societies: San Diego, CA, USA, 2000; p. 1162. [Google Scholar]
- Stenger, D.C.; Hein, G.L.; Gildow, F.E.; Horken, K.M.; French, R. Plant Virus HC-Pro Is a Determinant of Eriophyid Mite Transmission. J. Virol. 2005, 79, 9054–9061. [Google Scholar] [CrossRef]
- Jones, R.A.C. Plant and Insect Viruses in Managed and Natural Environments: Novel and Neglected Transmission Pathways. Adv. Virus Res. 2018, 101, 149–187. [Google Scholar] [CrossRef]
- García-Ordóñez, L.; Pagán, I. Vertical and Horizontal Transmission of Plant Viruses: Two Extremes of a Continuum? npj Viruses 2024, 2, 18. [Google Scholar] [CrossRef]
- Sastry, K.S. Plant Virus Transmission Through Vegetative Propagules (Asexual Reproduction). In Seed-borne Plant Virus Diseases; Springer: New Delhi, India, 2013; pp. 153–172. [Google Scholar] [CrossRef]
- Singh, S.; Awasthi, L.P.; Jangre, A. Transmission of Plant Viruses in Fields Through Various Vectors. In Applied Plant Virology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 313–334. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1983; Volume 3, pp. 273–286. [Google Scholar] [CrossRef]
- Peng, X.; Wu, H.; Chen, H.J.; Zhang, Y.J.; Qiu, D.; Zhang, Z.Y. Transcriptome Profiling Reveals Candidate Flavonol-Related Genes of Tetrastigma hemsleyanum Under Cold Stress. BMC Genom. 2019, 20, 687. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water Stress Induced Changes in Concentrations of Proline and Total Soluble Sugars in Nodulated Alfalfa (Medicago sativa) Plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Yao, X.Y.; Lan, H.J.; Deng, W.; Chen, H.P.; Luo, C.X.; Kuang, Z.; Luo, Z.M.; Wang, J.L.; Chen, D.Z. Determination of Chlorophyll Content and Comparative Analysis of Agronomic Traits in Pale-White-Leaf Mutant Rice. Acta Agric. Jiangxi 2020, 32, 12–15. [Google Scholar] [CrossRef]
- Chen, W.D.; Liu, Z.Y.; Cai, X.F.; Jin, S.Z. Study on the Determination Method of Total Saponins in Radix Pseudostellariae. Strait Pharm. J. 2014, 26, 77–78. [Google Scholar]
- Sun, H.C.; Zhang, L.Y.; Ma, S.B.; Jin, H.J.; Tang, X.Q.; Dong, H.; Li, J. Determination of Polysaccharide Content in Pseudostellariae Radix from Guizhou. Guizhou Sci. 2022, 40, 18–21. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, B.Q.; Zhang, Y.; Zeng, L.F.; Wang, Z.H.; Ye, Z.Y. Growth, Yield, and Quality of Pseudostellaria heterophylla Affected by Different Viruses Infecting Seed Roots. Fujian J. Agric. Sci. 2023, 38, 68–74. [Google Scholar] [CrossRef]
- Ertunç, F. Physiology of Virus-Infected Plants. In Applied Plant Virology: Advances, Detection and Antiviral Strategies; Awasthi, L.P., Ed.; Elsevier: London, UK, 2020; pp. 199–205. [Google Scholar]
- Chen, J.; Tang, H.H.; Li, L.; Qin, S.J.; Wang, G.P.; Hong, N. Effects of Virus Infection on Plant Growth, Root Development and Phytohormone Levels in In Vitro-Cultured Pear Plants. Plant Cell Tiss. Organ Cult. 2017, 131, 359–368. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin III, F.S.; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- González, E.; Mosquera, M.V.; San José, M.C.; Diaz, T. Influence of Virus on the Chlorophyll, Carotenoid and Polyamine Contents in Grapevine Microcuttings. J. Phytopathol. 1997, 145, 185–187. [Google Scholar] [CrossRef]
- Balachandran, S.; Hurry, V.M.; Kelley, S.E.; Osmond, C.B.; Robinson, S.A.; Rohozinski, J.; Seaton, G.G.R.; Sims, D.A. Concepts of Plant Biotic Stress. Some Insights into the Stress Physiology of Virus-Infected Plants, from the Perspective of Photosynthesis. Physiol. Plant. 1997, 100, 203–213. [Google Scholar] [CrossRef]
- Khan, A.; Luqman, S.; Masood, N.; Singh, D.K.; Saeed, S.T.; Samad, A. Eclipta Yellow Vein Virus Enhances Chlorophyll Destruction, Singlet Oxygen Production and Alters Endogenous Redox Status in Andrographis paniculata. Plant Physiol. Biochem. 2016, 104, 165–173. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Roháček, K. Chlorophyll Fluorescence Parameters: The Definitions, Photosynthetic Meaning, and Mutual Relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.Q.; Chai, J.K.; Wang, M.M.; Jia, X.J.; Guo, Z.F.; Sun, L.L.; Nie, X.M. Effect of Barley Yellow Dwarf Virus Infection on Photosynthesis and Chlorophyll Fluorescence Parameters of Oat. Acta Agrestia Sin. 2020, 28, 923–931. [Google Scholar] [CrossRef]
- Chen, Y.G.; Liang, Q.L.; Wei, L.X.; Zhou, X. Alfalfa Mosaic Virus and White Clover Mosaic Virus Combined Infection Leads to Chloroplast Destruction and Alterations in Photosynthetic Characteristics of Nicotiana benthamiana. Viruses 2024, 16, 1255. [Google Scholar] [CrossRef]
- Fu, F.Q.; Zhang, D.W.; Deng, X.G.; Li, J.Y.; Peng, X.J.; Tang, H.; Lin, H.H. Role of Plastid Signals in Modulating Arabidopsis Responses to Cucumber Mosaic Virus. Plant Growth Regul. 2015, 75, 761–769. [Google Scholar] [CrossRef]
- Rojas, M.R.; Maliano, M.R.; de Souza, J.O.; Vasquez-Mayorga, M.; de Macedo, M.A.; Ham, B.-K.; Gilbertson, R.L. Cell-to-Cell Movement of Plant Viruses: A Diversity of Mechanisms and Strategies. In Current Research Topics in Plant Virology; Wang, A., Zhou, X., Eds.; Springer: Cham, Switzerland, 2016; pp. 113–152. [Google Scholar]
- Prasad, A.; Sett, S.; Prasad, M. Plant-Virus-Abiotic stress interactions in plants: A complex Interplay. Environ. Exp. Bot. 2022, 199, 104869. [Google Scholar] [CrossRef]
- Hernández, J.A.; Gullner, G.; Clemente-Moreno, M.J.; Künstler, A.; Juhász, C.; Díaz-Vivancos, P.; Király, L. Oxidative Stress and Antioxidative Responses in Plant–Virus Interactions. Physiol. Mol. Plant Pathol. 2016, 94, 134–148. [Google Scholar] [CrossRef]
- Clarke, S.F.; Guy, P.L.; Burritt, D.J.; Jameson, P.E. Changes in the Activities of Antioxidant Enzymes in Response to Virus Infection and Hormone Treatment. Physiol. Plant. 2002, 114, 157–164. [Google Scholar] [CrossRef]
- Zhao, W.X.; Kang, L.Y.; Gao, N.N.; Liang, S.; Chang, G.Z.; Xu, X.L.; Li, H.L.; Wang, H.Y.; Li, X.H. Relationship Between Variation of Leaf Defensive Enzyme Activities and Resistance of Watermelon Cultivar to Zucchini Yellow Mosaic Virus. J. Henan Agric. Sci. 2021, 50, 92–98. [Google Scholar] [CrossRef]
- Li, J.X.; Bai, X.M.; Ran, F.; Zhang, C.Z.; Yan, Y.B.; Li, P.; Chen, H. Effects of Combined Extreme Cold and Drought Stress on Growth, Photosynthesis, and Physiological Characteristics of Cool-Season Grasses. Sci. Rep. 2024, 14, 116. [Google Scholar] [CrossRef]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Refaey, E.E.; Mohamed, H.I.; El-Dougdoug, N.K. Molecular Characterization of the Alfalfa Mosaic Virus Infecting Solanum melongena in Egypt and the Control of Its Deleterious Effects with Melatonin and Salicylic Acid. Plants 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.D.; Heilbronn, J.; Forrest, R.S.; Johnston, D.J. The Biochemical Basis of Resistance of Potato to Soft Rot Bacteria. Neth. J. Plant Pathol. 1992, 98, 127–133. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Sridhar, R. Physiology of Rice Tungro Virus Disease: Proline Accumulation Due to Infection. Physiol. Plant. 1982, 56, 89–93. [Google Scholar] [CrossRef]
- Xu, P.; Chen, F.; Mannas, J.P.; Feldman, T.; Sumner, L.W.; Roossinck, M.J. Virus Infection Improves Drought Tolerance. New Phytol. 2008, 180, 911–921. [Google Scholar] [CrossRef]
- Zhang, H.L.; Huang, Q.L.; Yi, L.; Song, X.N.; Li, L.; Deng, G.B.; Liang, J.J.; Chen, F.; Yu, M.Q.; Long, H. PAL-Mediated SA Biosynthesis Pathway Contributes to Nematode Resistance in Wheat. Plant J. 2021, 107, 698–712. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Qian, S.; Yao, S.; Wu, J.; Wang, L.; Mu, J.; Wang, Y.; Wang, J.; Zhang, Z.; Li, M. Long-Term Effects of Vegetative-Propagation-Mediated TuMV-ZR Transmission on Yield, Quality, and Stress Resistance in Pseudostellaria heterophylla. Pathogens 2025, 14, 353. https://doi.org/10.3390/pathogens14040353
Gu L, Qian S, Yao S, Wu J, Wang L, Mu J, Wang Y, Wang J, Zhang Z, Li M. Long-Term Effects of Vegetative-Propagation-Mediated TuMV-ZR Transmission on Yield, Quality, and Stress Resistance in Pseudostellaria heterophylla. Pathogens. 2025; 14(4):353. https://doi.org/10.3390/pathogens14040353
Chicago/Turabian StyleGu, Li, Sheng Qian, Shuting Yao, Jiaxin Wu, Lianghong Wang, Jing Mu, Yankun Wang, Jianming Wang, Zhongyi Zhang, and Mingjie Li. 2025. "Long-Term Effects of Vegetative-Propagation-Mediated TuMV-ZR Transmission on Yield, Quality, and Stress Resistance in Pseudostellaria heterophylla" Pathogens 14, no. 4: 353. https://doi.org/10.3390/pathogens14040353
APA StyleGu, L., Qian, S., Yao, S., Wu, J., Wang, L., Mu, J., Wang, Y., Wang, J., Zhang, Z., & Li, M. (2025). Long-Term Effects of Vegetative-Propagation-Mediated TuMV-ZR Transmission on Yield, Quality, and Stress Resistance in Pseudostellaria heterophylla. Pathogens, 14(4), 353. https://doi.org/10.3390/pathogens14040353




