Borrelia burgdorferi Strain-Specific Differences in Mouse Infectivity and Pathology
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cell Culture
2.2. Polymerase Chain Reaction (PCR) for Plasmid Characterization
2.3. Animal Care and Infectious Challenge
2.4. Tissue Culture for Confirmation of Infection
2.5. Quantitative PCR (qPCR) for Sprirochete Burden
2.6. Determination of Lymphadenopathy
2.7. Histopathology
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Multiplex Cytokine ELISA
2.10. Statistical Analysis
3. Results
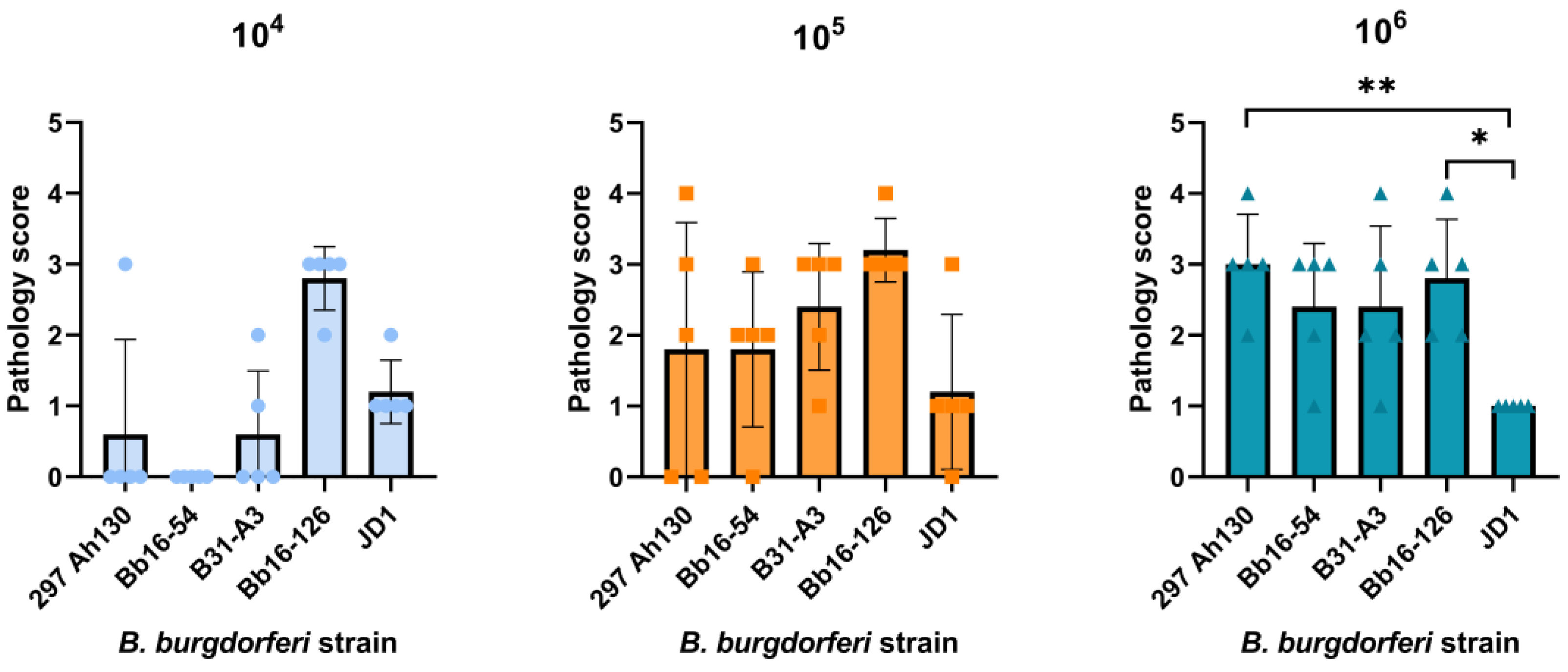
3.1. Strains Bb16-126 and JD1 Are Highly Infectious in C3H/HeN Mice
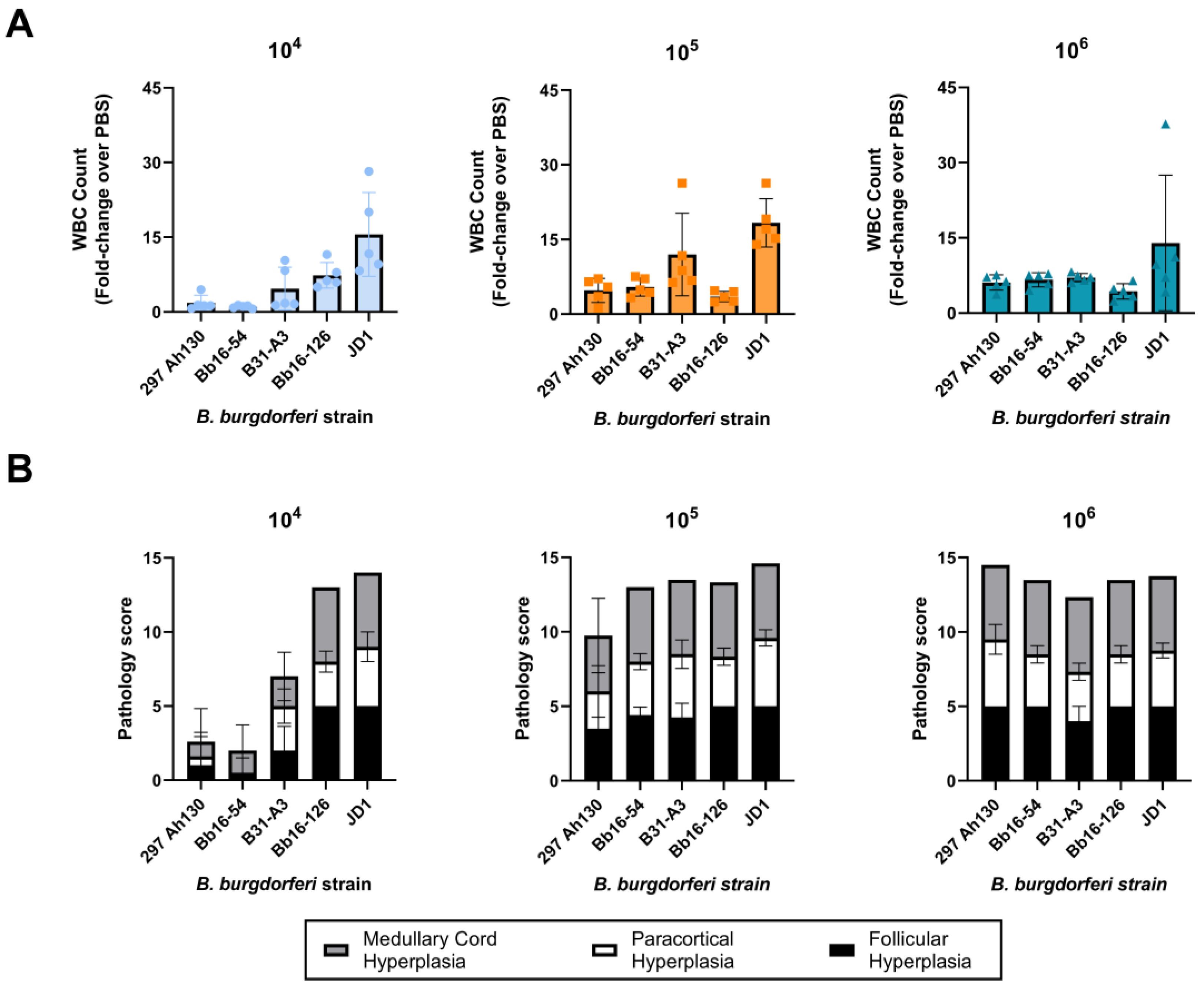
3.2. B. burgdorferi Strains Differ in Spirochete Load in Mouse Tissues
3.3. Strain JD1 Causes Minimal Carditis in C3H/HeN Mice
3.4. All B. burgdorferi Strains Cause Severe Lymph Node Hyperplasia
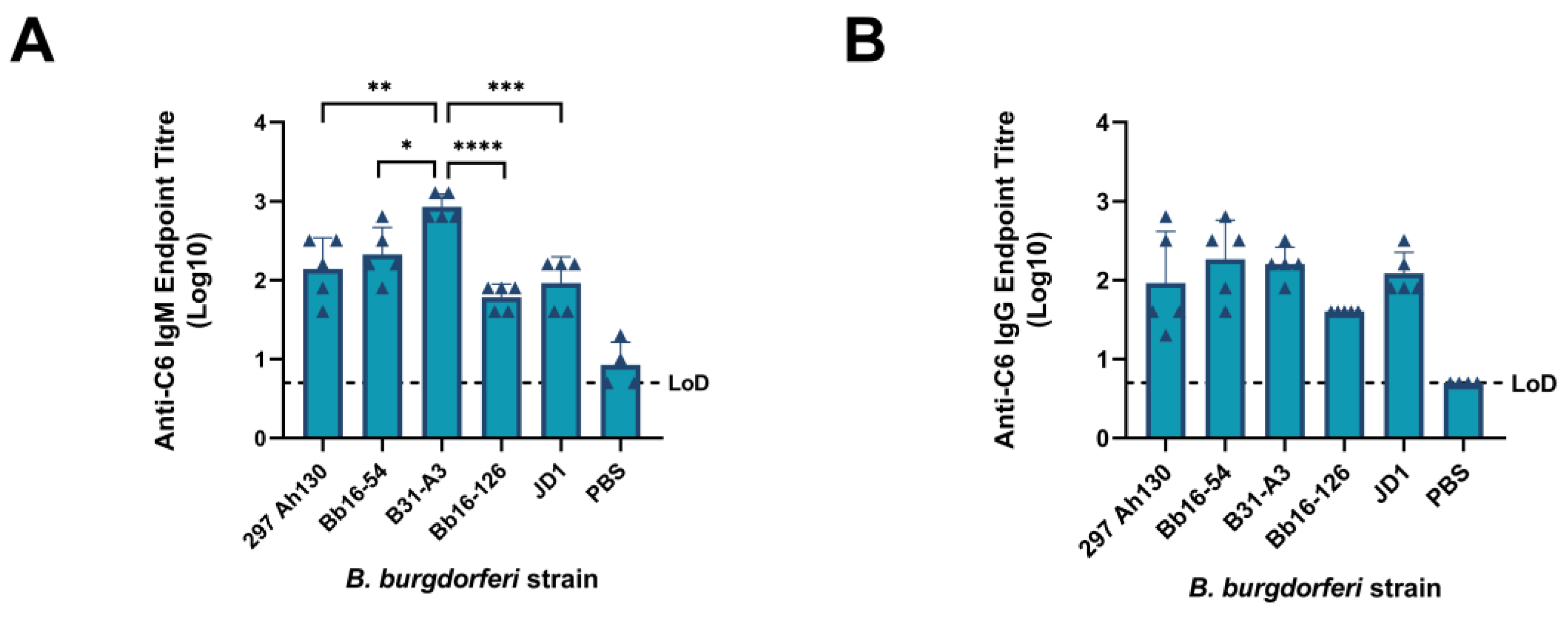
3.5. All B. burgdorferi Strains Induce Significant Host Immune Responses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LD | Lyme disease |
| Bbsl | Borrelia burgdorferi sensu lato |
| Bbss | Borrelia burgdorferi sensu stricto |
| MST | Multi-locus sequence typing |
| osp | Outer surface protein |
| RST | Ribosomal RNA intragenic spacer type |
| BSK | Barbour–Stoenner–Kelly |
| PCR | Polymerase chain reaction |
| qPCR | Quantitative polymerase chain reaction |
| WBC | White blood cell |
| FBS | Fetal bovine serum |
| ELISA | Enzyme-linked immunosorbent assay |
| Vls | Variable major protein (VMP)-like sequence |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| PBS | Phosphate-buffered saline |
| PBS-T | Phosphate-buffered saline-Tween |
| Lp | Linear plasmid |
| Cp | Circular plasmid |
References
- Kugeler, K.J.; Schwartz, A.M.; Delorey, M.J.; Mead, P.S.; Hinckley, A.F. Estimating the Frequency of Lyme Disease Diagnoses, United States, 2010-2018. Emerg. Infect. Dis. 2021, 27, 616–619. [Google Scholar] [CrossRef]
- Government of Canada Lyme Disease: Surveillance. Available online: https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease.html (accessed on 13 May 2024).
- Strnad, M.; Rudenko, N.; Rego, R.O.M. Pathogenicity and Virulence of Borrelia Burgdorferi. Virulence 2023, 14, 2265015. [Google Scholar] [CrossRef]
- Stanek, G.; Reiter, M. The Expanding Lyme Borrelia Complex--Clinical Significance of Genomic Species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Kurtenbach, K.; Hanincová, K.; Tsao, J.I.; Margos, G.; Fish, D.; Ogden, N.H. Fundamental Processes in the Evolutionary Ecology of Lyme Borreliosis. Nat. Rev. Microbiol. 2006, 4, 660–669. [Google Scholar] [CrossRef]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme Borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Sikand, V.K. The Presenting Manifestations of Lyme Disease and the Outcomes of Treatment. N. Engl. J. Med. 2003, 348, 2472–2474. [Google Scholar] [CrossRef] [PubMed]
- Malane, M.S.; Grant-Kels, J.M.; Feder, H.M.; Luger, S.W. Diagnosis of Lyme Disease Based on Dermatologic Manifestations. Ann. Intern. Med. 1991, 114, 490–498. [Google Scholar] [CrossRef]
- Steere, A.C.; Schoen, R.T.; Taylor, E. The Clinical Evolution of Lyme Arthritis. Ann. Intern. Med. 1987, 107, 725–731. [Google Scholar] [CrossRef]
- Halperin, J.J. Nervous System Lyme Disease. Infect. Dis. Clin. N. Am. 2015, 29, 241–253. [Google Scholar] [CrossRef]
- Radolf, J.D.; Strle, K.; Lemieux, J.E.; Strle, F. Lyme Disease in Humans. Curr. Issues Mol. Biol. 2021, 42, 333–384. [Google Scholar] [CrossRef]
- Cardenas-de la Garza, J.A.; De la Cruz-Valadez, E.; Ocampo-Candiani, J.; Welsh, O. Clinical Spectrum of Lyme Disease. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Strle, F. Lyme Borreliosis-from Tick Bite to Diagnosis and Treatment. FEMS Microbiol. Rev. 2018, 42, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.M.; Forsthuber, T.; Tary-Lehmann, M.; Etling, C.; Ito, K.; Nagy, Z.A.; Field, J.A.; Steere, A.C.; Huber, B.T. Identification of LFA-1 as a Candidate Autoantigen in Treatment-Resistant Lyme Arthritis. Science 1998, 281, 703–706. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme Borreliosis. Nat. Rev. Dis. Prim. 2016, 2, 16090. [Google Scholar] [CrossRef]
- van Dam, A.P.; Kuiper, H.; Vos, K.; Widjojokusumo, A.; de Jongh, B.M.; Spanjaard, L.; Ramselaar, A.C.; Kramer, M.D.; Dankert, J. Different Genospecies of Borrelia Burgdorferi Are Associated with Distinct Clinical Manifestations of Lyme Borreliosis. Clin. Infect. Dis. 1993, 17, 708–717. [Google Scholar] [CrossRef]
- Trevisan, G.; Bonin, S.; Ruscio, M. A Practical Approach to the Diagnosis of Lyme Borreliosis: From Clinical Heterogeneity to Laboratory Methods. Front. Med. 2020, 7, 265. [Google Scholar] [CrossRef]
- Pritt, B.S.; Mead, P.S.; Johnson, D.K.H.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a Novel Pathogenic Borrelia Species Causing Lyme Borreliosis with Unusually High Spirochaetaemia: A Descriptive Study. Lancet Infect. Dis. 2016, 16, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.M.; Stroeher, U.H.; Ogunniyi, A.D.; Smith-Vaughan, H.C.; Leach, A.J.; Paton, J.C. A Variable Region within the Genome of Streptococcus Pneumoniae Contributes to Strain-Strain Variation in Virulence. PLoS ONE 2011, 6, e19650. [Google Scholar] [CrossRef]
- Jones, K.R.; Jang, S.; Chang, J.Y.; Kim, J.; Chung, I.-S.; Olsen, C.H.; Merrell, D.S.; Cha, J.-H. Polymorphisms in the Intermediate Region of VacA Impact Helicobacter Pylori -Induced Disease Development. J. Clin. Microbiol. 2011, 49, 101–110. [Google Scholar] [CrossRef]
- Tantalo, L.C.; Lukehart, S.A.; Marra, C.M. Treponema Pallidum Strain-Specific Differences in Neuroinvasion and Clinical Phenotype in a Rabbit Model. J. Infect. Dis. 2005, 191, 75–80. [Google Scholar] [CrossRef]
- Israel, D.A.; Salama, N.; Arnold, C.N.; Moss, S.F.; Ando, T.; Wirth, H.P.; Tham, K.T.; Camorlinga, M.; Blaser, M.J.; Falkow, S.; et al. Helicobacter Pylori Strain-Specific Differences in Genetic Content, Identified by Microarray, Influence Host Inflammatory Responses. J. Clin. Investig. 2001, 107, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Lebech, A.M.; Hansen, K.; Wilske, B.; Theisen, M. Taxonomic Classification of 29 Borrelia Burgdorferi Strains Isolated from Patients with Lyme Borreliosis: A Comparison of Five Different Phenotypic and Genotypic Typing Schemes. Med. Microbiol. Immunol. 1994, 183, 325–341. [Google Scholar] [CrossRef]
- Picken, R.N.; Strle, F.; Picken, M.M.; Ruzic-Sabljic, E.; Maraspin, V.; Lotric-Furlan, S.; Cimperman, J. Identification of Three Species of Borrelia burgdorferi Sensu Lato (B. burgdorferi Sensu Stricto, B. Garinii, and B. Afzelii) among Isolates from Acrodermatitis Chronica Atrophicans Lesions. J. Investig. Dermatol. 1998, 110, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, D.A.; Oliver, J.H.; Kolbert, C.P.; Tullson, E.D.; Johnson, B.J.; Campbell, G.L.; Mitchell, P.D.; Reed, K.D.; Telford, S.R.; Anderson, J.F.; et al. Genetic Heterogeneity of Borrelia Burgdorferi in the United States. J. Infect. Dis. 1997, 175, 98–107. [Google Scholar] [CrossRef]
- Hanincova, K.; Mukherjee, P.; Ogden, N.H.; Margos, G.; Wormser, G.P.; Reed, K.D.; Meece, J.K.; Vandermause, M.F.; Schwartz, I. Multilocus Sequence Typing of Borrelia Burgdorferi Suggests Existence of Lineages with Differential Pathogenic Properties in Humans. PLoS ONE 2013, 8, e73066. [Google Scholar] [CrossRef]
- Brisson, D.; Baxamusa, N.; Schwartz, I.; Wormser, G.P. Biodiversity of Borrelia Burgdorferi Strains in Tissues of Lyme Disease Patients. PLoS ONE 2011, 6, e22926. [Google Scholar] [CrossRef]
- Wormser, G.P.; Schwartz, I. Antibiotic Treatment of Animals Infected with Borrelia Burgdorferi. Clin. Microbiol. Rev. 2009, 22, 387–395. [Google Scholar] [CrossRef]
- Schneider, B.S.; Schriefer, M.E.; Dietrich, G.; Dolan, M.C.; Morshed, M.G.; Zeidner, N.S. Borrelia Bissettii Isolates Induce Pathology in a Murine Model of Disease. Vector Borne Zoonotic Dis. 2008, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Graham, C.B.; Hojgaard, A.; Breuner, N.E.; Maes, S.E.; Boegler, K.A.; Replogle, A.J.; Kingry, L.C.; Petersen, J.M.; Eisen, L.; et al. Isolation of the Lyme Disease Spirochete Borrelia Mayonii From Naturally Infected Rodents in Minnesota. J. Med. Entomol. 2017, 54, 1088–1092. [Google Scholar] [CrossRef]
- Zinck, C.B.; Thampy, P.R.; Rego, R.O.M.; Brisson, D.; Ogden, N.H.; Voordouw, M. Borrelia Burgdorferi Strain and Host Sex Influence Pathogen Prevalence and Abundance in the Tissues of a Laboratory Rodent Host. Mol. Ecol. 2022, 31, 5872–5888. [Google Scholar] [CrossRef]
- Zinck, C.B.; Raveendram Thampy, P.; Uhlemann, E.-M.E.; Adam, H.; Wachter, J.; Suchan, D.; Cameron, A.D.S.; Rego, R.O.M.; Brisson, D.; Bouchard, C.; et al. Variation among Strains of Borrelia Burgdorferi in Host Tissue Abundance and Lifetime Transmission Determine the Population Strain Structure in Nature. PLOS Pathog. 2023, 19, e1011572. [Google Scholar] [CrossRef]
- Baum, E.; Hue, F.; Barbour, A.G. Experimental Infections of the Reservoir Species Peromyscus Leucopus with Diverse Strains of Borrelia Burgdorferi, a Lyme Disease Agent. MBio 2012, 3, e00434-12. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Awan, M.; Barthold, S.W.; Parveen, N. Comparative Molecular Analyses of Borrelia Burgdorferi Sensu Stricto Strains B31 and N40D10/E9 and Determination of Their Pathogenicity. BMC Microbiol. 2012, 12, 157. [Google Scholar] [CrossRef]
- Sertour, N.; Cotté, V.; Garnier, M.; Malandrin, L.; Ferquel, E.; Choumet, V. Infection Kinetics and Tropism of Borrelia Burgdorferi Sensu Lato in Mouse After Natural (via Ticks) or Artificial (Needle) Infection Depends on the Bacterial Strain. Front. Microbiol. 2018, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ojaimi, C.; Wu, H.; Saksenberg, V.; Iyer, R.; Liveris, D.; McClain, S.A.; Wormser, G.P.; Schwartz, I. Disease Severity in a Murine Model of Lyme Borreliosis Is Associated with the Genotype of the Infecting Borrelia Burgdorferi Sensu Stricto Strain. J. Infect. Dis. 2002, 186, 782–791. [Google Scholar] [CrossRef]
- Zeidner, N.S.; Schneider, B.S.; Dolan, M.C.; Piesman, J. An Analysis of Spirochete Load, Strain, and Pathology in a Model of Tick-Transmitted Lyme Borreliosis. Vector Borne Zoonotic Dis. 2001, 1, 35–44. [Google Scholar] [CrossRef]
- Anderson, J.M.; Norris, D.E. Genetic Diversity of Borrelia Burgdorferi Sensu Stricto in Peromyscus Leucopus, the Primary Reservoir of Lyme Disease in a Region of Endemicity in Southern Maryland. Appl. Environ. Microbiol. 2006, 72, 5331–5341. [Google Scholar] [CrossRef]
- Mongodin, E.F.; Casjens, S.R.; Bruno, J.F.; Xu, Y.; Drabek, E.; Riley, D.R.; Cantarel, B.L.; Pagan, P.E.; Hernandez, Y.A.; Vargas, L.C.; et al. Inter- and Intra-Specific Pan-Genomes of Borrelia Burgdorferi Sensu Lato: Genome Stability and Adaptive Radiation. BMC Genom. 2013, 14, 693. [Google Scholar] [CrossRef]
- Brisson, D.; Drecktrah, D.; Eggers, C.H.; Samuels, D.S. Genetics of Borrelia Burgdorferi. Annu. Rev. Genet. 2012, 46, 515–536. [Google Scholar] [CrossRef]
- Margos, G.; Gatewood, A.G.; Aanensen, D.M.; Hanincová, K.; Terekhova, D.; Vollmer, S.A.; Cornet, M.; Piesman, J.; Donaghy, M.; Bormane, A.; et al. MLST of Housekeeping Genes Captures Geographic Population Structure and Suggests a European Origin of Borrelia Burgdorferi. Proc. Natl. Acad. Sci. USA 2008, 105, 8730–8735. [Google Scholar] [CrossRef]
- Margos, G.; Vollmer, S.A.; Ogden, N.H.; Fish, D. Population Genetics, Taxonomy, Phylogeny and Evolution of Borrelia Burgdorferi Sensu Lato. Infect. Genet. Evol. 2011, 11, 1545–1563. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liveris, D.; Mukherjee, P.; Jungnick, S.; Margos, G.; Schwartz, I. Molecular Typing of Borrelia burgdorferi. Curr. Protoc. Microbiol. 2014, 34, 12C.1–12C.31. [Google Scholar] [CrossRef]
- Dykhuizen, D.E.; Brisson, D.; Sandigursky, S.; Wormser, G.P.; Nowakowski, J.; Nadelman, R.B.; Schwartz, I. The Propensity of Different Borrelia Burgdorferi Sensu Stricto Genotypes to Cause Disseminated Infections in Humans. Am. J. Trop. Med. Hyg. 2008, 78, 806–810. [Google Scholar]
- Seinost, G.; Dykhuizen, D.E.; Dattwyler, R.J.; Golde, W.T.; Dunn, J.J.; Wang, I.N.; Wormser, G.P.; Schriefer, M.E.; Luft, B.J. Four Clones of Borrelia Burgdorferi Sensu Stricto Cause Invasive Infection in Humans. Infect. Immun. 1999, 67, 3518–3524. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Tan, X.; Caine, J.A.; Castellanos, M.; Chaconas, G.; Coburn, J.; Leong, J.M. Strain-Specific Joint Invasion and Colonization by Lyme Disease Spirochetes Is Promoted by Outer Surface Protein C. PLoS Pathog. 2020, 16, e1008516. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Benoit, V.; Yang, X.; Martínez-Herranz, R.; Pal, U.; Leong, J.M. Strain-Specific Variation of the Decorin-Binding Adhesin DbpA Influences the Tissue Tropism of the Lyme Disease Spirochete. PLoS Pathog. 2014, 10, e1004238. [Google Scholar] [CrossRef]
- Walter, L.; Sürth, V.; Röttgerding, F.; Zipfel, P.F.; Fritz-Wolf, K.; Kraiczy, P. Elucidating the Immune Evasion Mechanisms of Borrelia Mayonii, the Causative Agent of Lyme Disease. Front. Immunol. 2019, 10, 2722. [Google Scholar] [CrossRef]
- Koloski, C.W.; Adam, H.; Hurry, G.; Foley-Eby, A.; Zinck, C.B.; Wei, H.; Hansra, S.; Wachter, J.; Voordouw, M.J. Adaptive Immunity in Mus Musculus Influences the Acquisition and Abundance of Borrelia Burgdorferi in Ixodes Scapularis Ticks. Appl. Environ. Microbiol. 2024, 90, e0129924. [Google Scholar] [CrossRef]
- Bockenstedt, L.K.; Wooten, R.M.; Baumgarth, N. Immune Response to Borrelia: Lessons from Lyme Disease Spirochetes. Curr. Issues Mol. Biol. 2021, 42, 145–190. [Google Scholar] [CrossRef]
- Wang, G.; Ojaimi, C.; Iyer, R.; Saksenberg, V.; McClain, S.A.; Wormser, G.P.; Schwartz, I. Impact of Genotypic Variation of Borrelia Burgdorferi Sensu Stricto on Kinetics of Dissemination and Severity of Disease in C3H/HeJ Mice. Infect. Immun. 2001, 69, 4303–4312. [Google Scholar] [CrossRef]
- Armstrong, A.L.; Barthold, S.W.; Persing, D.H.; Beck, D.S. Carditis in Lyme Disease Susceptible and Resistant Strains of Laboratory Mice Infected with Borrelia Burgdorferi. Am. J. Trop. Med. Hyg. 1992, 47, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.W. Infectivity of Borrelia Burgdorferi Relative to Route of Inoculation and Genotype in Laboratory Mice. J. Infect. Dis. 1991, 163, 419–420. [Google Scholar] [CrossRef]
- Ma, Y.; Seiler, K.P.; Eichwald, E.J.; Weis, J.H.; Teuscher, C.; Weis, J.J. Distinct Characteristics of Resistance to Borrelia Burgdorferi-Induced Arthritis in C57BL/6N Mice. Infect. Immun. 1998, 66, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wooten, R.M.; Weis, J.J. Host-Pathogen Interactions Promoting Inflammatory Lyme Arthritis: Use of Mouse Models for Dissection of Disease Processes. Curr. Opin. Microbiol. 2001, 4, 274–279. [Google Scholar] [CrossRef]
- Ma, Y.; Bramwell, K.K.C.; Lochhead, R.B.; Paquette, J.K.; Zachary, J.F.; Weis, J.H.; Teuscher, C.; Weis, J.J. Borrelia Burgdorferi Arthritis-Associated Locus Bbaa1 Regulates Lyme Arthritis and K/B×N Serum Transfer Arthritis through Intrinsic Control of Type I IFN Production. J. Immunol. 2014, 193, 6050–6060. [Google Scholar] [CrossRef]
- Nardelli, D.T.; Luedtke, J.O.; Munson, E.L.; Warner, T.F.; Callister, S.M.; Schell, R.F. Significant Differences between the Borrelia-Infection and Borrelia-Vaccination and -Infection Models of Lyme Arthritis in C3H/HeN Mice. FEMS Immunol. Med. Microbiol. 2010, 60, 78–89. [Google Scholar] [CrossRef]
- Bernard, Q.; Hu, L.T. Innate Immune Memory to Repeated Borrelia Burgdorferi Exposure Correlates with Murine In Vivo Inflammatory Phenotypes. J. Immunol. 2020, 205, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Pfeifle, A.; Thulasi Raman, S.N.; Lansdell, C.; Zhang, W.; Tamming, L.; Cecillon, J.; Laryea, E.; Patel, D.; Wu, J.; Gravel, C.; et al. DNA Lipid Nanoparticle Vaccine Targeting Outer Surface Protein C Affords Protection against Homologous Borrelia Burgdorferi Needle Challenge in Mice. Front. Immunol. 2023, 14, 1020134. [Google Scholar] [CrossRef]
- Pfeifle, A.; Zhang, W.; Cao, J.; Thulasi Raman, S.N.; Anderson-Duvall, R.; Tamming, L.; Gravel, C.; Coatsworth, H.; Chen, W.; Johnston, M.J.W.; et al. Novel Recombinant Vaccinia Virus-Vectored Vaccine Affords Complete Protection against Homologous Borrelia Burgdorferi Infection in Mice. Emerg. Microbes Infect. 2024, 13, 2399949. [Google Scholar] [CrossRef]
- Yang, X.F.; Pal, U.; Alani, S.M.; Fikrig, E.; Norgard, M. V Essential Role for OspA/B in the Life Cycle of the Lyme Disease Spirochete. J. Exp. Med. 2004, 199, 641–648. [Google Scholar] [CrossRef]
- Steere, A.C.; Grodzicki, R.L.; Kornblatt, A.N.; Craft, J.E.; Barbour, A.G.; Burgdorfer, W.; Schmid, G.P.; Johnson, E.; Malawista, S.E. The Spirochetal Etiology of Lyme Disease. N. Engl. J. Med. 1983, 308, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.F.; Stewart, P.E.; Grimm, D.; Caimano, M.J.; Eggers, C.H.; Tilly, K.; Bono, J.L.; Akins, D.R.; Radolf, J.D.; Schwan, T.G.; et al. Clonal Polymorphism of Borrelia Burgdorferi Strain B31 MI: Implications for Mutagenesis in an Infectious Strain Background. Infect. Immun. 2002, 70, 2139–2150. [Google Scholar] [CrossRef]
- Tyler, S.; Tyson, S.; Dibernardo, A.; Drebot, M.; Feil, E.J.; Graham, M.; Knox, N.C.; Lindsay, L.R.; Margos, G.; Mechai, S.; et al. Whole Genome Sequencing and Phylogenetic Analysis of Strains of the Agent of Lyme Disease Borrelia Burgdorferi from Canadian Emergence Zones. Sci. Rep. 2018, 8, 10552. [Google Scholar] [CrossRef]
- Piesman, J.; Mather, T.N.; Sinsky, R.J.; Spielman, A. Duration of Tick Attachment and Borrelia Burgdorferi Transmission. J. Clin. Microbiol. 1987, 25, 557–558. [Google Scholar] [CrossRef]
- Verhey, T.B.; Castellanos, M.; Chaconas, G. Antigenic Variation in the Lyme Spirochete: Detailed Functional Assessment of Recombinational Switching at VlsE in the JD1 Strain of Borrelia Burgdorferi. Mol. Microbiol. 2019, 111, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G. Isolation and Cultivation of Lyme Disease Spirochetes. Yale J. Biol. Med. 1984, 57, 521–525. [Google Scholar]
- Bunikis, I.; Kutschan-Bunikis, S.; Bonde, M.; Bergström, S. Multiplex PCR as a Tool for Validating Plasmid Content of Borrelia Burgdorferi. J. Microbiol. Methods 2011, 86, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Schriefer, M.; Aguero-Rosenfeld, M.E.; Levin, A.; Steere, A.C.; Nadelman, R.B.; Nowakowski, J.; Marques, A.; Johnson, B.J.B.; Dumler, J.S. Single-Tier Testing with the C6 Peptide ELISA Kit Compared with Two-Tier Testing for Lyme Disease. Diagn. Microbiol. Infect. Dis. 2013, 75, 9–15. [Google Scholar] [CrossRef]
- Wormser, G.P.; Liveris, D.; Hanincová, K.; Brisson, D.; Ludin, S.; Stracuzzi, V.J.; Embers, M.E.; Philipp, M.T.; Levin, A.; Aguero-Rosenfeld, M.; et al. Effect of Borrelia Burgdorferi Genotype on the Sensitivity of C6 and 2-Tier Testing in North American Patients with Culture-Confirmed Lyme Disease. Clin. Infect. Dis. 2008, 47, 910–914. [Google Scholar] [CrossRef]
- Steere, A.C.; Bartenhagen, N.H.; Craft, J.E.; Hutchinson, G.J.; Newman, J.H.; Rahn, D.W.; Sigal, L.H.; Spieler, P.N.; Stenn, K.S.; Malawista, S.E. The Early Clinical Manifestations of Lyme Disease. Ann. Intern. Med. 1983, 99, 76–82. [Google Scholar] [CrossRef]
- Summers, B.A.; Straubinger, A.F.; Jacobson, R.H.; Chang, Y.-F.; Appel, M.J.G.; Straubinger, R.K. Histopathological Studies of Experimental Lyme Disease in the Dog. J. Comp. Pathol. 2005, 133, 1–13. [Google Scholar] [CrossRef]
- Tunev, S.S.; Hastey, C.J.; Hodzic, E.; Feng, S.; Barthold, S.W.; Baumgarth, N. Lymphoadenopathy during Lyme Borreliosis Is Caused by Spirochete Migration-Induced Specific B Cell Activation. PLoS Pathog. 2011, 7, e1002066. [Google Scholar] [CrossRef]
- Public Health Agency of Canada Lyme Disease: For Health Professionals. Available online: https://www.canada.ca/en/public-health/services/diseases/lyme-disease/health-professionals-lyme-disease.html (accessed on 4 February 2025).
- Philipp, M.T.; Aydintug, M.K.; Bohm, R.P.; Cogswell, F.B.; Dennis, V.A.; Lanners, H.N.; Lowrie, R.C.; Roberts, E.D.; Conway, M.D.; Karaçorlu, M.; et al. Early and Early Disseminated Phases of Lyme Disease in the Rhesus Monkey: A Model for Infection in Humans. Infect. Immun. 1993, 61, 3047–3059. [Google Scholar] [CrossRef]
- Lardieri, G.; Salvi, A.; Camerini, F.; Cińco, M.; Trevisan, G. Isolation of Borrelia Burgdorferi from Myocardium. Lancet 1993, 342, 490. [Google Scholar] [CrossRef] [PubMed]
- Keane-Myers, A.; Maliszewski, C.R.; Finkelman, F.D.; Nickell, S.P. Recombinant IL-4 Treatment Augments Resistance to Borrelia Burgdorferi Infections in Both Normal Susceptible and Antibody-Deficient Susceptible Mice. J. Immunol. 1996, 156, 2488–2494. [Google Scholar] [PubMed]
- Moody, K.D.; Barthold, S.W.; Terwilliger, G.A. Lyme Borreliosis in Laboratory Animals: Effect of Host Species and in Vitro Passage of Borrelia Burgdorferi. Am. J. Trop. Med. Hyg. 1990, 43, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Koloski, C.W.; Hurry, G.; Foley-Eby, A.; Adam, H.; Goldstein, S.; Zvionow, P.; Detmer, S.E.; Voordouw, M.J. Male C57BL/6J Mice Have Higher Presence and Abundance of Borrelia Burgdorferi in Their Ventral Skin Compared to Female Mice. Ticks Tick. Borne. Dis. 2024, 15, 102308. [Google Scholar] [CrossRef]
- Littman, M.P.; Gerber, B.; Goldstein, R.E.; Labato, M.A.; Lappin, M.R.; Moore, G.E. ACVIM Consensus Update on Lyme Borreliosis in Dogs and Cats. J. Vet. Intern. Med. 2018, 32, 887–903. [Google Scholar] [CrossRef]
- Ivanescu, M.L.; Marinescu, G.; Miron, L.D. Effective Diagnostic Techniques in Borrelia Burgdorferi Infestation in Dogs. J. Appl. Life Sci. Environ. 2023, 55, 219–232. [Google Scholar] [CrossRef]
- Miraglia, C.M. A Review of the Centers for Disease Control and Prevention’s Guidelines for the Clinical Laboratory Diagnosis of Lyme Disease. J. Chiropr. Med. 2016, 15, 272–280. [Google Scholar] [CrossRef][Green Version]
- Barbour, A.G.; Garon, C.F. Linear Plasmids of the Bacterium Borrelia Burgdorferi Have Covalently Closed Ends. Science 1987, 237, 409–411. [Google Scholar] [CrossRef]
- Fuchs, R.; Jauris, S.; Lottspeich, F.; Preac-Mursic, V.; Wilske, B.; Soutschek, E. Molecular Analysis and Expression of a Borrelia Burgdorferi Gene Encoding a 22 KDa Protein (PC) in Escherichia Coli. Mol. Microbiol. 1992, 6, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.R.; Hardham, J.M.; Barbour, A.G.; Norris, S.J. Antigenic Variation in Lyme Disease Borreliae by Promiscuous Recombination of VMP-like Sequence Cassettes. Cell 1997, 89, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, J.E.; Huang, W.; Hill, N.; Cerar, T.; Freimark, L.; Hernandez, S.; Luban, M.; Maraspin, V.; Bogovič, P.; Ogrinc, K.; et al. Whole Genome Sequencing of Human Borrelia Burgdorferi Isolates Reveals Linked Blocks of Accessory Genome Elements Located on Plasmids and Associated with Human Dissemination. PLoS Pathog. 2023, 19, e1011243. [Google Scholar] [CrossRef]
- Purser, J.E.; Norris, S.J. Correlation between Plasmid Content and Infectivity in Borrelia Burgdorferi. Proc. Natl. Acad. Sci. USA 2000, 97, 13865–13870. [Google Scholar] [CrossRef]
- Casselli, T.; Tourand, Y.; Gura, K.; Stevenson, B.; Zückert, W.R.; Brissette, C.A. Endogenous Linear Plasmids Lp28-4 and Lp25 Are Required for Infectivity and Restriction Protection in the Lyme Disease Spirochete Borrelia Mayonii. Infect. Immun. 2023, 91, e0006123. [Google Scholar] [CrossRef]
- Dulebohn, D.P.; Bestor, A.; Rosa, P.A. Borrelia Burgdorferi Linear Plasmid 28-3 Confers a Selective Advantage in an Experimental Mouse-Tick Infection Model. Infect. Immun. 2013, 81, 2986–2996. [Google Scholar] [CrossRef]
- Casselli, T.; Crowley, M.A.; Highland, M.A.; Tourand, Y.; Bankhead, T. A Small Intergenic Region of Lp17 Is Required for Evasion of Adaptive Immunity and Induction of Pathology by the Lyme Disease Spirochete. Cell. Microbiol. 2019, 21, e13029. [Google Scholar] [CrossRef]
- Stewart, P.E.; Byram, R.; Grimm, D.; Tilly, K.; Rosa, P.A. The Plasmids of Borrelia Burgdorferi: Essential Genetic Elements of a Pathogen. Plasmid 2005, 53, 1–13. [Google Scholar] [CrossRef]
- Casjens, S.; Palmer, N.; van Vugt, R.; Huang, W.M.; Stevenson, B.; Rosa, P.; Lathigra, R.; Sutton, G.; Peterson, J.; Dodson, R.J.; et al. A Bacterial Genome in Flux: The Twelve Linear and Nine Circular Extrachromosomal DNAs in an Infectious Isolate of the Lyme Disease Spirochete Borrelia Burgdorferi. Mol. Microbiol. 2000, 35, 490–516. [Google Scholar] [CrossRef]
- Norris, S.J.; Howell, J.K.; Odeh, E.A.; Lin, T.; Gao, L.; Edmondson, D.G. High-Throughput Plasmid Content Analysis of Borrelia Burgdorferi B31 by Using Luminex Multiplex Technology. Appl. Environ. Microbiol. 2011, 77, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Marconi, R.T. Demonstration of Cotranscription and 1-Methyl-3-Nitroso-Nitroguanidine Induction of a 30-Gene Operon of Borrelia Burgdorferi: Evidence That the 32-Kilobase Circular Plasmids Are Prophages. J. Bacteriol. 2005, 187, 7985–7995. [Google Scholar] [CrossRef] [PubMed]
- Dolan, M.C.; Piesman, J.; Schneider, B.S.; Schriefer, M.; Brandt, K.; Zeidner, N.S. Comparison of Disseminated and Nondisseminated Strains of Borrelia Burgdorferi Sensu Stricto in Mice Naturally Infected by Tick Bite. Infect. Immun. 2004, 72, 5262–5266. [Google Scholar] [CrossRef] [PubMed]
- Hanincová, K.; Ogden, N.H.; Diuk-Wasser, M.; Pappas, C.J.; Iyer, R.; Fish, D.; Schwartz, I.; Kurtenbach, K. Fitness Variation of Borrelia Burgdorferi Sensu Stricto Strains in Mice. Appl. Environ. Microbiol. 2008, 74, 153–157. [Google Scholar] [CrossRef]




| Strain ID | Isolate Source | Year of Original Isolation | Geographic Region | RST [39] | OspC Type | Reference(s) |
|---|---|---|---|---|---|---|
| 297 Ah130 | Laboratory isolate derived from strain 297, originally obtained from Lyme borreliosis patient cerebrospinal fluid | 1982 | Connecticut, U.S. | 2 | K | [61,62] |
| B31-A3 | Laboratory isolate of strain B31, originally obtained from Ixodes dammini | 1981 | New York, U.S. | 1 | A | [63] |
| Bb16-54 | Ixodes scapularis | 2016 | Buffalo Point, MB, CA | Unknown | I | [31,64] |
| Bb16-126 | Ixodes scapularis | 2016 | Big Grassy, ON, CA | Unknown | N | [31,64] |
| JD1 (Clone SK143) | Passaged in C3H/HeN mouse, originally obtained from Ixodes dammini | 1986 | Crane’s Beach, Ipswich, MA, U.S. | 3 | C | [65,66] |
| Plasmid | 297 Ah130 | Bb16-54 | B31-A3 | Bb16-126 | JD1 |
|---|---|---|---|---|---|
| Lp21 | + | + | + | + | + |
| Lp28-3 | + | + | + | + | + |
| Lp38 | − | + | + | + | − |
| Lp28-1 | + | + | + | + | + |
| Lp25 | − | + | + | + | − |
| Lp36 | − | − | + | + | − |
| Lp17 | + | + | + | + | + |
| Lp56 | − | + | + | + | − |
| Lp54 | + | + | + | + | + |
| Lp28-2 | + | + | + | + | + |
| Lp28-4 | + | + | + | + | + |
| Lp5 | − | − | − | − | − |
| Cp32-8 | − | + | + | + | + |
| Cp32-1 | + | + | + | + | + |
| Cp26 | + | + | + | + | + |
| Cp32-4 | − | − | + | + | + |
| Cp32-6 | − | − | + | + | − |
| Cp32-9 | − | − | + | − | − |
| Cp9 | − | + | − | + | − |
| Cp32-7 | + | + | + | + | + |
| Cp32-3 | + | − | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeifle, A.; Anderson-Duvall, R.; Tamming, L.A.; Zhang, W.; Thulasi Raman, S.N.; Gravel, C.; Wu, J.; Coatsworth, H.; Voordouw, M.J.; Zhang, X.; et al. Borrelia burgdorferi Strain-Specific Differences in Mouse Infectivity and Pathology. Pathogens 2025, 14, 352. https://doi.org/10.3390/pathogens14040352
Pfeifle A, Anderson-Duvall R, Tamming LA, Zhang W, Thulasi Raman SN, Gravel C, Wu J, Coatsworth H, Voordouw MJ, Zhang X, et al. Borrelia burgdorferi Strain-Specific Differences in Mouse Infectivity and Pathology. Pathogens. 2025; 14(4):352. https://doi.org/10.3390/pathogens14040352
Chicago/Turabian StylePfeifle, Annabelle, Rose Anderson-Duvall, Levi A. Tamming, Wanyue Zhang, Sathya N. Thulasi Raman, Caroline Gravel, Jianguo Wu, Heather Coatsworth, Maarten J. Voordouw, Xu Zhang, and et al. 2025. "Borrelia burgdorferi Strain-Specific Differences in Mouse Infectivity and Pathology" Pathogens 14, no. 4: 352. https://doi.org/10.3390/pathogens14040352
APA StylePfeifle, A., Anderson-Duvall, R., Tamming, L. A., Zhang, W., Thulasi Raman, S. N., Gravel, C., Wu, J., Coatsworth, H., Voordouw, M. J., Zhang, X., Johnston, M. J. W., Chen, W., Sauve, S., Wang, L., & Li, X. (2025). Borrelia burgdorferi Strain-Specific Differences in Mouse Infectivity and Pathology. Pathogens, 14(4), 352. https://doi.org/10.3390/pathogens14040352









