Promising Role of Fruitless Wolfberry Bud Tea in Combating Nakaseomyces glabratus Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Chemicals
2.3. Preparation of the FWE
2.4. Evaluating Susceptibility to Antimicrobial Agents
2.5. Antifungal Curve of Tested Agents Against N. glabratus
2.6. The Examination of Rhodamine 6G Efflux
2.7. Gene Expression Measurement Using qRT-PCR
2.8. Statistical Analysis
3. Results
3.1. Antifungal Susceptibility Testing
3.2. Investigation of the Interaction of FWE with Azole Antifungals
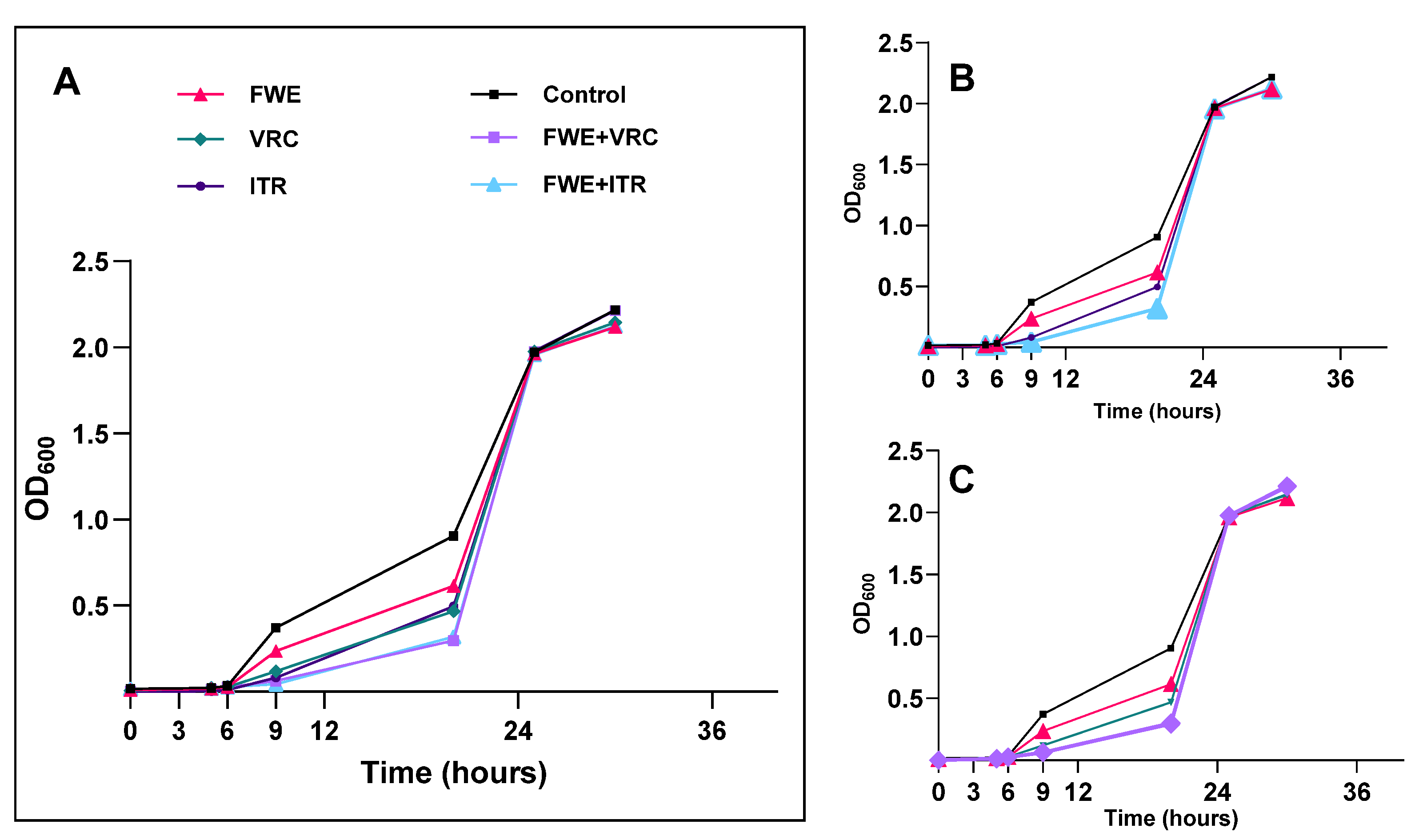
3.3. Determination of Growth Inhibition Curve
3.4. Assessment of Efflux Pump Activity
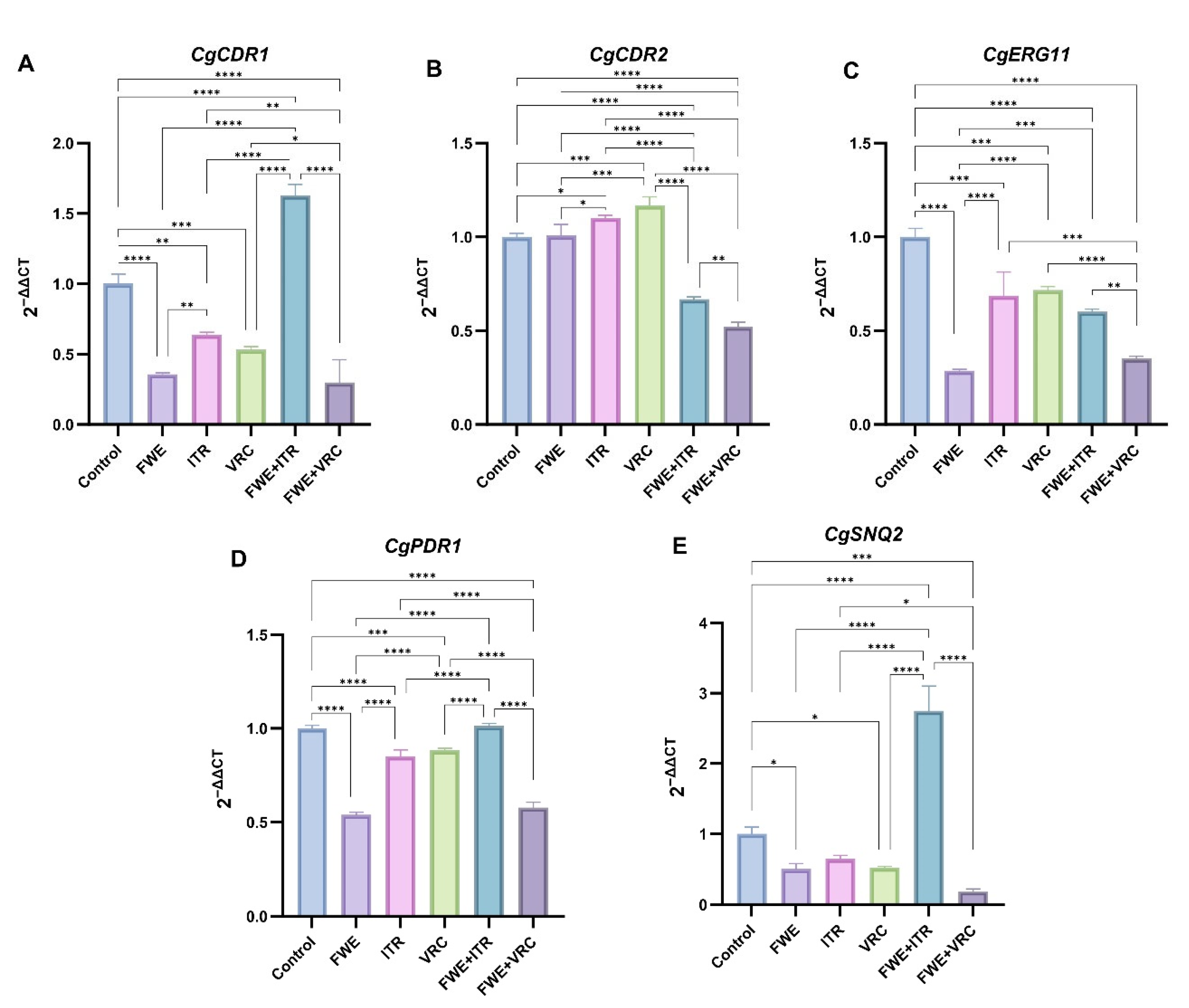
3.5. Determining Potential Drug-Resistance Gene Expression Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Ballou, E.R.; Bates, S.; Bignell, E.M.; Borman, A.M.; Brand, A.C.; Brown, A.J.P.; Coelho, C.; Cook, P.C.; Farrer, R.A.; et al. The Pathobiology of Human Fungal Infections. Nat. Rev. Microbiol. 2024, 22, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wijnants, S.; Feil, R.; Van Genechten, W.; Vergauwen, R.; Van Goethem, O.; Lunn, J.E.; Van Ende, M.; Van Dijck, P. The Stress-Protectant Molecule Trehalose Mediates Fluconazole Tolerance in Candida glabrata. Antimicrob. Agents Chemother. 2025, 69, e01349-24. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Lan, X.; Cai, M.; Liao, Y.; Zhang, J.; Ye, N.; Lu, X.; Wang, J.; Xiao, Y.; Zhang, Y.; et al. Nineteen Years Retrospective Analysis of Epidemiology, Antifungal Resistance and a Nomogram Model for 30-Day Mortality in Nosocomial Candidemia Patients. Front. Cell. Infect. Microbiol. 2025, 15, 1504866. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Katsipoulaki, M.; Stappers, M.H.T.; Malavia-Jones, D.; Brunke, S.; Hube, B.; Gow, N.A.R. Candida albicans and Candida glabrata: Global Priority Pathogens. Microbiol. Mol. Biol. Rev. 2024, 88, e0002123. [Google Scholar] [CrossRef]
- Wellington, M.; Koselny, K.; Sutterwala, F.S.; Krysan, D.J. Candida albicans Triggers NLRP3-Mediated Pyroptosis in Macrophages. Eukaryot. Cell 2014, 13, 329–340. [Google Scholar] [CrossRef]
- Abe, M.; Sekizuka, T.; Miyazaki, Y. Gastrointestinal Anaerobes and Enterococcus faecalis Promote Candida glabrata Gastrointestinal Colonization and Organ Dissemination. J. Infect. Chemother. 2025, 31, 102658. [Google Scholar] [CrossRef]
- Kwizera, R.; Abdolrasouli, A.; Garcia-Effron, G.; Denning, D.W. Antifungal Susceptibility Testing: Applicability of Methods and Strategies for Improving Access in Resource-Constrained Settings. Lancet Infect. Dis. 2024, 24, e782–e793. [Google Scholar] [CrossRef]
- Makled, A.F.; Ali, S.A.M.; Labeeb, A.Z.; Salman, S.S.; Shebl, D.Z.M.; Hegazy, S.G.; Sabal, M.S. Characterization of Candida Species Isolated from Clinical Specimens: Insights into Virulence Traits, Antifungal Resistance and Molecular Profiles. BMC Microbiol. 2024, 24, 388. [Google Scholar] [CrossRef]
- Douglas, A.P.; Stewart, A.G.; Halliday, C.L.; Chen, S.C.-A. Outbreaks of Fungal Infections in Hospitals: Epidemiology, Detection, and Management. J. Fungi 2023, 9, 1059. [Google Scholar] [CrossRef]
- Hato, H.; Sakata, K.; Sato, J.; Hasebe, A.; Yamazaki, Y.; Kitagawa, Y. Factor Associated with Oral Candidiasis Caused by Co-Infection of Candida albicans and Candida glabrata: A Retrospective Study. J. Dent. Sci. 2022, 17, 1458–1461. [Google Scholar] [CrossRef]
- Jampilek, J. Novel Avenues for Identification of New Antifungal Drugs and Current Challenges. Expert Opin. Drug Discov. 2022, 17, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.; Lopez-Ribot, J.L. Screening Repurposing Libraries for Identification of Drugs with Novel Antifungal Activity. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- de Lima Silva, M.G.; de Lima, L.F.; Alencar Fonseca, V.J.; Santos da Silva, L.Y.; Calixto Donelardy, A.C.; de Almeida, R.S.; de Morais Oliveira-Tintino, C.D.; Pereira Bezerra Martins, A.O.B.; Ribeiro-Filho, J.; Bezerra Morais-Braga, M.F.; et al. Enhancing the Antifungal Efficacy of Fluconazole with a Diterpene: Abietic Acid as a Promising Adjuvant to Combat Antifungal Resistance in Candida Spp. Antibiotics 2023, 12, 1565. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The Genus Lycium as Food and Medicine: A Botanical, Ethnobotanical and Historical Review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, S.; Lu, Y.; Hua, Y.; Zhang, F.; Yan, H.; Shang, E.; Wang, H.; Zhang, W.; Duan, J. Lycium barbarum L. Leaves Ameliorate Type 2 Diabetes in Rats by Modulating Metabolic Profiles and Gut Microbiota Composition. Biomed. Pharmacother. 2020, 121, 109559. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, X.; Cao, F.; Guo, Q.; Wang, J. Phytochemicals and Bioactivities of Goji (Lycium barbarum L. and Lycium chinense Mill.) Leaves and Their Potential Applications in the Food Industry: A Review. Int. J. Food Sci. Technol. 2022, 57, 1451–1461. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.; Bischin, C.; Hanganu, D.; Gheldiu, A.-M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic Content, Antioxidant and Antimicrobial Activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Mol. 2014, 19, 10056–10073. [Google Scholar] [CrossRef]
- Lee, S.R.; An, M.-Y.; Hwang, H.-J.; Yoon, J.-G.; Cho, J.A. Antioxidant Effect of Lycium barbarum Leaf through Inflammatory and Endoplasmic Reticulum Stress Mechanism. Antioxidants 2020, 10, 20. [Google Scholar] [CrossRef]
- Xiao, X.; Ren, W.; Zhang, N.; Bing, T.; Liu, X.; Zhao, Z.; Shangguan, D. Comparative Study of the Chemical Constituents and Bioactivities of the Extracts from Fruits, Leaves and Root Barks of Lycium barbarum. Molecules 2019, 24, 1585. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, S.; Yan, H.; Lu, Y.; Zhang, F.; Qian, D.; Wang, H.; Duan, J. Analysis of Phenolic Acids and Flavonoids in Leaves of Lycium barbarum from Different Habitats by Ultra-high-performance Liquid Chromatography Coupled with Triple Quadrupole Tandem Mass Spectrometry. Biomed. Chromatogr. 2019, 33, e4552. [Google Scholar] [CrossRef] [PubMed]
- Passero, P.; Muthular, M.; Barceló, S.; Miozza, V.; Pérez, C. Inhibition of Azole-Resistant Candida albicans ATPase and Oxidoreductase Activity by a Flavonoid from Dalea Elegans. J. Med. Mycol. 2022, 32, 101247. [Google Scholar] [CrossRef] [PubMed]
- Melchor-Martínez, E.M.; Tamez-Fernández, J.F.; González-González, G.M.; Silva-Mares, D.A.; Waksman-Minsky, N.; Pérez-López, L.A.; Rivas-Galindo, V.M. Active Flavonoids from Colubrina greggii Var. greggii S. Watson against Clinical Isolates of Candida spp. Molecules 2021, 26, 5760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Yan, Q.; Ning, Y.; Wang, Y.; Liu, K.; Qiang, Y.; Ma, X.; Sun, X. Establishment of High Performance Liquid Chromatographic Fingerprint and Determination of 4 Kinds of Phenolic Acid Bioactive Substances of Fruitless Lycium barbarum Leaves from Ningxia at Different Harvesting Periods. Heliyon 2024, 10, e24614. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI M27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; ISBN 1-56238-827-4. [Google Scholar]
- CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 4th ed.; CLSI M57S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; ISBN 978-1-68440-159-8. [Google Scholar]
- Yao, D.; Zhang, G.; Chen, W.; Chen, J.; Li, Z.; Zheng, X.; Yin, H.; Hu, X. Pyrogallol and Fluconazole Interact Synergistically In Vitro against Candida glabrata through an Efflux-Associated Mechanism. Antimicrob. Agents Chemother. 2021, 65, e00100-21. [Google Scholar] [CrossRef]
- Olfa, T.; Antonio, D.G.; Sana, A.; Imen, B.S.; Salem, E.; Mohamed Najib, A.; Bruno, C.; Vincenzo, L.; Ferid, L.; Maria Luisa, M. Synergistic Fungicidal Activity of the Lipopeptide Bacillomycin D with Amphotericin B against Pathogenic Candida Species. FEMS Yeast Res. 2015, 15, fov022. [Google Scholar] [CrossRef]
- Wei, C.; Cui, P.; Liu, X. Antibacterial Activity and Mechanism of Madecassic Acid against Staphylococcus aureus. Molecules 2023, 28, 1895. [Google Scholar] [CrossRef]
- Maesaki, S.; Marichal, P.; Bossche, H.V.; Sanglard, D.; Kohno, S. Rhodamine 6G Efflux for the Detection of CDR1-Overexpressing Azole-Resistant Candidaalbicans Strains. J. Antimicrob. Chemother. 1999, 44, 27–31. [Google Scholar] [CrossRef]
- Peng, Y.; Dong, D.; Jiang, C.; Yu, B.; Wang, X.; Ji, Y. Relationship between Respiration Deficiency and Azole Resistance in Clinical Candida glabrata. FEMS Yeast Res. 2012, 12, 719–727. [Google Scholar] [CrossRef]
- Hoenigl, M.; Sprute, R.; Egger, M.; Arastehfar, A.; Cornely, O.A.; Krause, R.; Lass-Flörl, C.; Prattes, J.; Spec, A.; Thompson, G.R.; et al. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs 2021, 81, 1703–1729. [Google Scholar] [CrossRef]
- Zobi, C.; Algul, O. The Significance of Mono- and Dual-Effective Agents in the Development of New Antifungal Strategies. Chem. Biol. Drug Des. 2025, 105, e70045. [Google Scholar] [CrossRef]
- Meccatti, V.M.; Santos, L.F.; de Carvalho, L.S.; Souza, C.B.; Carvalho, C.A.T.; Marcucci, M.C.; Abu Hasna, A.; de Oliveira, L.D. Antifungal Action of Herbal Plants’ Glycolic Extracts against Candida Species. Molecules 2023, 28, 2857. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.B.F.C.; de Oliveira Bento, A.; Lourenço, E.M.G.; Ferreira, M.R.A.; Oliveira, W.N.; Soares, L.A.L.; Barbosa, E.G.; Rocha, H.A.O.; Chaves, G.M. Mechanism of Action and Synergistic Effect of Eugenia Uniflora Extract in Candida Spp. PLoS ONE 2024, 19, e0303878. [Google Scholar] [CrossRef] [PubMed]
- Hervay, N.T.; Elias, D.; Habova, M.; Jacko, J.; Morvova, M.; Gbelska, Y. Catechin Potentiates the Antifungal Effect of Miconazole in Candida glabrata. Folia Microbiol. 2023, 68, 835–842. [Google Scholar] [CrossRef]
- Shen, J.-S.; Wang, Z.-J.; Duan, Y.; Mei, L.-N.; Zhu, Y.-Y.; Wei, M.-Z.; Wang, X.-H.; Luo, X.-D. Antifungal Bioactivity of Sarcococca hookeriana Var. Digyna Franch. against Fluconazole-Resistant Candida albicans in Vitro and in Vivo. J. Ethnopharmacol. 2024, 333, 118473. [Google Scholar] [CrossRef]
- Ma, D.-Y.; Wang, Z.-J.; Chen, Y.-C.; Qi, Z.-H.; Wang, H.; Zhu, Y.-Y.; Luo, X.-D. Antifungal Compounds of Chinese Prickly Ash against Drug-Resistant Candida albicans. Food Chem. X 2022, 15, 100400. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Cao, Y.L.; Wang, Y.H.; Chen, D.J.; Mi, J.; Li, X.Y.; Yan, Y.M.; Zeng, X.X. Analysis of Chemical Compositions and Antioxidant Activities of Lycium barbarum Bud and Leaf Teas. Sci. Technol. Food Ind. 2017, 10, 129–134. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Berman, J.; Krysan, D.J. Drug Resistance and Tolerance in Fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Nakano, K.; Okamoto, M.; Takahashi-Nakaguchi, A.; Sasamoto, K.; Yamaguchi, M.; Chibana, H. Evaluation of Antifungal Selective Toxicity Using Candida glabrata ERG25 and Human SC4MOL Knock-In Strains. J. Fungi 2023, 9, 1035. [Google Scholar] [CrossRef] [PubMed]
- Badrane, H.; Cheng, S.; Dupont, C.L.; Hao, B.; Driscoll, E.; Morder, K.; Liu, G.; Newbrough, A.; Fleres, G.; Kaul, D.; et al. Genotypic Diversity and Unrecognized Antifungal Resistance among Populations of Candida glabrata from Positive Blood Cultures. Nat. Commun. 2023, 14, 5918. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Deshpande, L.M.; Davis, A.P.; Carvalhaes, C.G.; Pfaller, M.A. Azole Resistance in Candida glabrata Clinical Isolates from Global Surveillance Is Associated with Efflux Overexpression. J. Glob. Antimicrob. Resist. 2022, 29, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Chen, J.; Chen, W.; Li, Z.; Hu, X. Mechanisms of Azole Resistance in Clinical Isolates of Candida glabrata from Two Hospitals in China. Infect. Drug Resist. 2019, 12, 771–781. [Google Scholar] [CrossRef]
- Moirangthem, R.; Kumar, K.; Kaur, R. Two Functionally Redundant FK506-Binding Proteins Regulate Multidrug Resistance Gene Expression and Govern Azole Antifungal Resistance. Antimicrob. Agents Chemother. 2021, 65, 10–1128. [Google Scholar] [CrossRef]
- Whaley, S.G.; Zhang, Q.; Caudle, K.E.; Rogers, P.D. Relative Contribution of the ABC Transporters Cdr1, Pdh1, and Snq2 to Azole Resistance in Candida glabrata. Antimicrob. Agents Chemother. 2018, 62, e01070-18. [Google Scholar] [CrossRef]
- Navarro-Rodríguez, P.; Martin-Vicente, A.; López-Fernández, L.; Guarro, J.; Capilla, J. Expression of ERG11 and Efflux Pump Genes CDR1, CDR2 and SNQ2 in Voriconazole Susceptible and Resistant Candida glabrata Strains. Med. Mycol. 2020, 58, 30–38. [Google Scholar] [CrossRef]
- Flowers, S.A.; Barker, K.S.; Berkow, E.L.; Toner, G.; Chadwick, S.G.; Gygax, S.E.; Morschhäuser, J.; Rogers, P.D. Gain-of-Function Mutations in UPC2 Are a Frequent Cause of ERG11 Upregulation in Azole-Resistant Clinical Isolates of Candida albicans. Eukaryot. Cell 2012, 11, 1289–1299. [Google Scholar] [CrossRef]
- Hsu, H.; Sheth, C.C.; Veses, V. Herbal Extracts with Antifungal Activity against Candida albicans: A Systematic Review. Mini-Rev. Med. Chem. 2021, 21, 90–117. [Google Scholar] [CrossRef]

| Genes | Nucleotide Sequence (5′-3′) |
|---|---|
| 18SrRNA-F | TCTTTCTTGATTTTGTGGGTGG |
| 18SrRNA-R | TCGATAGTCCCTCTAAGAAGT |
| CgCDR1-F | TAACCAGGTGGCAGAAGCAG |
| CgCDR1-R | CCACAAGTCAGGTTTGCAGC |
| CgCDR2-F | CAACGCTATGAGGGAAAA |
| CgCDR2-R | AACATAAGTGGCGTGGGT |
| CgPDR1-F | AACAGCTTGCTCTCGACGAA |
| CgPDR1-R | CTTCCACCATAGTAGCCGCC |
| CgERG11-F | ACCAAGAACAAATGCGCGTC |
| CgERG11-R | GTCCCTTGGGACAACGTAGG |
| CgSNQ2-F | CGATGCACCAACCAAGTATG |
| CgSNQ2-R | ACCACCGACAGTCATCAACA |
| Isolates | MIC Alone (μg/mL) | MIC Combination (μg/mL) | FICI | Interpretation | ||
|---|---|---|---|---|---|---|
| FLC | FWE | FLC | FWE | |||
| ATCC 22019 | 0.5 | 64 | 0.25 | 8 | 0.625 | Addition |
| Cg1 | 16 | 32 | 8 | 8 | 0.75 | Addition |
| Cg2 | 16 | 32 | 8 | 4 | 0.625 | Addition |
| Cg3 | 16 | 32 | 4 | 16 | 0.75 | Addition |
| Cg4 | 32 | 16 | 16 | 8 | 1.0 | Indifference |
| Cg5 | 16 | 16 | 4 | 8 | 0.75 | Addition |
| Cg6 | 16 | 32 | 2 | 16 | 0.625 | Addition |
| Cg7 | 8 | 32 | 4 | 8 | 0.75 | Addition |
| Cg8 | 8 | 32 | 4 | 8 | 0.75 | Addition |
| Isolates | MIC Alone (μg/mL) | MIC Combination (μg/mL) | FICI | Interpretation | ||
|---|---|---|---|---|---|---|
| ITR | FWE | ITR | FWE | |||
| ATCC 22019 | 0.125 | 64 | 0.0625 | 8 | 0.625 | Addition |
| Cg1 | 8 | 32 | 1.0 ↓ 8 | 8 | 0.375 | Synergism |
| Cg2 | 8 | 32 | 1.0 | 16 | 0.625 | Addition |
| Cg3 | 8 | 32 | 1.0 ↓ 8 | 8 | 0.375 | Synergism |
| Cg4 | 4 | 16 | 1.0 ↓ 4 | 8 | 0.75 | Addition |
| Cg5 | 1 | 16 | 0.25 ↓ 4 | 8 | 0.75 | Addition |
| Cg6 | 0.5 | 32 | 0.125 ↓ 4 | 16 | 0.75 | Addition |
| Cg7 | 2 | 32 | 0.5 ↓ 4 | 4 ↓ 8 | 0.375 | Synergism |
| Cg8 | 1 | 32 | 0.5 ↓ 2 | 8 ↓ 4 | 0.75 | Addition |
| Isolates | MIC Alone (μg/mL) | MIC Combination (μg/mL) | FICI | Interpretation | ||
|---|---|---|---|---|---|---|
| VRC | FWE | VRC | FWE | |||
| ATCC 22019 | 0.0625 | 64 | 0.0313 | 8 | 0.625 | Addition |
| Cg1 | 1 | 32 | 0.125 ↓ 8 | 8 ↓ 4 | 0.375 | Synergism |
| Cg2 | 0.5 | 32 | 0.125 ↓ 4 | 8 ↓ 4 | 0.5 | Synergism |
| Cg3 | 1 | 32 | 0.25 | 16 | 0.75 | Addition |
| Cg4 | 2 | 16 | 0.5 | 8 | 0.75 | Addition |
| Cg5 | 0.5 | 16 | 0.125 ↓ 4 | 8 | 0.75 | Addition |
| Cg6 | 2 | 32 | 0.25 ↓ 8 | 8 ↓ 4 | 0.375 | Synergism |
| Cg7 | 1 | 32 | 0.125 ↓ 8 | 16 ↓ 2 | 0.625 | Addition |
| Cg8 | 1 | 32 | 0.25 ↓ 4 | 8 ↓ 4 | 0.5 | Synergism |
| Groups | Efflux Level (%) | |
|---|---|---|
| Absence of Glucose | Addition of Glucose | |
| Control | 0.8 | 9.1 |
| FWE | 0.6 | 16.0 |
| ITR | 1.6 | 17.3 |
| VRC | 0.6 | 17.5 |
| FWE + ITR | 0.6 | 11.4 |
| FWE + VRC | 0.6 | 14.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ma, Z.; Zhou, X.; Zhang, Z.; Wu, T. Promising Role of Fruitless Wolfberry Bud Tea in Combating Nakaseomyces glabratus Resistance. Pathogens 2025, 14, 351. https://doi.org/10.3390/pathogens14040351
Zhang L, Ma Z, Zhou X, Zhang Z, Wu T. Promising Role of Fruitless Wolfberry Bud Tea in Combating Nakaseomyces glabratus Resistance. Pathogens. 2025; 14(4):351. https://doi.org/10.3390/pathogens14040351
Chicago/Turabian StyleZhang, Liping, Zhiyan Ma, Xuezhang Zhou, Ziping Zhang, and Tao Wu. 2025. "Promising Role of Fruitless Wolfberry Bud Tea in Combating Nakaseomyces glabratus Resistance" Pathogens 14, no. 4: 351. https://doi.org/10.3390/pathogens14040351
APA StyleZhang, L., Ma, Z., Zhou, X., Zhang, Z., & Wu, T. (2025). Promising Role of Fruitless Wolfberry Bud Tea in Combating Nakaseomyces glabratus Resistance. Pathogens, 14(4), 351. https://doi.org/10.3390/pathogens14040351






