Signaling Pathways Regulating Dimorphism in Medically Relevant Fungal Species
Abstract
1. Introduction
2. Fungal Dimorphism Regulators
2.1. Temperature-Responsive Genes
2.2. Mating Is Associated with Dimorphism
2.3. Signaling Cascades in Dimorphic Fungi
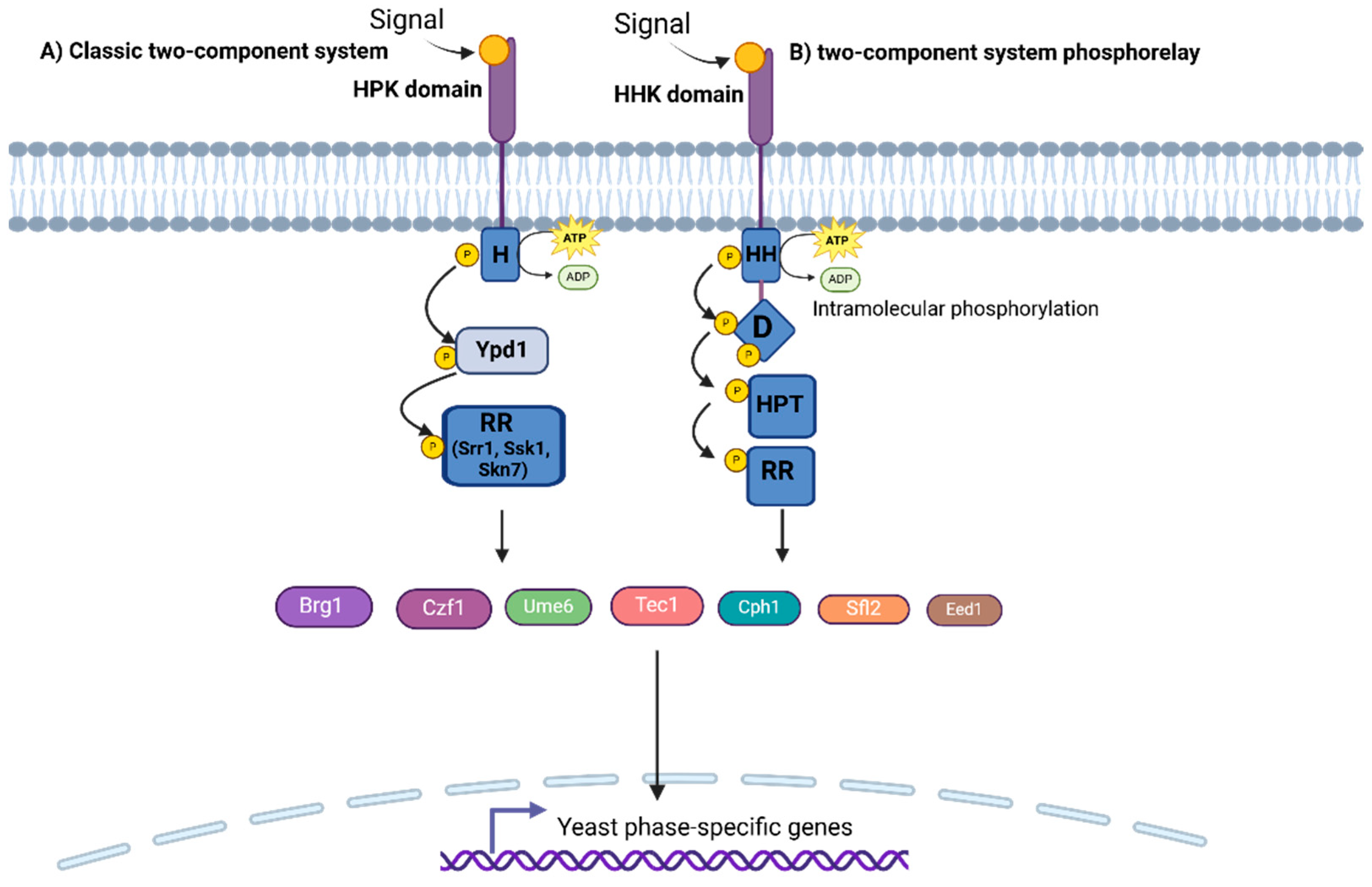
2.3.1. Two-Component Regulatory Systems
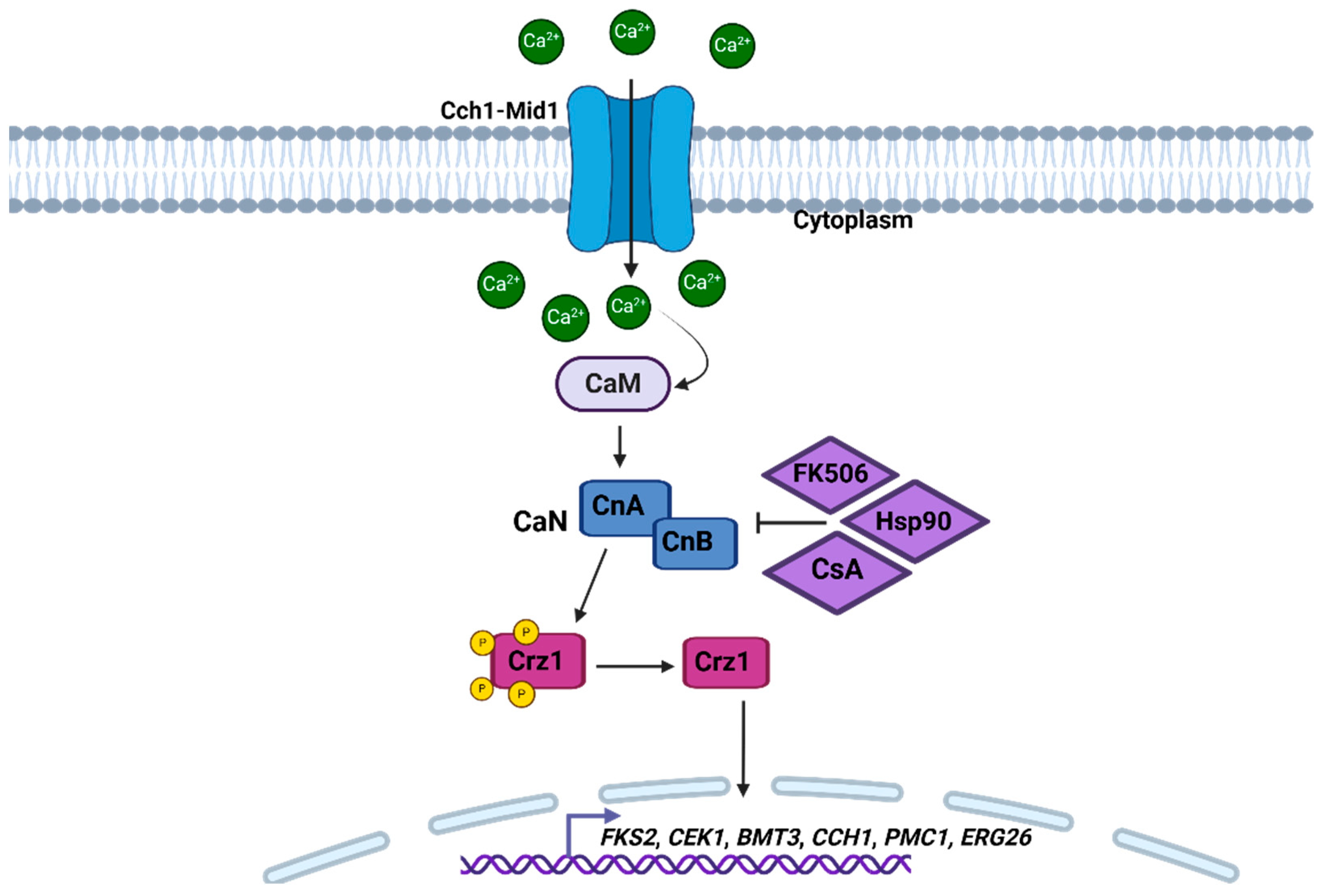
2.3.2. Calcium/Calcineurin Pathway
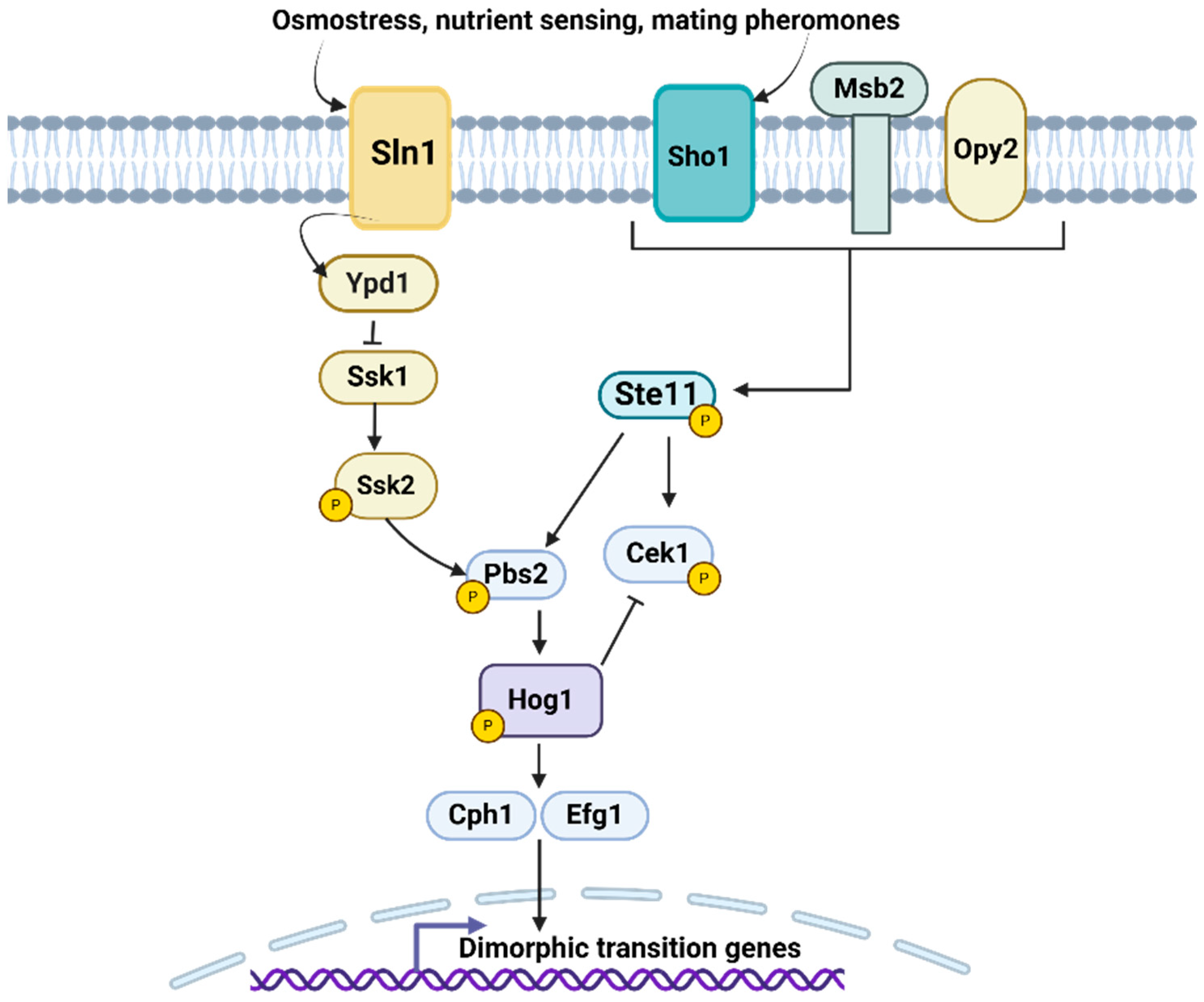
2.3.3. Mitogen-Activated Protein Kinase (MAPK)
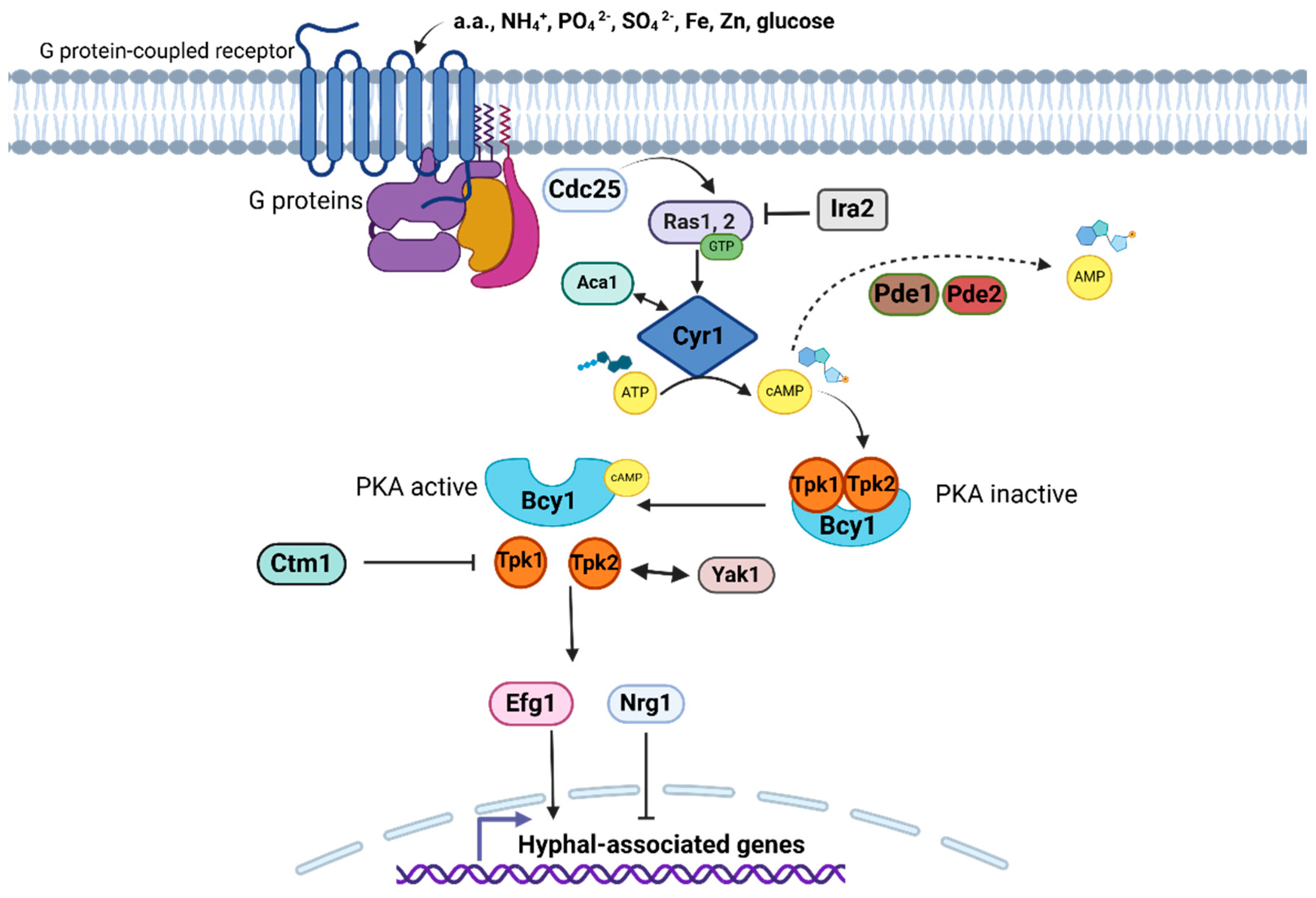
2.3.4. Cyclic AMP-Dependent Protein Kinase A
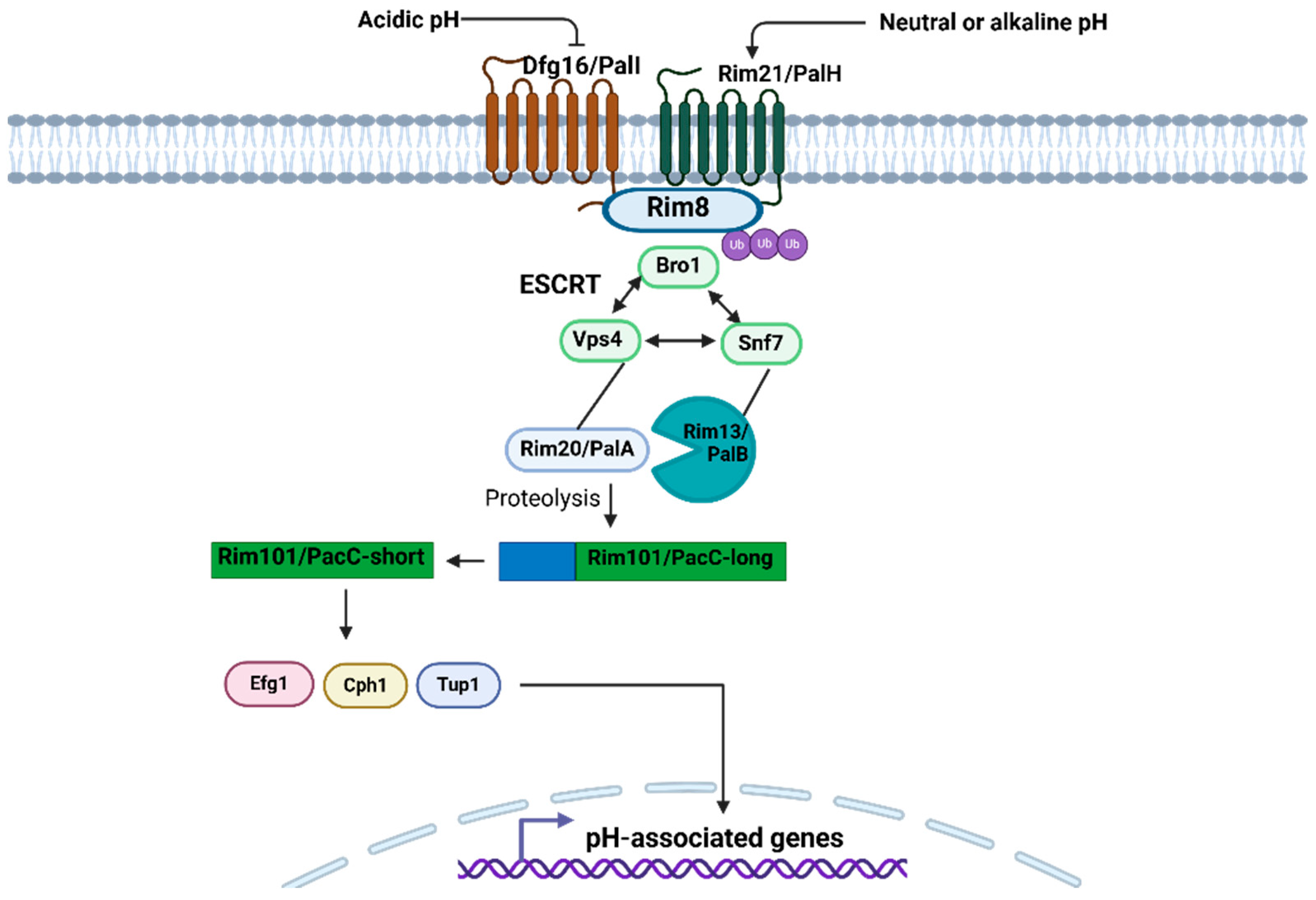
2.3.5. Pal/Rim Pathway
3. Transcriptional Regulation of Fungal Dimorphism
4. Dimorphism Regulators with Therapeutic Potential
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gauthier, G.M. Dimorphism in fungal pathogens of mammals, plants, and insects. PLoS Pathog. 2015, 11, e1004608. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Govender, N.P.; Corcoran, C.; Dlamini, S.; Prozesky, H.; Burton, R.; Mendelson, M.; Taljaard, J.; Lehloenya, R.; Calligaro, G.; et al. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: A retrospective case series. Clin. Infect. Dis. 2015, 61, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.M. Fungal dimorphism and virulence: Molecular mechanisms for temperature adaptation, immune evasion, and in vivo survival. Mediat. Inflamm. 2017, 2017, 8491383. [Google Scholar] [CrossRef]
- Gauthier, G.; Klein, B.S. Insights into fungal morphogenesis and immune evasion: Fungal conidia, when situated in mammalian lungs, may switch from mold to pathogenic yeasts or spore-forming spherules. Microbe 2008, 3, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [Google Scholar] [CrossRef]
- Lee, S.C.; Li, A.; Calo, S.; Heitman, J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 2013, 9, e1003625. [Google Scholar] [CrossRef]
- Jiménez Mdel, P.; Restrepo, A.; Radzioch, D.; Cano, L.E.; García, L.F. Importance of complement 3 and mannose receptors in phagocytosis of Paracoccidioides brasiliensis conidia by Nramp1 congenic macrophages lines. FEMS Immunol. Med. Microbiol. 2006, 47, 56–66. [Google Scholar] [CrossRef]
- Guzman-Beltran, S.; Perez-Torres, A.; Coronel-Cruz, C.; Torres-Guerrero, H. Phagocytic receptors on macrophages distinguish between different Sporothrix schenckii morphotypes. Microbes Infect. 2012, 14, 1093–1101. [Google Scholar] [CrossRef]
- McKenzie, C.G.; Koser, U.; Lewis, L.E.; Bain, J.M.; Mora-Montes, H.M.; Barker, R.N.; Gow, N.A.; Erwig, L.P. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect. Immun. 2010, 78, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.F.; Bain, J.M.; Erwig, L.P.; Brown, A.J.P.; Gow, N.A.R. Hyphal swelling induced in the phagosome of macrophages. Fungal Biol. 2024, 128, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- García-Carnero, L.C.; Martínez-Duncker, I.; Gómez-Gaviria, M.; Mora-Montes, H.M. Differential recognition of clinically relevant Sporothrix species by human mononuclear cells. J. Fungi 2023, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, J.A.; Pérez-García, L.A.; Mellado-Mojica, E.; López, M.G.; Martínez-Duncker, I.; Lópes-Bezerra, L.M.; Mora-Montes, H.M. Sporothrix schenckii sensu stricto and Sporothrix brasiliensis are differentially recognized by human peripheral blood mononuclear cells. Front. Microbiol. 2017, 8, 843. [Google Scholar] [CrossRef]
- Mukaremera, L.; Lee, K.K.; Mora-Montes, H.M.; Gow, N.A.R. Candida albicans yeast, pseudohyphal, and hyphal morphogenesis differentially affects immune recognition. Front. Immunol. 2017, 8, 629. [Google Scholar] [CrossRef]
- Villalobos-Duno, H.L.; Barreto, L.A.; Alvarez-Aular, Á.; Mora-Montes, H.M.; Lozoya-Pérez, N.E.; Franco, B.; Lopes-Bezerra, L.M.; Niño-Vega, G.A. Comparison of cell wall polysaccharide composition and structure between strains of Sporothrix schenckii and Sporothrix brasiliensis. Front. Microbiol. 2021, 12, 726958. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005, 24, 1277–1286. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef]
- Gilmore, S.A.; Naseem, S.; Konopka, J.B.; Sil, A. N-acetylglucosamine (GlcNAc) triggers a rapid, temperature-responsive morphogenetic program in thermally dimorphic fungi. PLoS Genet. 2013, 9, e1003799. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Sil, A. Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc. Natl. Acad. Sci. USA 2008, 105, 4880–4885. [Google Scholar] [CrossRef]
- Webster, R.H.; Sil, A. Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum. Proc. Natl. Acad. Sci. USA 2008, 105, 14573–14578. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.M.; Sullivan, T.D.; Gallardo, S.S.; Brandhorst, T.T.; Vanden Wymelenberg, A.J.; Cuomo, C.A.; Suen, G.; Currie, C.R.; Klein, B.S. SREB, a GATA transcription factor that directs disparate fates in Blastomyces dermatitidis including morphogenesis and siderophore biosynthesis. PLoS Pathog. 2010, 6, e1000846. [Google Scholar] [CrossRef]
- Chao, L.Y.; Marletta, M.A.; Rine, J. Sre1, an iron-modulated GATA DNA-binding protein of iron-uptake genes in the fungal pathogen Histoplasma capsulatum. Biochemistry 2008, 47, 7274–7283. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, C.; Konopka, J.B. A Candida albicans temperature-sensitive cdc12-6 mutant identifies roles for septins in selection of sites of germ tube formation and hyphal morphogenesis. Eukaryot. Cell 2012, 11, 1210–1218. [Google Scholar] [CrossRef]
- González-Novo, A.; Correa-Bordes, J.; Labrador, L.; Sánchez, M.; Vázquez de Aldana, C.R.; Jiménez, J. Sep7 is essential to modify septin ring dynamics and inhibit cell separation during Candida albicans hyphal growth. Mol. Biol. Cell 2008, 19, 1509–1518. [Google Scholar] [CrossRef]
- Warenda, A.J.; Konopka, J.B. Septin function in Candida albicans morphogenesis. Mol. Biol. Cell 2002, 13, 2732–2746. [Google Scholar] [CrossRef]
- Saraswat, D.; Kumar, R.; Pande, T.; Edgerton, M.; Cullen, P.J. Signalling mucin Msb2 regulates adaptation to thermal stress in Candida albicans. Mol. Microbiol. 2016, 100, 425–441. [Google Scholar] [CrossRef]
- Boyce, K.J.; Andrianopoulos, A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol. Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef]
- Beyhan, S.; Gutierrez, M.; Voorhies, M.; Sil, A. A temperature-responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol. 2013, 11, e1001614. [Google Scholar] [CrossRef]
- Cleare, L.G.; Zamith-Miranda, D.; Nosanchuk, J.D. Heat shock proteins in Histoplasma and Paracoccidioides. Clin. Vaccine Immunol. 2017, 24, e00221-00217. [Google Scholar] [CrossRef]
- Caruso, M.; Sacco, M.; Medoff, G.; Maresca, B. Heat shock 70 gene is differentially expressed in Histoplasma capsulatum strains with different levels of thermotolerance and pathogenicity. Mol. Microbiol. 1987, 1, 151–158. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.P.; Borges-Walmsley, M.I.; Pereira, I.S.; Soares, C.M.; Walmsley, A.R.; Felipe, M.S. Differential expression of an hsp70 gene during transition from the mycelial to the infective yeast form of the human pathogenic fungus Paracoccidioides brasiliensis. Mol. Microbiol. 1999, 31, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Cowen, L.E. Roles of Hsp90 in Candida albicans morphogenesis and virulence. Curr. Opin. Microbiol. 2023, 75, 102351. [Google Scholar] [CrossRef]
- Minchiotti, G.; Gargano, S.; Maresca, B. The intron-containing hsp82 gene of the dimorphic pathogenic fungus Histoplasma capsulatum is properly spliced in severe heat shock conditions. Mol. Cell Biol. 1991, 11, 5624–5630. [Google Scholar] [CrossRef]
- Edwards, J.A.; Zemska, O.; Rappleye, C.A. Discovery of a role for Hsp82 in Histoplasma virulence through a quantitative screen for macrophage lethality. Infect. Immun. 2011, 79, 3348–3357. [Google Scholar] [CrossRef]
- Rocha, M.C.; Minari, K.; Fabri, J.; Kerkaert, J.D.; Gava, L.M.; da Cunha, A.F.; Cramer, R.A.; Borges, J.C.; Malavazi, I. Aspergillus fumigatus Hsp90 interacts with the main components of the cell wall integrity pathway and cooperates in heat shock and cell wall stress adaptation. Cell Microbiol. 2021, 23, e13273. [Google Scholar] [CrossRef]
- Nicola, A.M.; Andrade, R.V.; Dantas, A.S.; Andrade, P.A.; Arraes, F.B.; Fernandes, L.; Silva-Pereira, I.; Felipe, M.S. The stress responsive and morphologically regulated hsp90 gene from Paracoccidioides brasiliensis is essential to cell viability. BMC Microbiol. 2008, 8, 158. [Google Scholar] [CrossRef]
- Matos, T.G.; Morais, F.V.; Campos, C.B. Hsp90 regulates Paracoccidioides brasiliensis proliferation and ROS levels under thermal stress and cooperates with calcineurin to control yeast to mycelium dimorphism. Med. Mycol. 2013, 51, 413–421. [Google Scholar] [CrossRef]
- Morrow, C.A.; Fraser, J.A. Sexual reproduction and dimorphism in the pathogenic basidiomycetes. FEMS Yeast Res. 2009, 9, 161–177. [Google Scholar] [CrossRef]
- Lee, H.; Chang, Y.C.; Kwon-Chung, K.J. TUP1 disruption reveals biological differences between MATa and MATα strains of Cryptococcus neoformans. Mol. Microbiol. 2005, 55, 1222–1232. [Google Scholar] [CrossRef]
- Fraser, J.A.; Heitman, J. Fungal mating-type loci. Curr. Biol. 2003, 13, R792–R795. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Penoyer, L.A.; Kwon-Chung, K.J. The second STE12 homologue of Cryptococcus neoformans is MATa-specific and plays an important role in virulence. Proc. Natl. Acad. Sci. USA 2001, 98, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Lin, X. Cryptococcus neoformans: Morphogenesis, infection, and evolution. Infect. Genet. Evol. 2009, 9, 401–416. [Google Scholar] [CrossRef]
- Roberts, R.L.; Fink, G.R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: Mating and invasive growth. Genes Dev. 1994, 8, 2974–2985. [Google Scholar] [CrossRef]
- Chang, Y.C.; Wickes, B.L.; Miller, G.F.; Penoyer, L.A.; Kwon-Chung, K.J. Cryptococcus neoformans STE12alpha regulates virulence but is not essential for mating. J. Exp. Med. 2000, 191, 871–882. [Google Scholar] [CrossRef]
- Hull, C.M.; Boily, M.J.; Heitman, J. Sex-specific homeodomain proteins Sxi1alpha and Sxi2a coordinately regulate sexual development in Cryptococcus neoformans. Eukaryot. Cell 2005, 4, 526–535. [Google Scholar] [CrossRef]
- Butler, G.; Rasmussen, M.D.; Lin, M.F.; Santos, M.A.; Sakthikumar, S.; Munro, C.A.; Rheinbay, E.; Grabherr, M.; Forche, A.; Reedy, J.L.; et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 2009, 459, 657–662. [Google Scholar] [CrossRef]
- Bennett, R.J.; Turgeon, B.G. Fungal sex: The Ascomycota. Microbiol. Spectr. 2016, 4, 10.1128. [Google Scholar] [CrossRef]
- Reedy, J.L.; Floyd, A.M.; Heitman, J. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr. Biol. 2009, 19, 891–899. [Google Scholar] [CrossRef]
- Sherwood, R.K.; Scaduto, C.M.; Torres, S.E.; Bennett, R.J. Convergent evolution of a fused sexual cycle promotes the haploid lifestyle. Nature 2014, 506, 387–390. [Google Scholar] [CrossRef]
- Kvaal, C.A.; Srikantha, T.; Soll, D.R. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect. Immun. 1997, 65, 4468–4475. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Johnson, A.D. Differential phagocytosis of white versus opaque Candida albicans by Drosophila and mouse phagocytes. PLoS ONE 2008, 3, e1473. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Hernday, A.D.; Hirakawa, M.P.; Johnson, A.D.; Bennett, R.J. Candida albicans white and opaque cells undergo distinct programs of filamentous growth. PLoS Pathog. 2013, 9, e1003210. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Nicolet, J. Specificity models in MAPK cascade signaling. FEBS Open Bio 2023, 13, 1177–1192. [Google Scholar] [CrossRef]
- Braunsdorf, C.; Mailänder-Sánchez, D.; Schaller, M. Fungal sensing of host environment. Cell Microbiol. 2016, 18, 1188–1200. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Xue, C.; Idnurm, A.; Rutherford, J.C.; Heitman, J.; Cardenas, M.E. Sensing the environment: Lessons from fungi. Nat. Rev. Microbiol. 2007, 5, 57–69. [Google Scholar] [CrossRef]
- Xue, C.; Hsueh, Y.P.; Heitman, J. Magnificent seven: Roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol. Rev. 2008, 32, 1010–1032. [Google Scholar] [CrossRef]
- Basso, V.; Znaidi, S.; Lagage, V.; Cabral, V.; Schoenherr, F.; LeibundGut-Landmann, S.; d’Enfert, C.; Bachellier-Bassi, S. The two-component response regulator Skn7 belongs to a network of transcription factors regulating morphogenesis in Candida albicans and independently limits morphogenesis-induced ROS accumulation. Mol. Microbiol. 2017, 106, 157–182. [Google Scholar] [CrossRef]
- Liao, B.; Ye, X.; Chen, X.; Zhou, Y.; Cheng, L.; Zhou, X.; Ren, B. The two-component signal transduction system and its regulation in Candida albicans. Virulence 2021, 12, 1884–1899. [Google Scholar] [CrossRef]
- Chaves, A.F.; Navarro, M.V.; Castilho, D.G.; Calado, J.C.; Conceição, P.M.; Batista, W.L. A conserved dimorphism-regulating histidine kinase controls the dimorphic switching in Paracoccidioides brasiliensis. FEMS Yeast Res. 2016, 16, fow047. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, Z.; Zheng, F.; Liu, X. Molecular cloning, characterization and differential expression of DRK1 in Sporothrix schenckii. Int. J. Mol. Med. 2013, 31, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hou, B.; Wu, Y.Z.; Wang, Y.; Liu, X.; Han, S. Two-component histidine kinase DRK1 is required for pathogenesis in Sporothrix schenckii. Mol. Med. Rep. 2018, 17, 721–728. [Google Scholar] [CrossRef]
- Desai, C.; Mavrianos, J.; Chauhan, N. Candida albicans SRR1, a putative two-component response regulator gene, is required for stress adaptation, morphogenesis, and virulence. Eukaryot. Cell 2011, 10, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Mavrianos, J.; Desai, C.; Chauhan, N. Two-component histidine phosphotransfer protein Ypd1 is not essential for viability in Candida albicans. Eukaryot. Cell 2014, 13, 452–460. [Google Scholar] [CrossRef]
- Kruppa, M.; Calderone, R. Two-component signal transduction in human fungal pathogens. FEMS Yeast Res. 2006, 6, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Okabe, T.; Mio, T.; Ono, N.; Kashima, Y.; Matsui, M.; Arisawa, M.; Yamada-Okabe, H. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J. Bacteriol. 1999, 181, 7243–7247. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, Y.J.; Kim, S.Y.; Bahn, Y.S. Multiple roles of Ypd1 phosphotransfer protein in viability, stress response, and virulence factor regulation in Cryptococcus neoformans. Eukaryot. Cell 2011, 10, 998–1002. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Ault, A.; Malone, C.L.; Raitt, D.; Dean, S.; Johnston, L.H.; Deschenes, R.J.; Fassler, J.S. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 1998, 17, 6952–6962. [Google Scholar] [CrossRef]
- Chauvel, M.; Nesseir, A.; Cabral, V.; Znaidi, S.; Goyard, S.; Bachellier-Bassi, S.; Firon, A.; Legrand, M.; Diogo, D.; Naulleau, C.; et al. A versatile overexpression strategy in the pathogenic yeast Candida albicans: Identification of regulators of morphogenesis and fitness. PLoS ONE 2012, 7, e45912. [Google Scholar] [CrossRef]
- Capra, E.J.; Laub, M.T. Evolution of two-component signal transduction systems. Annu. Rev. Microbiol. 2012, 66, 325–347. [Google Scholar] [CrossRef]
- Tebbets, B.; Yu, Z.; Stewart, D.; Zhao, L.X.; Jiang, Y.; Xu, L.H.; Andes, D.; Shen, B.; Klein, B. Identification of antifungal natural products via Saccharomyces cerevisiae bioassay: Insights into macrotetrolide drug spectrum, potency and mode of action. Med. Mycol. 2013, 51, 280–289. [Google Scholar] [CrossRef]
- Kennedy, E.N.; Menon, S.K.; West, A.H. Extended N-terminal region of the essential phosphorelay signaling protein Ypd1 from Cryptococcus neoformans contributes to structural stability, phosphostability and binding of calcium ions. FEMS Yeast Res. 2016, 16, fow068. [Google Scholar] [CrossRef] [PubMed]
- Colinet, A.S.; Sengottaiyan, P.; Deschamps, A.; Colsoul, M.L.; Thines, L.; Demaegd, D.; Duchêne, M.C.; Foulquier, F.; Hols, P.; Morsomme, P. Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci. Rep. 2016, 6, 24282. [Google Scholar] [CrossRef]
- Xu, J.R. Map kinases in fungal pathogens. Fungal Genet. Biol. 2000, 31, 137–152. [Google Scholar] [CrossRef]
- Miskei, M.; Karányi, Z.; Pócsi, I. Annotation of stress-response proteins in the aspergilli. Fungal Genet. Biol. 2009, 46 (Suppl. S1), S105–S120. [Google Scholar] [CrossRef]
- Matsumoto, T.K.; Ellsmore, A.J.; Cessna, S.G.; Low, P.S.; Pardo, J.M.; Bressan, R.A.; Hasegawa, P.M. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 33075–33080. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; Wu, J.; Han, J.; Li, S.; Shao, S. Transcriptomic analysis reveals genes mediating salt tolerance through calcineurin/CchA-Independent signaling in Aspergillus nidulans. Biomed. Res. Int. 2017, 2017, 4378627. [Google Scholar] [CrossRef]
- Mendoza, I.; Rubio, F.; Rodriguez-Navarro, A.; Pardo, J.M. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 1994, 269, 8792–8796. [Google Scholar]
- Fox, D.S.; Cruz, M.C.; Sia, R.A.; Ke, H.; Cox, G.M.; Cardenas, M.E.; Heitman, J. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 2001, 39, 835–849. [Google Scholar] [CrossRef]
- Stie, J.; Fox, D. Calcineurin regulation in fungi and beyond. Eukaryot. Cell 2008, 7, 177–186. [Google Scholar] [CrossRef]
- Kothe, G.O.; Free, S.J. Calcineurin subunit B is required for normal vegetative growth in Neurospora crassa. Fungal Genet. Biol. 1998, 23, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.C.; Goldstein, A.L.; Blankenship, J.R.; Del Poeta, M.; Davis, D.; Cardenas, M.E.; Perfect, J.R.; McCusker, J.H.; Heitman, J. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 2002, 21, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Chávez, J.A.; Ali, S.; Bakkeren, G. Response to environmental stresses, cell-wall integrity, and virulence are orchestrated through the calcineurin pathway in Ustilago hordei. Mol. Plant Microbe Interact. 2011, 24, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Garfoot, A.L.; Rappleye, C.A. Histoplasma capsulatum surmounts obstacles to intracellular pathogenesis. FEBS J. 2016, 283, 619–633. [Google Scholar] [CrossRef]
- Li, W.; Shrivastava, M.; Lu, H.; Jiang, Y. Calcium-calcineurin signaling pathway in Candida albicans: A potential drug target. Microbiol. Res. 2021, 249, 126786. [Google Scholar] [CrossRef]
- Bader, T.; Bodendorfer, B.; Schröppel, K.; Morschhäuser, J. Calcineurin is essential for virulence in Candida albicans. Infect. Immun. 2003, 71, 5344–5354. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot. Cell 2015, 14, 324–334. [Google Scholar] [CrossRef]
- Young, J.C.; Moarefi, I.; Hartl, F.U. Hsp90: A specialized but essential protein-folding tool. J. Cell Biol. 2001, 154, 267–273. [Google Scholar] [CrossRef]
- Onyewu, C.; Blankenship, J.R.; Del Poeta, M.; Heitman, J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 2003, 47, 956–964. [Google Scholar] [CrossRef]
- Li, L.; An, M.; Shen, H.; Huang, X.; Yao, X.; Liu, J.; Zhu, F.; Zhang, S.; Chen, S.; He, L.; et al. The non-Geldanamycin Hsp90 inhibitors enhanced the antifungal activity of fluconazole. Am. J. Transl. Res. 2015, 7, 2589–2602. [Google Scholar]
- Yu, Q.; Wang, H.; Cheng, X.; Xu, N.; Ding, X.; Xing, L.; Li, M. Roles of Cch1 and Mid1 in morphogenesis, oxidative stress response and virulence in Candida albicans. Mycopathologia 2012, 174, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Schlenstedt, G.; Flockerzi, V.; Beck, A. Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1. FEBS Lett. 2010, 584, 2028–2032. [Google Scholar] [CrossRef]
- Chen, Y.; Mallick, J.; Maqnas, A.; Sun, Y.; Choudhury, B.I.; Côte, P.; Yan, L.; Ni, T.J.; Li, Y.; Zhang, D.; et al. Chemogenomic profiling of the fungal pathogen Candida albicans. Antimicrob. Agents Chemother. 2018, 62, e02365-02317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, D.; Hameed, A.; Fang, T.; Bakr Ahmad Fazili, A.; Asghar, F. The plasma membrane protein Rch1 and the Golgi/ER calcium pump Pmr1 have an additive effect on filamentation in Candida albicans. Fungal Genet. Biol. 2018, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luna-Tapia, A.; DeJarnette, C.; Sansevere, E.; Reitler, P.; Butts, A.; Hevener, K.E.; Palmer, G.E. The vacuolar Ca(2+) ATPase pump Pmc1p is required for Candida albicans pathogenesis. mSphere 2019, 4, e00715-00718. [Google Scholar] [CrossRef]
- Karababa, M.; Valentino, E.; Pardini, G.; Coste, A.T.; Bille, J.; Sanglard, D. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 2006, 59, 1429–1451. [Google Scholar] [CrossRef]
- Blankenship, J.R.; Wormley, F.L.; Boyce, M.K.; Schell, W.A.; Filler, S.G.; Perfect, J.R.; Heitman, J. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2003, 2, 422–430. [Google Scholar] [CrossRef]
- Chen, Y.L.; Brand, A.; Morrison, E.L.; Silao, F.G.; Bigol, U.G.; Malbas, F.F., Jr.; Nett, J.E.; Andes, D.R.; Solis, N.V.; Filler, S.G.; et al. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot. Cell 2011, 10, 803–819. [Google Scholar] [CrossRef]
- Odom, A.; Muir, S.; Lim, E.; Toffaletti, D.L.; Perfect, J.; Heitman, J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997, 16, 2576–2589. [Google Scholar] [CrossRef]
- Kraus, P.R.; Nichols, C.B.; Heitman, J. Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot. Cell 2005, 4, 1079–1087. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Cramer, R.A., Jr.; Perfect, B.Z.; Asfaw, Y.G.; Sauer, T.C.; Najvar, L.K.; Kirkpatrick, W.R.; Patterson, T.F.; Benjamin, D.K., Jr.; Heitman, J.; et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 2006, 5, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Fortwendel, J.R.; Rogg, L.E.; Burns, K.A.; Randell, S.H.; Steinbach, W.J. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol. Microbiol. 2011, 82, 1235–1259. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Lamoth, F.; Steinbach, W.J. Calcineurin as a multifunctional regulator: Unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol. Rev. 2014, 28, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.B.; Di Benedette, J.P.; Morais, F.V.; Ovalle, R.; Nobrega, M.P. Evidence for the role of calcineurin in morphogenesis and calcium homeostasis during mycelium-to-yeast dimorphism of Paracoccidioides brasiliensis. Eukaryot. Cell 2008, 7, 1856–1864. [Google Scholar] [CrossRef]
- García-Carnero, L.C.; Martínez-Álvarez, J.A. Virulence factors of Sporothrix schenckii. J. Fungi 2022, 8, 318. [Google Scholar] [CrossRef]
- Zhao, X.; Mehrabi, R.; Xu, J.R. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell 2007, 6, 1701–1714. [Google Scholar] [CrossRef]
- May, G.S.; Xue, T.; Kontoyiannis, D.P.; Gustin, M.C. Mitogen activated protein kinases of Aspergillus fumigatus. Med. Mycol. 2005, 43, S83–S86. [Google Scholar] [CrossRef]
- Monge, R.A.; Román, E.; Nombela, C.; Pla, J. The MAP kinase signal transduction network in Candida albicans. Microbiology 2006, 152, 905–912. [Google Scholar] [CrossRef]
- Román, E.; Correia, I.; Prieto, D.; Alonso, R.; Pla, J. The HOG MAPK pathway in Candida albicans: More than an osmosensing pathway. Int. Microbiol. 2020, 23, 23–29. [Google Scholar] [CrossRef]
- Lo, H.J.; Köhler, J.R.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef]
- Braun, B.R.; Head, W.S.; Wang, M.X.; Johnson, A.D. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 2000, 156, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Csank, C.; Schröppel, K.; Leberer, E.; Harcus, D.; Mohamed, O.; Meloche, S.; Thomas, D.Y.; Whiteway, M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 1998, 66, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, K.; Kim, J.; Yee, S.; Kim, W.; Choi, W. A MAP kinase pathway is implicated in the pseudohyphal induction by hydrogen peroxide in Candica albicans. Mol. Cells 2012, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Arana, D.M.; Nombela, C.; Alonso-Monge, R.; Pla, J. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology 2005, 151, 1033–1049. [Google Scholar] [CrossRef]
- Román, E.; Nombela, C.; Pla, J. The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans. Mol. Cell Biol. 2005, 25, 10611–10627. [Google Scholar] [CrossRef]
- Alonso-Monge, R.; Navarro-García, F.; Molero, G.; Diez-Orejas, R.; Gustin, M.; Pla, J.; Sánchez, M.; Nombela, C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 1999, 181, 3058–3068. [Google Scholar] [CrossRef]
- Du, C.; Sarfati, J.; Latge, J.P.; Calderone, R. The role of the sakA (Hog1) and tcsB (sln1) genes in the oxidant adaptation of Aspergillus fumigatus. Med. Mycol. 2006, 44, 211–218. [Google Scholar] [CrossRef]
- Ma, D.; Li, R. Current understanding of HOG-MAPK pathway in Aspergillus fumigatus. Mycopathologia 2013, 175, 13–23. [Google Scholar] [CrossRef]
- Jang, Y.-B.; Kim, J.-Y.; Bahn, Y.-S. Unraveling the cryptic functions of mitogen-activated protein kinases Cpk2 and Mpk2 in Cryptococcus neoformans. mBio 2024, 15, e01156-01124. [Google Scholar] [CrossRef]
- González-Rubio, G.; Fernández-Acero, T.; Martín, H.; Molina, M. Mitogen-activated protein kinase phosphatases (MKPs) in fungal signaling: Conservation, function, and regulation. Int. J. Mol. Sci. 2019, 20, 1709. [Google Scholar] [CrossRef]
- Sil, A. Molecular regulation of Histoplasma dimorphism. Curr. Opin. Microbiol. 2019, 52, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jung, W.H.; Kronstad, J.W. The cAMP/protein kinase A signaling pathway in pathogenic basidiomycete fungi: Connections with iron homeostasis. J. Microbiol. 2015, 53, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Leberer, E.; Harcus, D.; Dignard, D.; Johnson, L.; Ushinsky, S.; Thomas, D.Y.; Schröppel, K. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 2001, 42, 673–687. [Google Scholar] [CrossRef]
- Caza, M.; Kronstad, J.W. The cAMP/protein kinase a pathway regulates virulence and adaptation to host conditions in Cryptococcus neoformans. Front. Cell Infect. Microbiol. 2019, 9, 212. [Google Scholar] [CrossRef]
- Chen, D.; Janganan, T.K.; Chen, G.; Marques, E.R.; Kress, M.R.; Goldman, G.H.; Walmsley, A.R.; Borges-Walmsley, M.I. The cAMP pathway is important for controlling the morphological switch to the pathogenic yeast form of Paracoccidioides brasiliensis. Mol. Microbiol. 2007, 65, 761–779. [Google Scholar] [CrossRef] [PubMed]
- Zaremberg, V.; Donella-Deana, A.; Moreno, S. Mechanism of activation of cAMP-dependent protein kinase: In Mucor rouxii the apparent specific activity of the cAMP-activated holoenzyme is different than that of its free catalytic subunit. Arch. Biochem. Biophys. 2000, 381, 74–82. [Google Scholar] [CrossRef][Green Version]
- Zeng, G.; Xu, X.; Kok, Y.J.; Deng, F.S.; Ling Chow, E.W.; Gao, J.; Bi, X.; Wang, Y. Cytochrome c regulates hyphal morphogenesis by interfering with cAMP-PKA signaling in Candida albicans. Cell Rep. 2023, 42, 113473. [Google Scholar] [CrossRef]
- Zuber, S.; Hynes, M.J.; Andrianopoulos, A. G-protein signaling mediates asexual development at 25 degrees C but has no effect on yeast-like growth at 37 degrees C in the dimorphic fungus Penicillium mameffei. Eukaryot. Cell 2002, 1, 440–447. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Q.; Wei, Y.; Wang, Y.; Du, H. Multiple roles and diverse regulation of the Ras/cAMP/protein kinase A pathway in Candida albicans. Mol. Microbiol. 2019, 111, 6–16. [Google Scholar] [CrossRef]
- Wijnants, S.; Vreys, J.; Nysten, J.; Van Dijck, P. The Cdc25 and Ras1 proteins of Candida albicans Influence epithelial toxicity in a niche-specific way. J. Fungi 2023, 9, 201. [Google Scholar] [CrossRef]
- Piispanen, A.E.; Bonnefoi, O.; Carden, S.; Deveau, A.; Bassilana, M.; Hogan, D.A. Roles of Ras1 membrane localization during Candida albicans hyphal growth and farnesol response. Eukaryot. Cell 2011, 10, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fang, H.M.; Wang, Y.M.; Zeng, G.S.; Zheng, X.D.; Wang, Y. Ras1 and Ras2 play antagonistic roles in regulating cellular cAMP level, stationary-phase entry and stress response in Candida albicans. Mol. Microbiol. 2009, 74, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Ramírez-Sotelo, U.; Mora-Montes, H.M. Non-albicans Candida species: Immune response, evasion mechanisms, and new plant-derived alternative therapies. J. Fungi 2022, 9, 11. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Hicks, J.K.; Giles, S.S.; Cox, G.M.; Heitman, J. Adenylyl cyclase-associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade. Eukaryot. Cell 2004, 3, 1476–1491. [Google Scholar] [CrossRef]
- Mogensen, E.G.; Janbon, G.; Chaloupka, J.; Steegborn, C.; Fu, M.S.; Moyrand, F.; Klengel, T.; Pearson, D.S.; Geeves, M.A.; Buck, J.; et al. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot. Cell 2006, 5, 103–111. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bahn, Y.S.; Heitman, J. Pde1 phosphodiesterase modulates cyclic AMP levels through a protein kinase A-mediated negative feedback loop in Cryptococcus neoformans. Eukaryot. Cell 2005, 4, 1971–1981. [Google Scholar] [CrossRef]
- Davis-Hanna, A.; Piispanen, A.E.; Stateva, L.I.; Hogan, D.A. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol. Microbiol. 2008, 67, 47–62. [Google Scholar] [CrossRef]
- MacAlpine, J.; Liu, Z.; Hossain, S.; Whitesell, L.; Robbins, N.; Cowen, L.E. DYRK-family kinases regulate Candida albicans morphogenesis and virulence through the Ras1/PKA pathway. mBio 2023, 14, e0218323. [Google Scholar] [CrossRef]
- Giusani, A.D.; Vinces, M.; Kumamoto, C.A. Invasive filamentous growth of Candida albicans is promoted by Czf1p-dependent relief of Efg1p-mediated repression. Genetics 2002, 160, 1749–1753. [Google Scholar] [CrossRef]
- Biswas, S.; Van Dijck, P.; Datta, A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 2007, 71, 348–376. [Google Scholar] [CrossRef]
- Glazier, V.E. EFG1, everyone’s favorite gene in Candida albicans: A comprehensive literature review. Front. Cell Infect. Microbiol. 2022, 12, 855229. [Google Scholar] [CrossRef]
- Su, C.; Yu, J.; Lu, Y. Hyphal development in Candida albicans from different cell states. Curr. Genet. 2018, 64, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.; Ko, Y.J.; Kim, G.B.; Jung, K.W.; Floyd, A.; Heitman, J.; Bahn, Y.S. Comparative transcriptome analysis reveals novel roles of the Ras and cyclic AMP signaling pathways in environmental stress response and antifungal drug sensitivity in Cryptococcus neoformans. Eukaryot. Cell 2010, 9, 360–378. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Jung, K.W. Stress signaling pathways for the pathogenicity of Cryptococcus. Eukaryot. Cell 2013, 12, 1564–1577. [Google Scholar] [CrossRef]
- Maliehe, M.; Ntoi, M.A.; Lahiri, S.; Folorunso, O.S.; Ogundeji, A.O.; Pohl, C.H.; Sebolai, O.M. Environmental factors that contribute to the maintenance of Cryptococcus neoformans pathogenesis. Microorganisms 2020, 8, 180. [Google Scholar] [CrossRef]
- Selvig, K.; Alspaugh, J.A. pH Response pathways in fungi: Adapting to host-derived and environmental signals. Mycobiology 2011, 39, 249–256. [Google Scholar] [CrossRef]
- Cervantes-Chávez, J.A.; Ortiz-Castellanos, L.; Tejeda-Sartorius, M.; Gold, S.; Ruiz-Herrera, J. Functional analysis of the pH responsive pathway Pal/Rim in the phytopathogenic basidiomycete Ustilago maydis. Fungal Genet. Biol. 2010, 47, 446–457. [Google Scholar] [CrossRef]
- Davis, D.; Wilson, R.B.; Mitchell, A.P. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol. Cell Biol. 2000, 20, 971–978. [Google Scholar] [CrossRef]
- Davis, D.; Edwards, J.E., Jr.; Mitchell, A.P.; Ibrahim, A.S. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 2000, 68, 5953–5959. [Google Scholar] [CrossRef]
- Ost, K.S.; O’Meara, T.R.; Huda, N.; Esher, S.K.; Alspaugh, J.A. The Cryptococcus neoformans alkaline response pathway: Identification of a novel rim pathway activator. PLoS Genet. 2015, 11, e1005159. [Google Scholar] [CrossRef]
- Davis, D.A. How human pathogenic fungi sense and adapt to pH: The link to virulence. Curr. Opin. Microbiol. 2009, 12, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; He, X.Y.; Chen, J.W.; Mao, Y.S.; Gao, X.D. The pH-responsive transcription factors YlRim101 and Mhy1 regulate alkaline pH-induced filamentation in the dimorphic yeast Yarrowia lipolytica. mSphere 2021, 6, e00179-00121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.B.; Wang, Y.; Zhang, Z.B.; Yang, H.L.; Yan, R.M.; Zhu, D. Influence of environmental and nutritional conditions on yeast–mycelial dimorphic transition in Trichosporon cutaneum. Biotechnol. Biotechnol. Equip. 2017, 31, 516–526. [Google Scholar] [CrossRef]
- Kullas, A.L.; Li, M.; Davis, D.A. Snf7p, a component of the ESCRT-III protein complex, is an upstream member of the RIM101 pathway in Candida albicans. Eukaryot. Cell 2004, 3, 1609–1618. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Norton, D.; Price, M.S.; Hay, C.; Clements, M.F.; Nichols, C.B.; Alspaugh, J.A. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog. 2010, 6, e1000776. [Google Scholar] [CrossRef]
- Odorizzi, G.; Katzmann, D.J.; Babst, M.; Audhya, A.; Emr, S.D. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J. Cell Sci. 2003, 116, 1893–1903. [Google Scholar] [CrossRef]
- Sentandreu, M.; Elorza, M.V.; Sentandreu, R.; Fonzi, W.A. Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans. J. Bacteriol. 1998, 180, 282–289. [Google Scholar] [CrossRef]
- Saporito-Irwin, S.M.; Birse, C.E.; Sypherd, P.S.; Fonzi, W.A. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol. Cell Biol. 1995, 15, 601–613. [Google Scholar] [CrossRef]
- Liu, H. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 2001, 4, 728–735. [Google Scholar] [CrossRef]
- Cornet, M.; Gaillardin, C. pH signaling in human fungal pathogens: A new target for antifungal strategies. Eukaryot. Cell 2014, 13, 342–352. [Google Scholar] [CrossRef]
- Zhao, R.; Lockhart, S.R.; Daniels, K.; Soll, D.R. Roles of TUP1 in switching, phase maintenance, and phase-specific gene expression in Candida albicans. Eukaryot. Cell 2002, 1, 353–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marcos, C.M.; de Oliveira, H.C.; Assato, P.A.; de Oliveira, L.T.; Fregonezi, N.; Dos Santos, K.S.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Polypeptides targeting Paracoccidioides brasiliensis Drk1. J. Fungi 2023, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, K.T.; Lee, M.H.; Cheong, E.; Bahn, Y.S. Adenylyl cyclase and protein kinase A play redundant and distinct roles in growth, differentiation, antifungal drug resistance, and pathogenicity of Candida auris. mBio 2021, 12, e0272921. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; Bezerra, B.T.; Rodrigues, M.L. Antifungal development and the urgency of minimizing the impact of fungal diseases on public health. ACS Bio Med. Chem. Au 2023, 3, 137–146. [Google Scholar] [CrossRef]
- Marcos, C.M.; de Oliveira, H.C.; Assato, P.A.; Castelli, R.F.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Drk1, a Dimorphism Histidine Kinase, contributes to morphology, virulence, and stress adaptation in Paracoccidioides brasiliensis. J. Fungi 2021, 7, 852. [Google Scholar] [CrossRef]
- Navarro, M.V.; de Barros, Y.N.; Segura, W.D.; Chaves, A.F.A.; Jannuzzi, G.P.; Ferreira, K.S.; Xander, P.; Batista, W.L. The Role of Dimorphism Regulating Histidine Kinase (Drk1) in the pathogenic fungus Paracoccidioides brasiliensis cell wall. J. Fungi 2021, 7, 1014. [Google Scholar] [CrossRef]
- Martin, D.C.; Kim, H.; Mackin, N.A.; Maldonado-Báez, L.; Evangelista, C.C., Jr.; Beaudry, V.G.; Dudgeon, D.D.; Naiman, D.Q.; Erdman, S.E.; Cunningham, K.W. New regulators of a high affinity Ca2+ influx system revealed through a genome-wide screen in yeast. J. Biol. Chem. 2011, 286, 10744–10754. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Marchetti, O.; Entenza, J.; Bille, J. Calcineurin A of Candida albicans: Involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 2003, 48, 959–976. [Google Scholar] [CrossRef]
- Whitesell, L.; Robbins, N.; Huang, D.S.; McLellan, C.A.; Shekhar-Guturja, T.; LeBlanc, E.V.; Nation, C.S.; Hui, R.; Hutchinson, A.; Collins, C.; et al. Structural basis for species-selective targeting of Hsp90 in a pathogenic fungus. Nat. Commun. 2019, 10, 402. [Google Scholar] [CrossRef]
- Cornet, M.; Gaillardin, C.; Richard, M.L. Deletions of the endocytic components VPS28 and VPS32 in Candida albicans lead to echinocandin and azole hypersensitivity. Antimicrob. Agents Chemother. 2006, 50, 3492–3495. [Google Scholar] [CrossRef]
- Garnaud, C.; García-Oliver, E.; Wang, Y.; Maubon, D.; Bailly, S.; Despinasse, Q.; Champleboux, M.; Govin, J.; Cornet, M. The Rim pathway mediates antifungal tolerance in Candida albicans through newly identified Rim101 transcriptional targets, including Hsp90 and Ipt1. Antimicrob. Agents Chemother. 2018, 62, e01785-01717. [Google Scholar] [CrossRef]
- Iyer, K.R.; Robbins, N.; Cowen, L.E. The role of Candida albicans stress response pathways in antifungal tolerance and resistance. iScience 2022, 25, 103953. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Sotelo, U.; Gómez-Gaviria, M.; Mora-Montes, H.M. Signaling Pathways Regulating Dimorphism in Medically Relevant Fungal Species. Pathogens 2025, 14, 350. https://doi.org/10.3390/pathogens14040350
Ramírez-Sotelo U, Gómez-Gaviria M, Mora-Montes HM. Signaling Pathways Regulating Dimorphism in Medically Relevant Fungal Species. Pathogens. 2025; 14(4):350. https://doi.org/10.3390/pathogens14040350
Chicago/Turabian StyleRamírez-Sotelo, Uriel, Manuela Gómez-Gaviria, and Héctor M. Mora-Montes. 2025. "Signaling Pathways Regulating Dimorphism in Medically Relevant Fungal Species" Pathogens 14, no. 4: 350. https://doi.org/10.3390/pathogens14040350
APA StyleRamírez-Sotelo, U., Gómez-Gaviria, M., & Mora-Montes, H. M. (2025). Signaling Pathways Regulating Dimorphism in Medically Relevant Fungal Species. Pathogens, 14(4), 350. https://doi.org/10.3390/pathogens14040350






