Abstract
Infectious diseases, the second leading cause of death worldwide, have traditionally been treated with antimicrobials. However, the emergence of drug-resistant microorganisms has driven the need for alternative therapies. This study aimed to assess the antibacterial efficacy of Capparis spinosa crude extracts and five essential oils (EOs) derived from Salvia officinalis, Eucalyptus globulus, Micromeria barbata, Origanum vulgare, and Juniperus excelsa. The EOs were extracted using hydro-distillation, and C. spinosa extracts were obtained using ethanol and acetone solvents. Microdilution assays revealed that O. vulgare EO exhibited the strongest activity against Listeria monocytogenes, Escherichia coli, Salmonella spp., and Brucella melitensis, while C. spinosa demonstrated significant antibacterial effects against L. monocytogenes and notable inhibition of Pseudomonas aeruginosa. The combination of EOs with antibiotics, including M. barbata, J. excelsa, S. officinalis, and E. globulus, enhanced the efficacy of the antibiotics against recalcitrant bacterial strains. The synergistic effects were evaluated through Fractional Inhibitory Concentration Index (FICI) analysis. These findings confirm that the antibacterial efficacy observed in the tested EOs, especially when used in synergy with antibiotics, offers a promising therapeutic strategy to combat antimicrobial resistance.
1. Introduction
The discovery of penicillin started a golden age in antibiotic research and development, which revolutionized modern medicine and significantly extended the lifespan of humans [1]. Antibiotics mainly function by suppressing bacterial cell wall construction, altering membrane integrity, inhibiting nucleic acid and protein production, and interfering with metabolic processes [2].
The overuse of antibiotics in various sectors, such as medicine, agriculture, and the animal food industry, has led to bacterial resistance under selective pressure, representing a new paradigm in pathogenesis, transmission, and resistance [3,4]. This resistance initially developed in staphylococci, streptococci, and gonococci, following the release of the first commercial antibiotic, penicillin, in 1941, and penicillin-resistant Staphylococcus aureus emerged a year later, in 1942 [5]. Nowadays, addressing patients infected with multidrug-resistant (MDR), extensively drug-resistant (XDR), or pandrug-resistant (PDR) bacteria poses a serious challenge [6]. As a result, pharmaceutical companies face financial and regulatory challenges, and significant investments in scientific research were allocated to combat the resistant bacteria [5].
Acinetobacter baumannii, commonly found in hospital environments, particularly in intensive care units, is a major cause of ventilator-associated pneumonia, bloodstream infections, and wound infections, with many strains exhibiting resistance to colistin and other antibiotics [7]. Similarly, XDR Escherichia coli, which is linked to severe urinary tract infections, often exhibits resistance to carbapenems, leading to treatment failures and increased mortality in critically ill patients [8]. Both pathogens pose significant challenges to effective infection management in healthcare settings [9].
Campylobacter coli has developed increasing resistance to antibiotics, particularly those that are typically used as first-line treatments for campylobacteriosis [10]. These resistance patterns complicate treatment strategies for infections, particularly in the healthcare setting, where C. coli can lead to severe gastrointestinal and systemic infections [11].
Pseudomonas aeruginosa is known for acquiring resistance to β-lactams, including carbapenems, ceftazidime, and piperacillin-tazobactam, particularly in healthcare settings [12]. It is responsible for severe infections such as those associated with burns, catheterization, and neutropenia, leading to septicemia, urinary tract infections, and bacteremia [13]. P. aeruginosa represents approximately 7.3% of all healthcare-associated infections [14].
The 2021–2022 European Union report highlighted the growing issue of antibiotic resistance in Salmonella, particularly in poultry-associated strains [15]. Resistance to critical antibiotics, such as third-generation cephalosporins, is increasing, with extended-spectrum β-lactamase (ESBL)-producing strains becoming more widespread. This rise in resistance presents a significant challenge to both healthcare and food safety, as these strains complicate treatment options and elevate the risk of foodborne outbreaks [15].
The global priority pathogens list published by the World Health Organization (WHO) classified antibiotic-resistant bacteria into critical, high, and medium priority tiers, emphasizing the immediate requirement for research and developing new treatment options [16]. New strategies are being implemented to overcome antimicrobial resistance, some of which focus on the antibacterial effect of essential oils (EOs) and their synergism with antibiotics [17].
EOs are aromatic and volatile liquids derived from plant materials such as bulbs, roots, bark, leaves, seeds, peels, fruits, and wood that can give plants their unique scent, taste, or both. EOs are hydrophobic, soluble in alcohol, non-polar or weakly polar solvents, waxes, and oils, but only partly soluble in water, many of which are uncolored or pale yellow [18,19]. EOs have been used for centuries in medicine, cosmetics, perfumes, and aromatherapy and have been applied to food as part of spices or herbs [20]. Recent research has demonstrated their potential as antimicrobial agents, with studies showing their efficacy against foodborne pathogens such as Listeria monocytogenes and Salmonella Typhimurium [21]. Additionally, certain components, such as thymol and carvacrol from oregano oil, have exhibited significant antibacterial properties by disrupting bacterial cell membranes [22], where pathogens cannot simply develop resistance to the EO because of its complex mixture of multiple compounds [23]. EOs influence bacterial cells through many pathways, including targeting cell membrane phospholipids, affecting enzyme processes, damaging genetic material, and producing fatty acid hydroperoxide triggered by the oxygenation of unsaturated fatty acids [4,24].
The floristic richness in Lebanon is estimated to be around 2600 plant species, with endemic plants accounting for 12% of the total, all within a relatively small area of 10,452 square kilometers [25].
Lebanese plants, including Origanum vulgare (Linnaeus, 1753), Micromeria spp. (Linnaeus, 1753), Salvia officinalis (Linnaeus, 1753), Eucalyptus globulus (L’Héritier 1788), and Juniperus excelsa (Pallas, 1771), are valued for their EOs with antimicrobial properties. Capparis spinosa (Linnaeus, 1753), native to the Mediterranean and present in Lebanon, demonstrates a wide range of bioactivities, such as antibacterial, antifungal, antioxidant, anti-inflammatory, and anticarcinogenic effects [26,27]. O. vulgare has been used since ancient times as a food seasoning and effectively targets foodborne pathogens [28], while Micromeria species are particularly effective against Gram-positive bacteria and exhibit significant free radical scavenging properties [29]. S. officinalis is widely used in traditional medicine, especially for women’s health, and has attracted attention for its therapeutic potential [30,31]. In Tunisian folk medicine, inhalation of E. globulus EO has been traditionally used to manage respiratory disorders such as pharyngitis, bronchitis, and sinusitis [32]. J. excelsa offers antimicrobial and antioxidant benefits, primarily used in traditional medicine to address conditions like cough, dysmenorrhea, jaundice, tuberculosis, bronchitis, and colds, as well as to induce menstruation and expel the fetus [33].
The present study aims to evaluate the antibacterial effect of O. vulgare, S. officinalis, E. globulus, J. excelsa EOs, and Capparis spinosa crude extracts against L. monocytogenes, XDR P. aeruginosa, XDR E. coli, ESBL-producing Salmonella spp., and Brucella melitensis. Also, the synergistic antibacterial effect of J. excelsa, S. officinalis, Micromeria barbata, and E. globulus EOs associated with commercial antibiotics against E. coli, P. aeruginosa, XDR A. baumannii strains, and MDR C. coli strains is evaluated. This assessment could offer a novel approach to enhancing the efficacy of existing antimicrobial agents, providing a promising alternative to combat resistant infections.
2. Materials and Methods
2.1. Preparation and Extraction of Plant Materials
Plant materials of Salvia officinalis, E. globulus, Micromeria barbata, Origanum vulgare, Juniperus excelsa, and Capparis spinosa were obtained through wild harvest from the North Lebanon Region. Specifically, M. barbata and J. excelsa were harvested in Denniyeh, O. vulgare and C. spinosa in Abu Samra, during August, S. officinalis, and E. globulus in Tripoli during September. Whole plant parts were used for essential oil (EO) extraction, except for C. spinosa, where only the leaves were utilized. The plant materials were air-dried at room temperature (28 °C) for approximately 4 days, and EOs were extracted using the hydro-distillation method with a stainless steel hydrodistillator at the Chamber of Commerce, Industry, and Agriculture in Tripoli. After weighing, each batch of plant samples was submerged in 18 L of clean water heated to 95 °C for 4 h and separated into aqueous and oil-rich layers. The oil was collected using a separating funnel, stored in opaque glass bottles, and refrigerated at 4 °C until analysis [34]. The yield of EOs from J. excelsa, M. barbata, and E. globulus was 2% when extracted from 2000 g of the plants on a moisture-free basis, while O. vulgare yielded 0.6%, and S. officinalis yielded 1.2% from the same weight of whole plant material. Leaves from C. spinosa were pulverized into a coarse powder using a pestle and mortar. For extraction, 10 g of the powder was mixed with 100 mL of 100% ethanol or 200 mL of 80% acetone [35,36]. The mixtures were kept at 4 °C for three days. The extracts were filtered through Whatman No.1 filter paper. Solvents were removed at 40 °C to obtain C. spinosa acetonic extracts (C. spinosa-ac) and C. spinosa ethanolic extracts (C. spinosa-etOH).
2.2. Antibiotics
The selected antibiotics were gentamicin (GEN, Panpharma, Brombach, Germany), tetracycline (TET, Pharmadex S.A.L, Kahaleh, Lebanon), ciprofloxacin (CIP, Ladinin200, Athens, Greece), levofloxacin (LEV), amikacin (AMI), cefepime (CEF), and amoxicillin–clavulanic acid (AMC), all obtained from SIGMA. Antibiotics were used as recommended by the Clinical and Laboratory Standards Institute (CLSI) [37].
2.3. Bacterial Strains
The bacterial strains examined, as listed in Table 1, were obtained from the Microbial Collection of the Lebanese University (CMUL) at the Laboratoire Microbiologie Santé et Environnement (LMSE). These strains were selected based on their clinical significance and antimicrobial resistance profiles. The collection includes MDR, XDR, and ESBL-producing strains, which are frequently implicated in hospital-acquired infections and pose major treatment challenges.

Table 1.
Characteristics of the tested bacterial strains.
The inoculum was prepared from a pure culture incubated for 24 h on Mueller–Hinton II agar medium (Bio-Rad, Marnes-la-Coquette, France) for the bacterial strains. The microorganisms were then suspended in sterile brain heart infusion (BHI) broth (Scharlau, Barcelona, Spain) supplemented with 0.2% agar (Bio-Rad, Marnes-la-Coquette, France) to achieve turbidity levels approximating 0.5 and 1 McFarland.
Antimicrobial susceptibility was assessed using phenotypic testing on Mueller–Hinton II agar, with results interpreted according to EUCAST guidelines 2023 (https://www.eucast.org/clinical_breakpoints, accessed on 14 March 2025).
2.4. Determination of Minimum Inhibitory Concentration (MIC)
MIC of EOs, C. spinosa-ac, and C. spinosa-etOH were determined by serial microdilution in 96-well microplates, as described by Diniz et al. Also, 200 µL of the broth was pipetted in the well of the first row as a negative control (A1), and 200 µL of bacterial inoculum of 106 CFU/mL with BHI was inoculated in the well of the second row as a positive control (A2) [38]. EOs and extracts were prepared using a diluent containing 0.5% TWEEN 80 (Fluka, Darmstadt, Germany). Serial dilutions of 1/2, 1/4, 1/8, 1/16, 1/32, 1/64, 1/128, 1/256, and 1/1024 resulted in final concentrations ranging from 50% v/v to 0.10% v/v. The plates were incubated at 35 °C for 18–24 h. At last, 10 μL from each well were deposited in streaks on the surface of the Mueller–Hinton agar. The Petri dishes were incubated at 35 °C for 18 h before analysis, as shown in Figure 1. The experiment was conducted three times independently to ensure the reliability of the results.
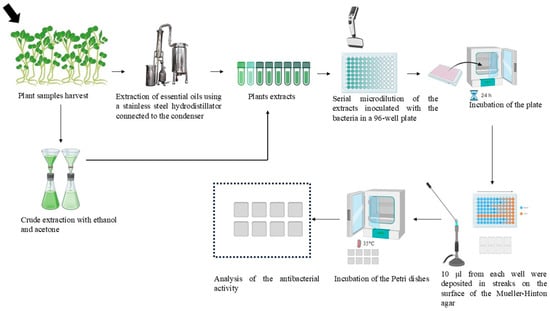
Figure 1.
Extraction methods of essential oils, Capparis spinosa crude extracts, and evaluation of the antibacterial activity by microdilution.
2.5. Synergistic Effect of Essential Oils and Antibiotics
The synergistic effect of EOs and antibiotics was analyzed using a serial twofold microdilution method in 96-well microplates to determine the MIC [39]. Antibiotics were tested at the following concentration ranges: AMI and AMC, 8 to 2 mg/L; LEV, 1 to 0.25 mg/L; GEN and TET, 2 to 0.5 mg/L; CIP, 0.5 to 0.125 mg/L.
For EOs, concentrations were prepared using a stepwise dilution from 45% v/v to 25% v/v. Each well contained a total volume of 200 µL, consisting of 100 µL of bacterial inoculum (10⁶ CFU/mL), EO, and an antibiotic. The EO volume was adjusted according to its concentration, such that for 45% EO, the well contained 90 µL of EO and 10 µL of antibiotic, while proportional adjustments were applied for lower EO concentrations.
Plates were incubated at 35 °C for 18–24 h. Following incubation, 10 µL from each well was streaked onto Mueller–Hinton agar plates and incubated at 35 °C for 18 h. The experiment was conducted three times independently to ensure the reliability of the results.
2.6. Fractional Inhibitory Concentration Index (FICI) Analysis
The FICI of EOs combined with antibiotics was calculated to evaluate their interactions against resistant bacterial strains. The FICI was calculated using the following formula: FICI = (MIC of EO in combination/MIC of EO alone) + (MIC of antibiotic in combination/MIC of antibiotic alone) [40]. The highest tested concentration was considered MIC when the EO showed no activity [41]. The concentrations of EOs and antibiotics were expressed in mg/L for consistency, with FICI values interpreted as follows: FICI ≤ 0.50 indicates synergy, 0.50 < FICI ≤ 1.00 is additive, 1.00 < FICI ≤ 4.00 indicates indifference, and FICI > 4.00 suggests antagonism [42].
2.7. Statistical Analysis
Statistical analyses were performed using IBM SPSS software, version 26. The mean and standard deviation were analyzed by mean comparison to determine the most effective EO and crude extract, as well as the most susceptible bacterial strains. The Kruskal–Wallis test, a non-parametric method, was used to assess the significance of differences between the EOs and bacterial strains. Since SPSS version 26 does not include pairwise post-hoc comparisons for Kruskal–Wallis, the Mann–Whitney U test was applied for pairwise comparisons. All tests were two-sided, with a significance level set at α = 0.05.
2.8. Chemical Analysis
The chemical composition and concentrations of the component in the EOs of M. barbata, J. excelsa, O. vulgare, and E. globulus were determined through capillary column GC-MS (Shimadzu QP 2010), with injection conducted in splitless mode [29].
3. Results
The results obtained from the three independent repetitions were consistent, with no significant variations observed among them.
3.1. Antibacterial Activity of the Essential Oils
The results of the microdilution of the EOs against E. coli CMUL 260, E. coli CMUL 096, P. aeruginosa CMUL 122, Salmonella spp. CMUL 216, L. monocytogenes AL004, and B. melitensis CMUL 05 are shown in Table 2.

Table 2.
Minimum inhibitory concentration of Salvia officinalis, Eucalyptus globulus, Origanum vulgare, and Juniperus excelsa EOs against clinical strains.
S. officinalis essential oil showed antibacterial activity against E. coli, Salmonella spp., L. monocytogenes, and P. aeruginosa. However, E. coli CMUL 096 showed the highest sensitivity to the EO.
E. globulus EO exhibited antibacterial activity against E. coli, Salmonella spp., L. monocytogenes, P. aeruginosa, and B. melitensis strains. Among them, L. monocytogenes showed the highest sensitivity, with a 0.78% v/v MIC value, while the Gram-negative bacteria displayed a MIC range of 1.56% v/v to 50% v/v. Additionally, E. coli CMUL 260 was more sensitive to the EO than E. coli CMUL 096.
The antibacterial activity of O. vulgare EO was also observed against the bacterial strains. The EO inhibited E. coli CMUL 260, Salmonella spp., and L. monocytogenes at a 0.10% v/v MIC value. Notably, E. coli CMUL 260 showed higher sensitivity than E. coli CMUL 096.
J. excelsa EO demonstrated antibacterial activity against B. melitensis and E. coli CMUL 096, with B. melitensis showing sensitivity to the EO at a MIC value of 6.25% v/v.
Kruskal–Wallis tests revealed statistically significant differences in the antibacterial activities of the EOs (p-value = 0.035). O. vulgare EO was the most effective, exhibiting the lowest mean percentage of activity (4.28%) and minimal variability (standard deviation = 10.15%), followed by E. globulus EO, which showed a significant difference (p-value = 0.029). S. officinalis EO exhibited significantly lower activity than O. vulgare (p-value = 0.019). J. excelsa EO was the least effective, showing markedly lower activity than O. vulgare EO, though the difference was not statistically significant (p-value = 0.086).
There were no statistically significant differences in the susceptibility of the bacterial strains (p-value = 0.255). The highest susceptibility was observed in E. coli CMUL 260, with a minimal mean percentage (mean = 2.64%) and minimal variability (standard deviation = 3.21), indicating highly consistent results. Salmonella spp. also showed strong susceptibility, though the difference compared to E. coli CMUL 260 was not significant (p-value = 0.822).
When comparing the two E. coli strains, E. coli CMUL 096 exhibited markedly lower susceptibility than E. coli CMUL 260, but this difference was also not statistically significant (p-value = 0.289).
L. monocytogenes exhibited moderate susceptibility (p-value = 1.00), while B. melitensis showed lower susceptibility, with no statistically significant difference (p-value = 0.212) compared to E. coli CMUL 260. Finally, P. aeruginosa exhibited the least susceptibility to the EOs, with a statistically significant difference in effectiveness compared to E. coli CMUL 260 (p-value = 0.046), indicating its higher resistance relative to the other bacterial strains.
3.2. Antibacterial Activity of the Crude Extracts
C. spinosa acetonic extracts (C. spinosa-ac) and C. spinosa ethanolic extracts (C. spinosa-etOH) were prepared and evaluated against E. coli CMUL 260, Salmonella spp., L. monocytogenes, and P. aeruginosa CMUL 122 strains. L. monocytogenes exhibited the highest sensitivity, requiring the lowest concentration for inhibition for both extracts (mean = 3.13%, standard deviation < 0.001). P. aeruginosa also showed high susceptibility, with a 12.50% v/v MIC value (mean = 12.50%), while E. coli demonstrated moderate susceptibility (mean = 18.75%). Salmonella spp. was the least susceptible, requiring the highest concentration for inhibition (mean = 37.50%), as shown in Figure 2. There were no statistically significant differences in the susceptibility of the bacterial strains (p-value = 0.101). Notably, C. spinosa-EtOH (mean = 13.28%) exhibited greater overall efficacy compared to C. spinosa-ac (mean = 22.66%), although the difference was not statistically significant (p-value = 0.549). This result highlights the potential of C. spinosa-EtOH as a highly effective extract, particularly against L. monocytogenes and P. aeruginosa.
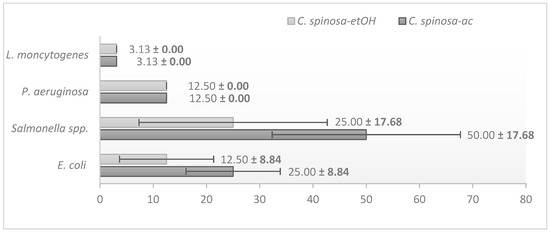
Figure 2.
Minimum inhibitory concentration (%) of Capparis spinosa crude extracts, showing the highest antibacterial activity against Listeria monocytogenes, as indicated by the lowest MIC value. Error bars represent the mean ± standard deviation (%) for the susceptibility of bacterial strains to the crude extracts.
3.3. Effect of Associating Levofloxacin and Amikacin with M. barbata, J. excelsa, and E. globulus
The control of the three EOs against XDR A. baumannii and P. aeruginosa CMUL 122 strains showed bacterial growth, except for M. barbata, which showed antibacterial activity against A. baumannii till 0.20% v/v MIC. So, its tested concentrations were 0.39% v/v, 0.20% v/v, and 0.10% v/v.
Total inhibition of P. aeruginosa colonies with 45% v/v of M. barbata EO and each 2, 4, 8 mg/L of AMI, 35% v/v of the EO combined with each 4 and 8 mg/L of AMI. The significant antibacterial activity was for 4 mg/L of AMI associated with 35% v/v of M. barbata EO. FICI results showed that this combination had an additive action against the bacterial strain (FICI = 0.85), as shown in Table 3.

Table 3.
Fractional inhibitory concentration index (FICI) of Micromeria barbata EO tested in combination with amikacin against extensively drug-resistant (XDR) Pseudomonas aeruginosa strain.
Total inhibition of A. baumannii colonies was observed with all combinations of 25, 35, and 45% v/v of J. excelsa EO with 2, 4, and 8 mg/L of AMI, and 0.25, 0.5, and 1 mg/L of LEV. The highest antibacterial activity was noted when 25% v/v of J. escelsa was combined with each 2 mg/L AMI and 0.25 mg/L LEV. FICI results showed that these combinations synergized against the bacterial strain (FICI = 0.50). Same for all E. globulus concentrations combined with LEV dilutions, where 25% v/v of the oil with 0.25 mg/L of the antibiotic showed optimal inhibition and synergistic action (FICI = 0.50). However, only 2 mg/L of AMI with all E. globulus concentrations, especially 25% v/v, showed total inhibition, with synergistic activity (FICI = 0.50). A. baumannii colonies inhibition for the following associations: 1 mg/L of LEV and 0.10% v/v, 0.20% v/v, and 0.39% v/v of M. barbata EO, 0.5 mg/L LEV and 0.10% v/v and 0.39% v/v of M. barbata EO. The optimal bacterial inhibition and synergistic action were marked when combining 0.25 mg/L of LEV with 0.10% v/v of M. barbata EO (FICI = 0.50). Moreover, additive actions were noted against XDR A. baumannii, as demonstrated in Table 4.

Table 4.
Fractional inhibitory concentration index (FICI) of Juniperus excelsa, Micromeria barbata, and Eucalyptus globulus EOs tested in combination with levofloxacin and amikacin against extensively drug-resistant (XDR) A. baumannii strain.
3.4. Effect of Associating AMC with J. excelsa, M. barbata, and E. globulus
A control of J. excelsa, M. barbata, and E. globulus EOs against XDR E. coli CMUL 260 showed zero antibacterial activity.
Antibacterial activity was observed when both 35% v/v and 45% v/v of E. globulus EO dilutions were associated with 2, 4, and 8 mg/L of AMC, also with 25% v/v of the EO combined with 8 mg/L of AMC.
Both 45% v/v and 25% v/v concentrations of M. barbata EO with 2 mg/L AMC showed an optimal inhibition of E. coli. Also, 35% v/v of the EO combined with all 2, 4, and 8 mg/L of AMC resulted in total bacterial inhibition.
On the contrary, all combinations of AMC concentrations and J. excelsa EO showed total bacterial growth.
The combination of M. barbata and E. globulus EOs with AMC showed a synergistic effect against XDR E. coli (FICI = 0.50) when 25% of the EO was associated with 2 mg/L of AMC, as shown in Table 5. Additive action was also noted, with FCI values ranging from 0.60 to 0.90.

Table 5.
Fractional inhibitory concentration index (FICI) of Micromeria barbata and Eucalyptus globulus EOs tested in combination with amoxicillin–clavulanic acid against extensively drug-resistant (XDR) Escherichia coli strain.
3.5. Effect of Associating Tetracycline, Gentamicin, and Ciprofloxacin with S. officinalis, J. excelsa, and M. barbata
In a control of the three EOs against MDR C. coli AX031, all the EOs showed almost total antimicrobial activity. All the combinations of TET, CIP, and GEN concentrations with the four tested Eos showed a total inhibition of C. coli.
The FICI values of EOs combined with antibiotics are shown in Table 6. A synergistic effect was highlighted (FICI = 0.50), and an additive action (FICI = 0.60–0.90) against MDR C. coli. A neutral effect (FICI = 1.25–1.45) when EOs were combined with the highest antibiotic concentration.

Table 6.
Fractional inhibitory concentration index (FICI) of Juniperus excelsa, Micromeria barbata, and Salvia officinalis EOs tested in combination with ciprofloxacin, gentamicin, and tetracycline against multidrug-resistant Campylobacter coli strain.
3.6. Effect of Associating Cefepime and Ciprofloxacin with M. barbata, J. excelsa, and E. globulus
All the combinations of the EOs with the antibiotic dilutions were ineffective and showed a total growth of P. aeruginosa CMUL 120 colonies.
3.7. Chemical Composition of the Essential Oils Tested in This Study
The EO of M. barbata is composed of various components, including pulegone, limonene, menthol, neomenthol, β-pinene, and piperitone, along with other ketones that contribute to its characteristic odor (Supplementary Table S1). The EO of J. excelsa was found to contain high amounts of α-pinene, followed by α-cedrol, limonene, and other compounds (Supplementary Table S2). The chemical analysis of O. vulgare EO revealed a total of 20 distinct components. The major constituents identified include carvacrol, p-cymene, and thymol, which collectively account for a significant proportion of the oil (Supplementary Table S3). Lastly, the EO of E. globulus exhibited a diverse composition with various identified compounds (Supplementary Table S4).
4. Discussion
Several studies have reported the antimicrobial activity of EOs and their bioactive compounds against Gram-negative and Gram-positive bacteria. However, data on the effectiveness of Lebanese EOs remain limited. Our findings highlight their notable antibacterial activity, particularly against MDR and XDR Gram-negative species. Antimicrobial resistance to bacterial pathogens is a critical issue associated with high morbidity and mortality rates [43]. The emergence of multidrug-resistant patterns in Gram-positive and Gram-negative bacteria poses significant challenges in treatment, often rendering conventional antibiotics ineffective or inadequate [43]. In recent years, researchers have explored alternative strategies to combat AMR, including the use of EOs or their bioactive constituents. The combinatorial approach, which leverages synergistic interactions between volatile EO compounds and antibiotics, has demonstrated enhanced antimicrobial efficacy [44].
Plant samples of S. officinalis, E. globulus, M. barbata, O. vulgare, J. excelsa, and C. spinosa were collected from the North Lebanon Region. EOs were extracted by hydro-distillation, and the resulting oils gave off a pleasant odor. Crude extracts of C. spinosa leaves were obtained using acetone and ethanol.
The antimicrobial activity results indicated that the oils exhibited significant effectiveness against bacterial strains with diverse resistance profiles. EOs showed a MIC range from 0.10% v/v to 50% v/v for the tested bacterial strains. O. vulgare revealed the highest antibacterial activity against L. monocytogenes, E. coli, and Salmonella spp., with a MIC of 0.10% v/v. The study conducted by Xiao et al. highlighted that oregano oil was the most effective agent in inhibiting E. coli UTI 189, with the lowest MIC (0.015% v/v) [45]. Recently, Pinto et al. found that oregano EO or carvacrol at 0.05% v/v could eradicate S. Typhimurium and L. monocytogenes at 0.5% v/v MIC. The differences in the oregano oil’s MIC against the strains can be due to the different resistance profiles of the bacteria. They also reported that the main antibacterial components were α-pinene, p-cymene, β-myrcene, and camphene. Nakas et al. identified carvacrol, γ-terpinene, p-cymene, and β-myrcene as the primary components of O. vulgare EO [46]. Our EO analysis revealed that carvacrol, p-cymene, and thymol were the main components. The different EO chemotypes, EO origin, and conditions for the EO application might have affected the composition of the EOs [47].
In this study, O. vulgare EO showed significant activity against B. melitensis at very low concentrations (0.20% v/v), followed by J. excelsa and E. globulus EOs. Studies on the bacterial effect of EOs against B. melitensis are limited compared to those conducted on other bacteria. Al-Mariri et al. confirmed the activity of Origanum syriacum against tetracycline-resistant B. melitensis [48]. Additionally, C. spinosa acetonic extracts (C. spinosa-ac) and C. spinosa ethanolic extracts (C. spinosa-etOH) from leaves showed the highest activity against P. aeruginosa among all tested plant extracts, with a MIC value of 12.5% v/v. The significant activity of C. spinosa extracts was against L. monocytogenes. On the other hand, the results showed modest activity of the extracts at high concentrations against E. coli and Salmonella spp. strains. The stronger antimicrobial activity of ethanolic extracts is likely attributed to the higher extraction efficiency of bioactive compounds in ethanol, resulting in higher concentrations of antimicrobial agents [49]. Gull et al. reported that C. spinosa-ac and C. spinosa-etOH extracts from roots exhibited the best antibacterial activity against E. coli and Bacillus subtilis [50]. In the recent research conducted by Al-Khafagi et al., C. spinosa-etOH had great antibacterial activity against Helicobacter pylori [51]. C. spinosa extracts exhibited variable degrees of antimicrobial activity related to its bioactive compounds, mainly polyphenols and flavonoids [52].
The findings regarding the combination of EOs with antibiotics are interesting. Several studies suggest that this approach significantly diminishes bacterial resistance and broadens the spectrum of antibiotic activity [53]. Moreover, this combination has been observed to lower the concentration of antibiotics required, consequently reducing their toxicity [53]. For instance, a study demonstrated that thymol, when combined with penicillin, showed synergistic activity against E. coli, reducing resistance and broadening the antibiotic’s effectiveness [54].
The results showed that the MIC of LEV decreased from 1 mg/L to 0.25 mg/L in the presence of E. globulus and M. barbata EOs, demonstrating a synergistic effect against the A. baumannii strain (FICI = 0.50). These combinations reduced the MIC values of LEV fourfold. The observed antibacterial activity of E. globulus EO is likely due to the presence of key bioactive compounds, such as p-cymene and eucalyptol (1,8-cineol), which are known for their antimicrobial properties. Iseppi et al. observed, using a checkerboard assay, the efficacity of oxacillin combined with E. globulus against methicillin-resistant S. aureus strains, with FICI values between 0.15 and 0.50 [53]. In a recent study, Santos et al. claimed the synergistic and additive effects after combining CRO and Eucalyptus radiata against E. coli strains isolated from meat products [55].
Despite the limited data available on the antimicrobial activity of J. excelsa and M. barbata EOs, El Omari et al. reported the efficacy of Lebanese J. excelsa, M. barbata, and E. globulus EOs against Mycobacterium tuberculosis and atypical mycobacteria [56]. Moreover, Alwan et al. showed a significant effect of M. barbata on Candida albicans [57]. In the same context, the present study revealed the antibacterial activity of J. excelsa EO against Brucella melitensis. According to El Omari et al., GC-MS analysis of J. excelsa EO identified 44 components, with high concentrations of α-pinene and α-cedrol, both known for their antimicrobial properties [56]. Similarly, M. barbata EO was characterized by a diverse composition, including pulegone, limonene, menthol, neomenthol, β-pinene, and piperitone, along with other ketone compounds [57].
The synergistic activity of LEV and AMI, when associated with J. excelsa EO against the A. baumannii strain, resulted in a reduction of MIC values of the antibiotics fourfold, with MIC values ranging from 1 mg/L to 0.25 mg/L and 8 mg/L to 2 mg/L, respectively. Furthermore, the additive action between AMI and M. barbata EO was obtained against the P. aeruginosa strain, with a decrease in MIC from 8 mg/L to 4 mg/L, and the reduction was twofold. Moreover, the decrease in the MIC of AMC was also associated with M. barbata EO against the E. coli strain from 8 mg/L to 2 mg/L. This synergistic combination reduced the MIC values of AMC fourfold.
In this research, Salvia officinalis demonstrated antimicrobial activity against E. coli, P. aeruginosa, L. monocytogenes, and Salmonella spp. This activity is primarily attributed to the presence of major components such as α-thujone, camphor, eucalyptol, α-humulene, and camphene in Lebanese S. officinalis EO [58]. A significant decrease in the MIC of TET, GEN, and CIP was observed when combined with S. officinalis, J. excelsa, and M. barbata EOs against the MDR C. coli strain. These synergistic combinations reduced the antibiotics’ MIC values fourfold. The association of GEN and TET with S. officinalis was also effective against S. aureus strains, as reported by Silva et al. [59]. Our data also showed no synergistic effect of CEF and CIP with EOs from M. barbata, J. excelsa, and E. globulus against P. aeruginosa. This lack of effect is related to the resistant nature of P. aeruginosa and its XDR phenotypic profile.
5. Conclusions
This study demonstrates that all the EOs tested exhibited antibacterial activity against the bacterial strains. Among the five EOs, O. vulgare showed the highest activity against L. monocytogenes, E. coli, Salmonella spp., and B. melitensis at very low concentrations. C. spinosa leaf extracts also displayed significant antibacterial activity, particularly against L. monocytogenes, with notable inhibition of P. aeruginosa, despite limited previous reports.
Notably, this research is the first to investigate the synergistic antibacterial effects of Lebanese M. barbata and J. excelsa EOs in combination with antibiotics against resistant bacterial strains. Given the growing concern about global antimicrobial resistance, the synergism observed between these EOs and antibiotics presents a promising strategy to address bacterial resistance.
However, while the findings indicate some significant differences in antibacterial activity, not all EOs exhibited consistent or statistically significant effectiveness across all bacterial strains. For instance, while S. officinalis and J. excelsa EOs showed significantly lower activities in some cases, others, such as O. vulgare and E. globulus, did not show significant differences in comparison. These nuances are required to understand the broader applicability of these EOs as potential antibiotic alternatives.
Although the results are promising, further investigation into the practical application of these EOs as alternatives to traditional antibiotics is essential. Considerations regarding their safety, efficacy, and clinical feasibility are paramount. Cytotoxicity studies and additional in vivo testing are necessary to assess better their potential for widespread clinical use.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pathogens14040348/s1, Table S1. Chemical composition of the essential oil of Micromeria barbata. Table S2. Chemical composition of the essential oil of Juniperus excelsa. Table S3. Chemical composition of the essential oil of Origanum vulgare. Table S4. Chemical composition of the essential oil of Eucalyptus globulus.
Author Contributions
Conceptualization, K.E.O.; formal analysis, H.H.S. and K.E.O.; investigation, H.H.S., M.B., B.I. and R.S.; writing—original draft, H.H.S.; writing—review and editing, H.H.S., F.D., B.I., R.S. and M.O.; supervision, M.B., F.D., M.O. and K.E.O.; funding acquisition, M.O. and K.E.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the valuable assistance and technical collaboration of Taha Abdou and Sara Wraydeh, as well as the contributions of Nour Kabbara, Yasser Aboud, Badria Chaaban, and Hayat Khoder to the project as part of their master’s research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Goldman, E. Antibiotic Abuse in Animal Agriculture: Exacerbating Drug Resistance in Human Pathogens. Hum. Ecol. Risk Assess. 2004, 10, 121–134. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Hsieh, Y.-H.; Powers, Z.M.; Kao, C.-Y. Defeating Antibiotic-Resistant Bacteria: Exploring Alternative Therapies for a Post-Antibiotic Era. Int. J. Mol. Sci. 2020, 21, 1061. [Google Scholar] [CrossRef] [PubMed]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- Whelan, S.; Lucey, B.; Finn, K. Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment. Microorganisms 2023, 11, 2169. [Google Scholar] [CrossRef]
- Nasr, J.; Abdessamad, H.; Mina, J.; Haykal, T.; Jamil, Y.; Abboud, E.; Mahdi, A.; Asmar, R.; Abi Assaad, R.; Alameddine, D.; et al. The epidemiology of gram-negative bacteremia in Lebanon: A study in four hospitals. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 90. [Google Scholar] [CrossRef]
- Portes, A.B.; Panzenhagen, P.; dos Santos, A.M.P.; Junior, C.A.C. Antibiotic Resistance in Campylobacter: A Systematic Review of South American Isolates. Antibiotics 2023, 12, 548. [Google Scholar] [CrossRef]
- Yaqub, M.O.; Joseph, C.E.; Jain, A.; Edison, L.K. Resistome Mapping in Foodborne Pathogens: Understanding Role in the Transmission Dynamics of Resistance Genes. Appl. Microbiol. 2024, 4, 1476–1492. [Google Scholar] [CrossRef]
- Schwartz, B.; Klamer, K.; Zimmerman, J.; Kale-Pradhan, P.B.; Bhargava, A. Multidrug Resistant Pseudomonas aeruginosa in Clinical Settings: A Review of Resistance Mechanisms and Treatment Strategies. Pathogens 2024, 13, 975. [Google Scholar] [CrossRef]
- Glen, K.A.; Lamont, I.L. β-lactam Resistance in Pseudomonas aeruginosa: Current Status, Future Prospects. Pathogens 2021, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022. EFSA J. 2024, 22, e8583. [Google Scholar] [CrossRef]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules 2020, 25, 2888. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Luo, J.; Deng, F.; Huang, Y.; Zhou, H. Antibiotic Combination Therapy: A Strategy to Overcome Bacterial Resistance to Aminoglycoside Antibiotics. Front. Pharmacol. 2022, 13, 839808. [Google Scholar]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Sobhy, M.; Abdelkarim, E.A.; Hussein, M.A.; Aziz, T.; Al-Asmari, F.; Alabbosh, K.F.; Cui, H.; Lin, L. Essential oils as antibacterials against multidrug-resistant foodborne pathogens: Mechanisms, recent advances, and legal considerations. Food Biosci. 2025, 64, 105937. [Google Scholar] [CrossRef]
- Khwaza, V.; Aderibigbe, B.A. Antibacterial Activity of Selected Essential Oil Components and Their Derivatives: A Review. Antibiotics 2025, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Rosato, A.; Carrieri, A.; Tardugno, R.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carocci, A. Antifungal Biofilm Inhibitory Effects of Combinations of Diclofenac and Essential Oils. Antibiotics 2023, 12, 1673. [Google Scholar] [CrossRef]
- Lewis, K. Antibiotics: Recover the lost art of drug discovery. Nature 2012, 485, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Zein, S.; Awada, S.; Rachidi, S.; Hajje, A.H.; Krivoruschko, E.; Kanaan, H. Chemical analysis of essential oil from Lebanese wild and cultivated Origanum syriacum L. (Lamiaceae) before and after flowering. J. Med. Plants Res. 2011, 5, 379–387. [Google Scholar]
- Chedraoui, S.; Abi-Rizk, A.; El-Beyrouthy, M.; Chalak, L.; Ouaini, N.; Rajjou, L. Capparis spinosa L. in A Systematic Review: A Xerophilous Species of Multi Values and Promising Potentialities for Agrosystems under the Threat of Global Warming. Front. Plant Sci. 2017, 8, 1845. [Google Scholar] [CrossRef]
- Kulisic-Bilusic, T.; Schmöller, I.; Schnäbele, K.; Siracusa, L.; Ruberto, G. The anticarcinogenic potential of essential oil and aqueous infusion from caper (Capparis spinosa L.). Food Chem. 2012, 132, 261–267. [Google Scholar] [CrossRef]
- Özkalp, B.; Sevgi, F.; Özcan, M.; Özcan, M. The antibacterial activity of essential oil of oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 8, 272–274. [Google Scholar]
- Bakkour, Y.; Alwan, S.; Soufi, H.; El Achi, N.; Tabcheh, M.; El Omar, F. Chemical composition of essential oil extracted from Micromeria Barbata growing in Lebanon and their antimicrobial and antioxidant properties. J. Nat. Prod. 2012, 5, 116–120. [Google Scholar]
- Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef]
- Bommer, S.; Klein, P.; Suter, A. First time proof of sage’s tolerability and efficacy in menopausal women with hot flushes. Adv. Ther. 2011, 28, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Loyen, G.; Boukef, M.K. Les Plantes Dans la Médecine Traditionnelle Tunisienne; Agence de Coopération Culturelle et Technique: Paris, France, 1986. [Google Scholar]
- Emami, S.A.; Abedindo, B.F.; Hassanzadeh-Khayyat, M. Antioxidant Activity of the Essential Oils of Different Parts of Juniperus excelsa M. Bieb. subsp. excelsa and J. excelsa M. Bieb. subsp. polycarpos (K. Koch) Takhtajan (Cupressaceae). Iran. J. Pharm. Res. IJPR 2011, 10, 799–810. [Google Scholar]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Chapter 15—Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 243–259. ISBN 978-0-12-813768-0. [Google Scholar]
- Shami, A.-M.M.; Philip, K.; Muniandy, S. Synergy of antibacterial and antioxidant activities from crude extracts and peptides of selected plant mixture. BMC Complement. Altern. Med. 2013, 13, 360. [Google Scholar] [CrossRef]
- El Amri, N.; Errachidi, F.; Bour, A.; Bouhaddaoui, S.; Chabir, R. Morphological and Nutritional Properties of Moroccan Capparis spinosa Seeds. Sci. World J. 2019, 2019, 8594820. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Lewis, J.S. The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, Organization, Functions, and Processes. J. Clin. Microbiol. 2020, 58, e01864-19. [Google Scholar] [CrossRef]
- Diniz, A.F.; Santos, B.; Nóbrega, L.M.M.O.; Santos, V.R.L.; Mariz, W.S.; Cruz, P.S.C.; Nóbrega, R.O.; Silva, R.L.; Paula, A.F.R.; Santos, J.R.D.A.; et al. Antibacterial activity of Thymus vulgaris (thyme) essential oil against strains of Pseudomonas aeruginosa, Klebsiella pneumoniae and Staphylococcus saprophyticus isolated from meat product. Braz. J. Biol. Rev. Brasleira Biol. 2023, 83, e275306. [Google Scholar] [CrossRef]
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The agar microdilution method—A new method for antimicrobial susceptibility testing for essential oils and plant extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [CrossRef]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh, T.J. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010, 54, 602–609. [Google Scholar] [CrossRef]
- Van, N.T.B.; Vi, O.T.; Yen, N.T.P.; Nhung, N.T.; Cuong, N.V.; Kiet, B.T.; Hoang, N.V.; Hien, V.B.; Thwaites, G.; Campell, J.; et al. Minimum inhibitory concentrations of commercial essential oils against common chicken pathogenic bacteria and their relationship with antibiotic resistance. J. Appl. Microbiol. 2022, 132, 1025–1035. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Luca, S.V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A.C. Recent advances in tackling microbial multidrug resistance with essential oils: Combinatorial and nano-based strategies. Crit. Rev. Microbiol. 2020, 46, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cui, P.; Shi, W.; Zhang, Y. Identification of Essential Oils with Strong Activity Against Stationary Phase Uropathogenic Escherichia coli. Discov. Med. 2019, 28, 179–188. [Google Scholar]
- Nakas, A.; Giannarelli, G.; Fotopoulos, I.; Chainoglou, E.; Peperidou, A.; Kontogiannopoulos, K.N.; Tsiaprazi-Stamou, A.; Varsamis, V.; Gika, H.; Hadjipavlou-Litina, D.; et al. Optimizing the Distillation of Greek Oregano—Do Process Parameters Affect Bioactive Aroma Constituents and In Vitro Antioxidant Activity? Molecules 2023, 28, 971. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Cervellieri, S.; Netti, T.; Lippolis, V.; Baruzzi, F. Antibacterial Activity of Oregano (Origanum vulgare L.) Essential Oil Vapors against Microbial Contaminants of Food-Contact Surfaces. Antibiotics 2024, 13, 371. [Google Scholar] [CrossRef]
- Al-Mariri, A.; Safi, M. The Antibacterial Activity of Selected Labiatae (Lamiaceae) Essential Oils against Brucella melitensis. Iran. J. Med. Sci. 2013, 38, 44–50. [Google Scholar]
- Adwan, G.M.; Omar, G.I. Evaluation of Antimicrobial activity and Genotoxic Potential of Capparis spinosa (L.) Plant Extracts. Microbiol. Res. J. Int. 2021, 31, 48–57. [Google Scholar] [CrossRef]
- Gull, T.; Sultana, B.; Bhatti, I.; Jamil, A. Antibacterial Potential of Capparis spinosa and Capparis decidua Extracts. Int. J. Agric. Biol. 2015, 17, 727–733. [Google Scholar] [CrossRef]
- Al-Khafagi, M.F.J.; Mohammed, D.Y. Study Antibacterial Activity of Crude Capparis spinosa L. Extracts Against Helicobacter pylori Infection and Determine Their Bioactive Compounds. Iraqi J. Sci. 2023, 64, 503–512. [Google Scholar] [CrossRef]
- Abu-Shama, H. Effect of Caper (Capparis spinosa) Extracts as a Natural Antimicrobial Agent. J. Food Dairy Sci. 2019, 10, 209–216. [Google Scholar] [CrossRef]
- Iseppi, R.; Condò, C.; Messi, P. Synergistic Inhibition of Methicillin-Resistant Staphylococcus aureus (MRSA) by Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils in Association with Oxacillin. Antibiotics 2023, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Ilić, B.S.; Kocić, B.D.; Ćirić, V.M.; Cvetković, O.G.; Miladinović, D.L. An In Vitro Synergistic Interaction of Combinations of Thymus glabrescens Essential Oil and Its Main Constituents with Chloramphenicol. Sci. World J. 2014, 2014, 826219. [Google Scholar] [CrossRef]
- Santos, B.; Farias, J.H.A.; Simões, M.M.; Medeiros, M.A.A.; Alves, M.S.; Diniz, A.F.; Soares, A.P.O.; Cavalcante, A.P.T.M.; Silva, B.J.N.; Almeida, J.C.S.; et al. Evaluation of the antimicrobial activity of Eucalyptus radiata essential oil against Escherichia coli strains isolated from meat products. Braz. J. Biol. Rev. Brasleira Biol. 2024, 84, e281361. [Google Scholar] [CrossRef]
- El Omari, K.; Hamze, M.; Alwan, S.; Osman, M.; Jama, C.; Chihib, N.-E. In-vitro evaluation of the antibacterial activity of the essential oils of Micromeria barbata, Eucalyptus globulus and Juniperus excelsa against strains of Mycobacterium tuberculosis (including MDR), Mycobacterium kansasii and Mycobacterium gordonae. J. Infect. Public Health 2019, 12, 615–618. [Google Scholar] [CrossRef]
- Alwan, S.; El Omari, K.; Soufi, H.; Zreika, S.; Soukarieh, I.; Chihib, N.E.; Jama, C.; Hamze, M. Evaluation of the Antibacterial Activity of Micromeria barbata in Lebanon. J. Essent. Oil Bear. Plants 2016, 19, 321–327. [Google Scholar] [CrossRef]
- Chehade, S.; Kobeissy, M.; Kanaan, H.; Haddad, M. Comparison Between the Chemical Compositions and the in-Vitro Antidiabetic and Anti-Inflammatory Activities Of Salvia Libanotica’ And Salvia Officinalis’ Leaves Essential Oils. Eur. J. Pharm. Med. Res. 2022, 9, 34–43. [Google Scholar]
- Silva, D.M.; Costa, P.A.D.; Ribon, A.O.B.; Purgato, G.A.; Gaspar, D.-M.; Diaz, M.A.N. Plant Extracts Display Synergism with Different Classes of Antibiotics. An. Acad. Bras. Cienc. 2019, 91, e20180117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).